Abstract
Molecular targeted therapies are becoming ubiquitous in cancer treatment. These drugs may cause gastrointestinal toxicities including perforation, pneumatosis, enteritis, colitis and fistula formation. Knowledge of these complications and their management enables early radiological identification and appropriate intervention, reducing patient morbidity and mortality.
Targeted therapies, which attack the molecular weaknesses of cancers, are revolutionising cancer treatment, yet they bring toxicities very different from traditional chemotherapies. Bowel toxicities associated with these drugs may cause significant morbidity/mortality. Bowel toxicity associated with antiangiogenic agents, which function by inhibiting vascular endothelial growth factor (VEGF) receptors, is well documented [1]. It has been hypothesised that the decreased blood supply caused by angiogenesis inhibition may increase tumoral necrosis, leading to perforation [1]. Bevacizumab (Avastin; Genentech Inc., San Francisco, CA), a monoclonal antibody to VEGF, was the first antiangiogenic therapy that was shown to increase the risk of bowel perforation. It is now appreciated that increased risk of bowel perforation is a class effect of antiangiogenic inhibitors. Sunitinib (Sutent; Pfizer, New York, NY) and sorafenib (Nexavar; Bayer, Leverkusen, Germany), multitargeted tyrosine kinase inhibitors that inhibit VEGF receptors, have also been linked to bowel complications [2]. In addition to bowel perforation, several targeted therapies have been linked to pneumatosis. While the mechanism of pneumatosis is not well understood, antiangiogenic drugs and drugs targeting the epidermal growth factor receptor (EGFR), such as erlotinib (Tarceva; Genentech Inc.), have been associated with pneumatosis [2,3]. Mammalian target of rapamycin (mTOR) inhibitors, which block a kinase involved in cell growth, metabolism and angiogenesis, have also been associated with perforation and enteritis [4]. Immune modulators, a new class of targeted therapies that increase the antitumour immune response, have been associated with several autoimmune-mediated toxicities. A dreaded toxicity of ipilimumab (Yervoy; Bristol-Myers Squibb Pharmaceuticals, Uxbridge, UK), a drug that was approved for melanoma in 2011 by the Food and Drug Administration of the USA, is colitis and severe diarrhoea [5]. This paper familiarises radiologists with these side effects, allowing them to alert clinicians to drug toxicity, which will facilitate rapid drug cessation, and thus decrease morbidity and mortality. Table 1 summarises potential bowel complications of various molecular targeted agents by mechanism of action.
Table 1. Potential bowel complications of various molecular targeted therapies by mechanism of action.
| Mechanism of action | Generic name | Potential side effect of drugs with this mechanism of action |
| EGFR inhibitor | Cetuximab | Pneumatosis, colitis |
| Erlotinib | Pneumatosis, colitis | |
| VEGF inhibitor | Bevacizumab | Bowel perforation |
| Multityrosine kinase inhibitor | Sorafenib | Bowel perforation |
| Sunitinib | Bowel perforation | |
| mTOR inhibitor | Everolimus | Bowel perforation and enteritis |
| Temsirolimus | Bowel perforation and enteritis | |
| Immune modulator | Ipilimumab | Colitis/severe diarrhoea |
EGFR, epidermal growth factor receptor; mTOR, mammalian target of rapamycin; VEGF, vascular endothelial growth factor.
Gastrointestinal perforation
Gastrointestinal perforation occurs in 0.9–1.7% of patients on bevacizumab, with a 60-day mortality of approximately 25% [1,6]. Primary tumour in situ, radiation treatment, peritoneal carcinomatosis, recent colonoscopy, bowel surgery and higher antiangiogenic drug doses increase the risk of perforation [1,7,8]. Tumour type also predicts perforation risk, with higher rates reported in patients with ovarian cancer (6%).
Perforation may occur at surgical anastomoses (Figures 1 and 2), at sites of tumour in/on the bowel wall (primary or metastatic) or at otherwise normal bowel segments. Perforation may also occur at sites remote from tumour, and in association with enteritis or colitis (Figure 3). Patients with perforation typically present with abdominal pain, nausea or vomiting, or fever; however, large case studies have found up to 12.5% of patients to be asymptomatic, with perforation detected on surveillance imaging [6]. Given their long half-life (20 days), antiangiogenic agents should be held for 4–6 weeks before and after surgery to minimise perforation risk, bleeding and wound healing complications [7]. While antiangiogenic agents are routinely held before elective surgery, recent drug administration is not an absolute contraindication to emergent surgery. Other antiangiogenic agents such as sunitinib and sorafenib [9] may also cause perforation, and the same surgical precautions apply.
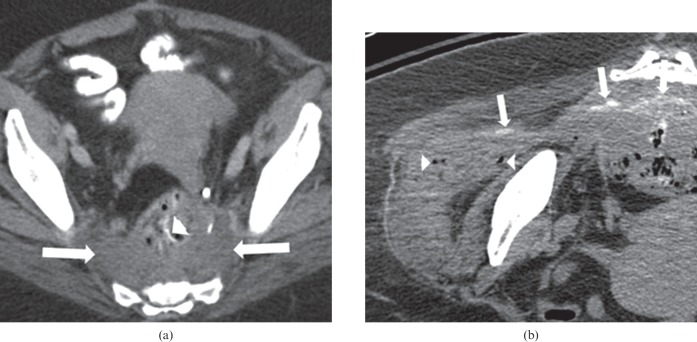
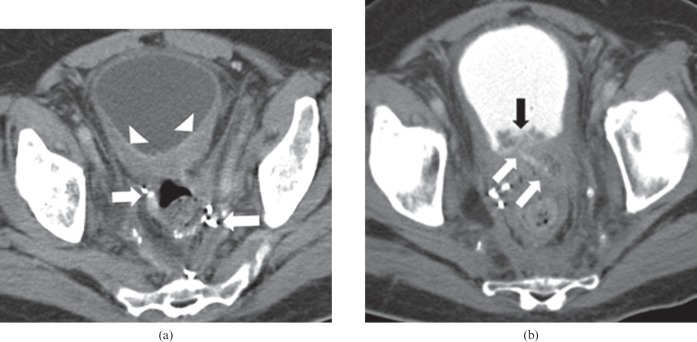
Figure 1.
66-year-old female undergoing treatment with bevacizumab and FOLFOX (folinic acid, fluorouracil and oxaliplatin), for locally recurrent rectal carcinoma at the site of the surgical anastomosis. The patient developed right hip pain, caused by perforation at the site of recurrence. (a) Pre-treatment axial contrast-enhanced CT of the pelvis demonstrates a soft tissue mass (arrows) in the pre-sacral space close to the surgical anastomosis (arrowhead), representing the site of tumour recurrence. (b) Non-contrast CT of the right hip after commencement of therapy and symptoms reveals a fistulous tract containing oral contrast (arrows) extending from the pre-sacral mass into the right gluteus medius muscle. Several locules of air (arrowheads) are also seen in the right gluteus medius muscle.
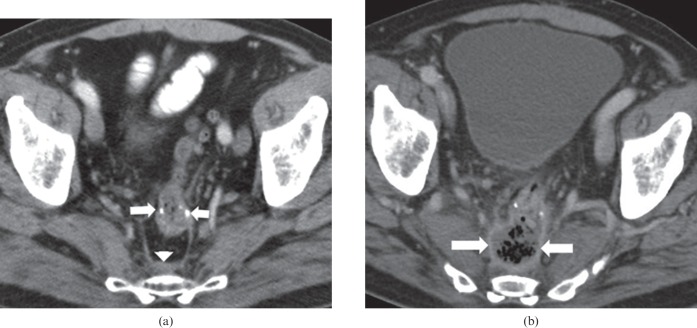
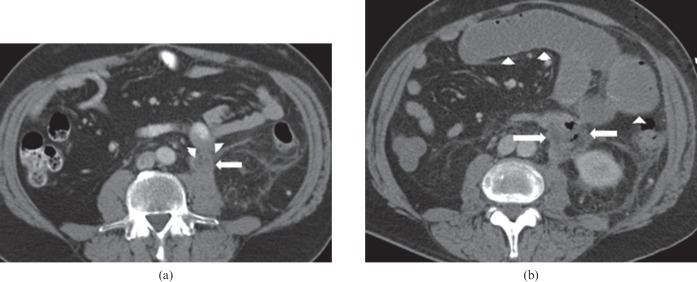
Figure 2.
57-year-old male on bevacizumab, cetuximab and irinotecan for metastatic rectal cancer. The patient had a previous history of anterior resection and adjuvant radiation treatment. (a) Pre-treatment axial contrast-enhanced CT of the pelvis demonstrates the surgical anastomosis (arrows) prior to commencement of treatment, with mild pre-sacral soft tissue thickening (arrowhead). (b) Post-treatment axial contrast-enhanced CT of the pelvis demonstrates dehiscence of the surgical anastomosis, with a predominantly air-filled collection in the pre-sacral space with an enhancing rim (arrows). Bevacizumab was discontinued and the patient was commenced on broad-spectrum antibiotics.
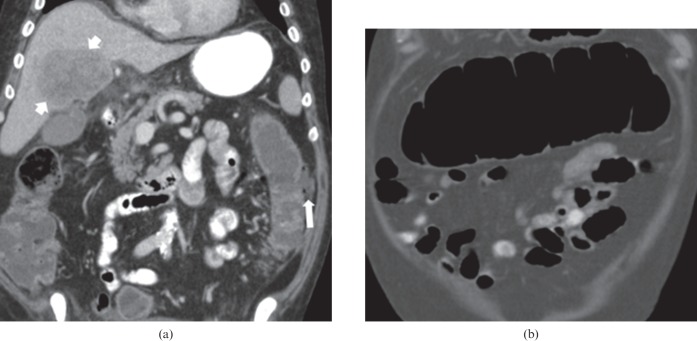
Figure 3.
71-year-old male with metastatic melanoma receiving ipilimumab. The patient developed abdominal pain and diarrhoea. (a) Coronal contrast-enhanced CT of the abdomen shows a fluid-filled colon, with focal perforation of the lateral wall of the left colon and a small collection containing a locule of extraluminal air (arrow). A defect can be seen in the wall of the left colon at the site of perforation. A large liver metastasis is also present (short arrows). (b) Coronal contrast-enhanced CT abdomen shows the dilated transverse colon, measuring up to 8 cm. The wall of the transverse colon is mildly thickened. The patient subsequently underwent a total colectomy and ileostomy. Pathology revealed severe active colitis, perforation and serositis, consistent with autoimmune colitis secondary to ipilimumab. Ipilimumab was discontinued permanently.
On CT, perforation may be seen as localised extraluminal air or frank pneumoperitoneum. Unstable patients should be considered for surgery. Stable patients can be managed with less invasive treatment, including bowel rest and broad-spectrum antibiotics, with or without percutaneous abscess drainage [6]. Antiangiogenic cancer treatments should be discontinued at diagnosis of perforation [1].
Pneumatosis with/without pneumoperitoneum
Pneumatosis intestinalis is characterised by subserosal and submucosal gas-filled cysts in the gastrointestinal tract, which may be associated with pneumoperitoneum and/or portal venous gas. It has been reported in patients treated with a variety of multikinase inhibitors [2,10]. The mechanism of pneumatosis is not well understood. One hypothesis is that antiangiogenic treatments reduce capillary density of intestinal villi, probably causing microperforation, and possibly allow air to infiltrate the bowel wall [2]. Longer exposure to these agents may increase the likelihood of pneumatosis [2]. Patients may be asymptomatic, with diagnosis made on routine surveillance scans [10]. Patients without pneumoperitoneum, peritonitis or sepsis may be managed by stopping the offending agent and close monitoring [10]. Figures 4 and 5 show the development of pneumatosis in patients on erlotinib and sunitinib. Figure 6 shows development of pneumatosis in a patient on sorafenib, which resolved upon drug discontinuation. Given prior treatment response, the drug was restarted; however, within 1 month of reinitiation the patient returned with severe abdominal pain, and more extensive pneumatosis forced drug cessation.
Figure 4.
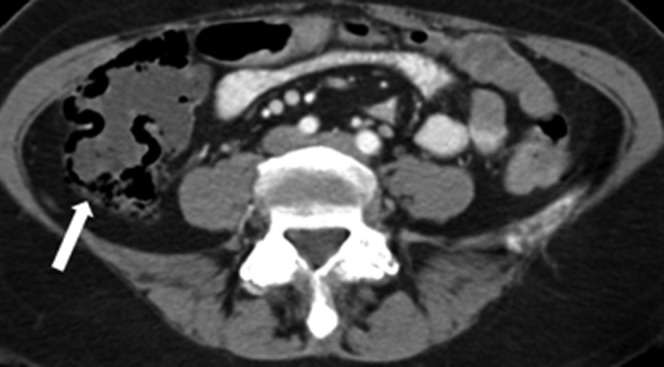
50-year-old female with multifocal bronchoalveolar carcinoma on single agent erlotinib for 5 months. Axial contrast-enhanced routine staging CT abdomen demonstrates pneumatosis of the wall of the fluid-filled right colon (arrow). CT abdomen performed 2 weeks later while patient remained off erlotinib (not shown) revealed complete resolution of pneumatosis, with normal colonic stool. Erlotinib was discontinued permanently.
Figure 5.
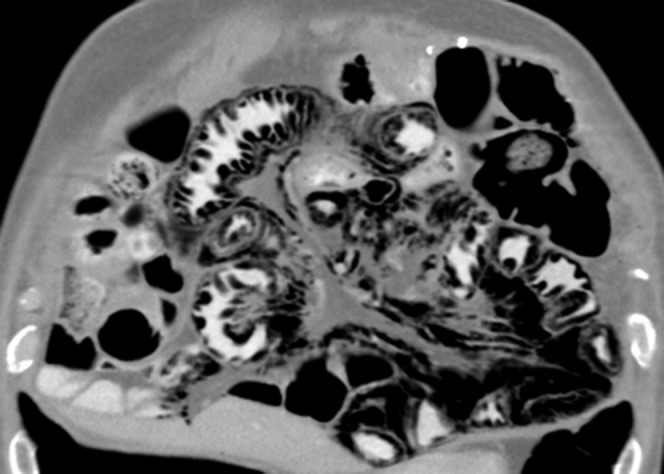
68-year-old male with metastatic renal cell carcinoma on sunitinib treatment. The patient developed abdominal discomfort. Coronal reformatted contrast-enhanced CT of the abdomen shows severe pneumatosis of the small bowel and pneumoperitoneum, with air tracking along the mesenteric vessels. No air is seen in the superior mesenteric vein or portal vein. Sunitinib was discontinued immediately, and the patient was placed on bowel rest. 10 weeks later, CT of the abdomen (not shown) revealed complete resolution of the pneumatosis and pneumoperitoneum following conservative management.
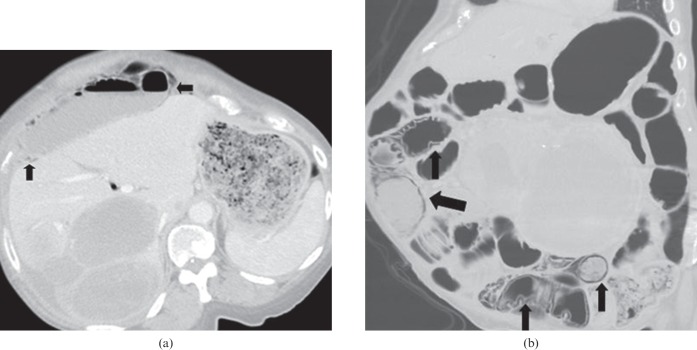
Figure 6.
57-year-old female with metastatic gastrointestinal stromal tumour undergoing treatment with sorafenib monotherapy, who presented with a 1-week history of increasing abdominal pain. (a) Axial CT scan of the abdomen performed with oral and intravenous contrast reveals pneumatosis along a colonic loop in the right upper quadrant (black arrow). Sorafenib was discontinued. CT scan (not shown) performed 1 week after cessation of therapy revealed resolution of pneumatosis. As the patient's disease had responded to sorafenib, therapy was reinstituted at the same dose. 1 month after reinitiation of therapy the patient again developed abdominal pain. (b) Coronal CT of the abdomen with oral and intravenous contrast then revealed extensive pneumatosis involving multiple bowel loops (black arrows). Sorafenib was permanently discontinued, and symptoms resolved.
Enteritis and colitis
Enteritis has been reported in patients undergoing treatment with mTOR inhibitors, immune modulators and tyrosine kinase inhibitors [4,5]. Patients may present with diffuse abdominal pain and diarrhoea, which typically resolves following drug discontinuation and supportive care. On CT, the wall of the small bowel is diffusely thickened and may be mildly dilated, with or without ascites (Figure 7).
Figure 7.
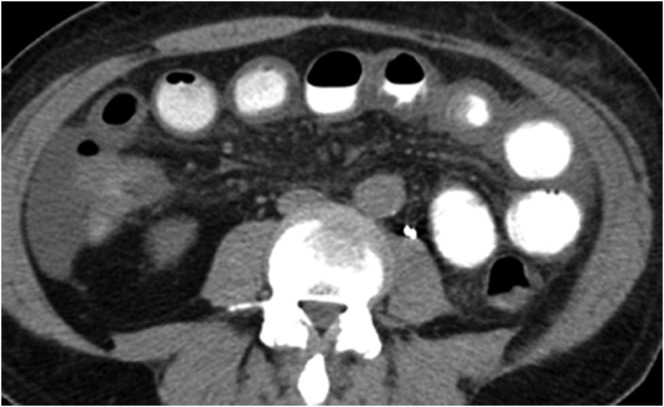
66-year-old male with metastatic renal cell carcinoma on temsirolimus and bevacizumab for 14 months who developed nausea, vomiting, diarrhoea and periumbilical pain. Axial contrast-enhanced CT abdomen demonstrates thickened loops of small bowel, consistent with enteritis, and low volume ascites. There was no pneumatosis or evidence of perforation. The patient was admitted and managed conservatively with bowel rest and intravenous fluids. Symptoms resolved following conservative treatment. Temsirolimus and bevacizumab were discontinued permanently (owing to disease progression). CT abdomen performed 18 days later (not shown) revealed resolution of the small bowel wall thickening and ascites.
Immune-mediated colitis occurs in 7.6% of patients receiving ipilimumab [11]. The hallmark presenting symptom is diarrhoea; however, patients may also present with abdominal pain, nausea, vomiting, fever or anal pain [5]. Development of colitis appears to be an indicator of treatment response, with significantly higher response rates in patients who developed colitis, when compared with their unaffected counterparts [5]. Patients can be managed with high-dose corticosteroids or with infliximab [5]. Administration of either of these treatments does not negatively impact tumour response [5]. Surgery may be required if colitis is complicated by perforation.
In very rare instances, antiangiogenic agents may cause an ischaemic colitis (Figure 8). The mechanism of this is unclear, but some have hypothesised that this could be secondary to reduced capillary density within the bowel wall [9]. Tyrosine kinase inhibitors targeting EGFR, such as erlotonib, have been shown to cause such inflammation, resulting in diarrhoea [12]. CT features of colitis include a fluid-filled lumen, colonic wall thickening, pericolonic fat stranding, mesenteric hyperemia and dilation of the affected bowel.
Figure 8.
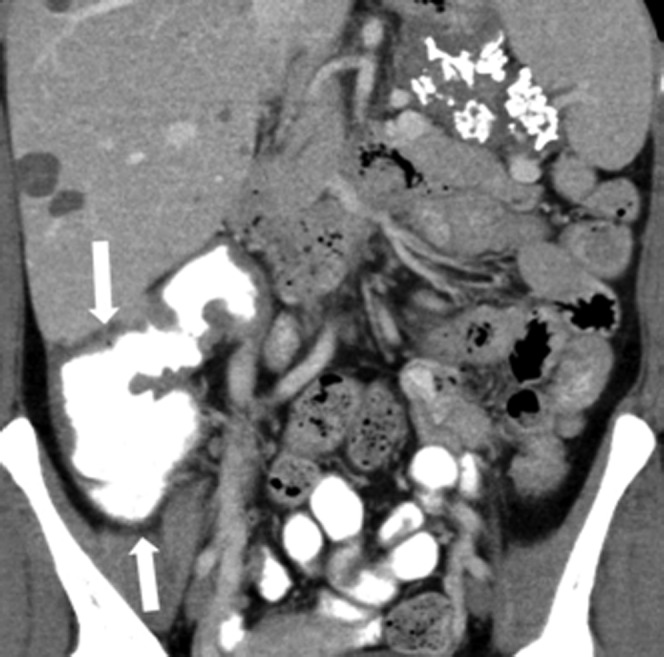
43-year-old female with metastatic gastrointestinal stromal tumour on sunitinib treatment. The patient developed diarrhoea and was feeling generally unwell. Coronal reformatted contrast-enhanced CT image of the abdomen shows severe thickening of the wall of the right colon (arrows) representing right-sided colitis. Small cystic liver lesions representing treated metastases are present in the right lobe of the liver. A soft tissue mass arising from the greater curvature of the stomach with internal calcifications represents the treated primary tumour. Sunitinib was discontinued, and the diarrhoea improved off treatment. 10 days later treatment was recommenced without dose reduction. CT of the abdomen performed 8 weeks later (not shown) demonstrated resolution of the right-sided colitis.
Fistula formation
Fistulae may develop between tumours and the urinary bladder (Figure 9), bowel (Figure 10), vagina, uterus, gallbladder or skin (Figure 1) during treatment with antiangiogenic agents [7]. Large metastatic deposits in the abdomen are at increased risk of developing fistulisation [13].
Figure 9.
68-year-old male with rectal carcinoma, post-low anterior resection and adjuvant radiation. (a) Pre-treatment axial contrast-enhanced CT of the pelvis demonstrates thickening of the posterior wall of the urinary bladder (arrowheads), which is probably radiation associated. Arrows point to surgical anastomosis from the previous anterior resection. The patient was treated with bevacizumab, fluorouracil and leucovorin when he developed haematuria, pneumaturia and fecaluria during treatment. (b) Post-treatment axial contrast-enhanced CT cystogram, with retrograde contrast instillation into the urinary bladder via a Foley catheter (not seen), shows contrast flowing along the fistulous tract from the urinary bladder to the rectum (white arrows). New irregular soft tissue thickening of the posterior wall of the urinary bladder is present (black arrow). Chemotherapy and bevacizumab were discontinued. The patient was treated with antibiotics. However, following recurrent episodes of urosepsis he required a diverting end colostomy.
Figure 10.
53-year-old male with metastatic rectal carcinoma, on cetuximab and FOLFOX (folinic acid, fluorouracil and oxaliplatin). (a) Pre-treatment axial contrast-enhanced CT abdomen shows a left para-aortic metastatic tumour deposit (arrow), which extends anteriorly to involve an adjacent small bowel loop, with associated thickening of the posterior wall of this loop of small bowel (arrowheads). (b) The patient developed symptoms of small bowel obstruction, and subsequent axial contrast-enhanced CT abdomen shows development of a tumour–bowel fistula (arrows) and small bowel obstruction (arrowheads). Oral contrast was not administered owing to the patient's symptoms of small bowel obstruction. The patient was managed conservatively, with intravenous fluids and nil per oral, as well as discontinuation of all chemotherapy and MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide].
Tumour–bowel fistulae may occur secondary to radiation treatment, progression of the cancer, in the setting of infection or secondary to antiangiogenic agents [13]. Tumour–bowel fistulae may also form as a tumour responds to treatment. As the mass necroses, a fistula may form from the bowel lumen into the lesion's necrotic centre [13]. These fistulae are more common in patients with epithelial ovarian cancer receiving bevacizumab, occurring in 1.8% of patients [8]. Rectovaginal nodularity is a risk factor [8].
Fistulae may be clinically silent, with surveillance imaging leading to diagnosis. Once identified, the antiangiogenic agent should be discontinued, and the patient should be closely observed. When fistulous tracks fail to heal following conservative measures, or when a patient develops sepsis or peritonitis, surgery or radiation may be considered [13].
Conclusion
Gastrointestinal complications, including perforation, pneumatosis, enterocolitis and the development of tumour–bowel fistulae may occur in patients undergoing treatment with molecular targeted therapies, and should be promptly identified by the radiologist to minimise patient morbidity and mortality.
References
- 1.Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol 2009;10:559–68 [DOI] [PubMed] [Google Scholar]
- 2.Coriat R, Ropert S, Mir O, Billemont B, Chaussade S, Massault PP, et al. Pneumatosis intestinalis associated with treatment of cancer patients with the vascular growth factor receptor tyrosine kinase inhibitors sorafenib and sunitinib. Invest New Drugs 2011;29:1090–3 [DOI] [PubMed] [Google Scholar]
- 3.Messersmith WA, Jimeno A, Jacene H, Zhao M, Kulesza P, Laheru DA, et al. Phase 1 trial of oxaliplatin, infusional 5-fluorouracil, and leucovorin (FOLFOX4) with erlotinib and bevacizumab in colorectal cancer. Clin Colorectal Cancer 2010;9:297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parithivel K, Ramaiya N, Jagannathan JP, O'Regan K, Krajewski K, Fisher D, et al. Everolimus- and temsirolimus-associated enteritis: report of three cases. J Clin Oncol 2011;29:e404–6 [DOI] [PubMed] [Google Scholar]
- 5.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol 2006;24:2283–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badgwell BD, Camp ER, Feig B, Wolff RA, Eng C, Ellis LM, et al. Management of bevacizumab-associated bowel perforation: a case series and review of the literature. Ann Oncol 2008;19:577–82 [DOI] [PubMed] [Google Scholar]
- 7.Saif MW. Managing bevacizumab-related toxicities in patients with colorectal cancer. J Support Oncol 2009;7:245–51 [PubMed] [Google Scholar]
- 8.Richardson DL, Backes FJ, Hurt JD, Seamon LG, Copeland LJ, Fowler JM, et al. Which factors predict bowel complications in patients with recurrent epithelial ovarian cancer being treated with bevacizumab? Gynecol Oncol 2010;118:47–51 [DOI] [PubMed] [Google Scholar]
- 9.Walraven M, Witteveen PO, Lolkema MP, van Hillegersberg R, Voest EE, Verheul HM. Antiangiogenic tyrosine kinase inhibition related gastrointestinal perforations: a case report and literature review. Angiogenesis 2011;14:135–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asmis TR, Chung KY, Teitcher JB, Kelsen DP, Shah MA. Pneumatosis intestinalis: a variant of bevacizumab related perforation possibly associated with chemotherapy related GI toxicity. Invest New Drugs 2008;26:95–6 [DOI] [PubMed] [Google Scholar]
- 11.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spigel DR, Burris HA, 3rd, Greco FA, Shipley DL, Friedman EK, Waterhouse DM, et al. Randomized, double-blind, placebo-controlled, phase II trial of sorafenib and erlotinib or erlotinib alone in previously treated advanced non-small-cell lung cancer. J Clin Oncol 2011;29:2582–9 [DOI] [PubMed] [Google Scholar]
- 13.Chow H, Jung A, Talbott J, Lin AM, Daud AI, Coakley FV, et al. Tumor fistulization associated with targeted therapy: computed tomographic findings and clinical consequences. J Comput Assist Tomogr 2011;35:86–90 [DOI] [PMC free article] [PubMed] [Google Scholar]