Abstract
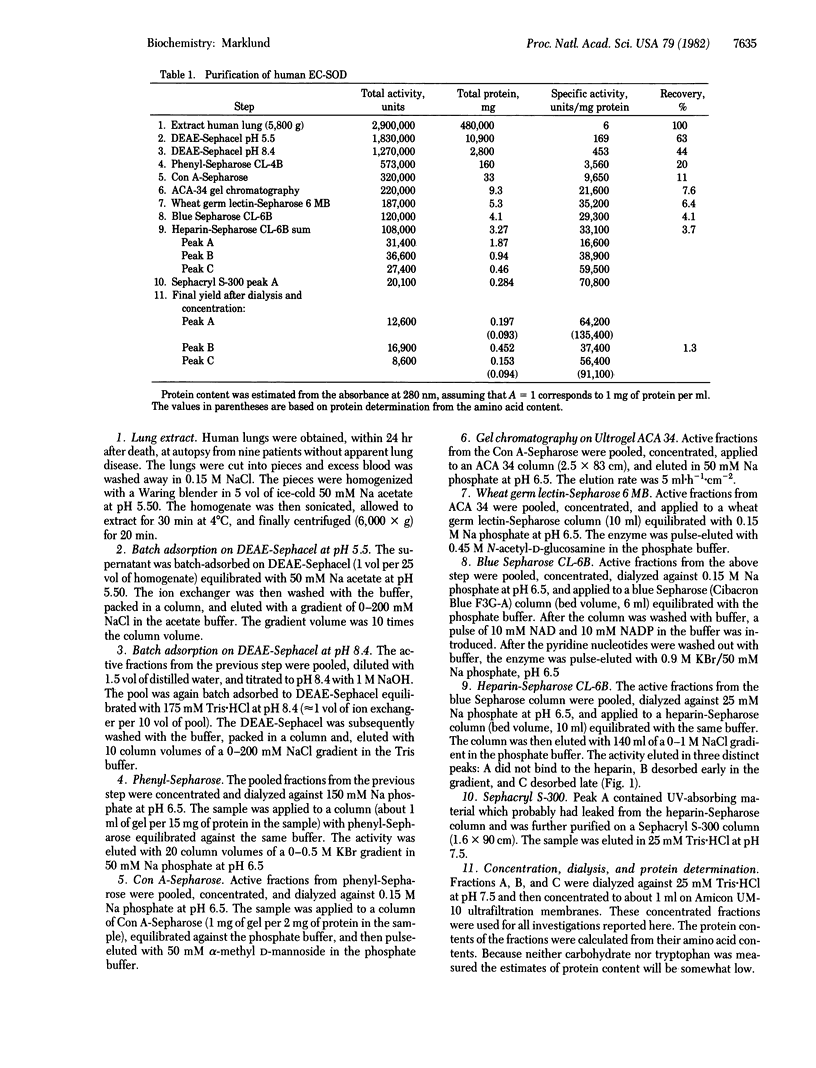
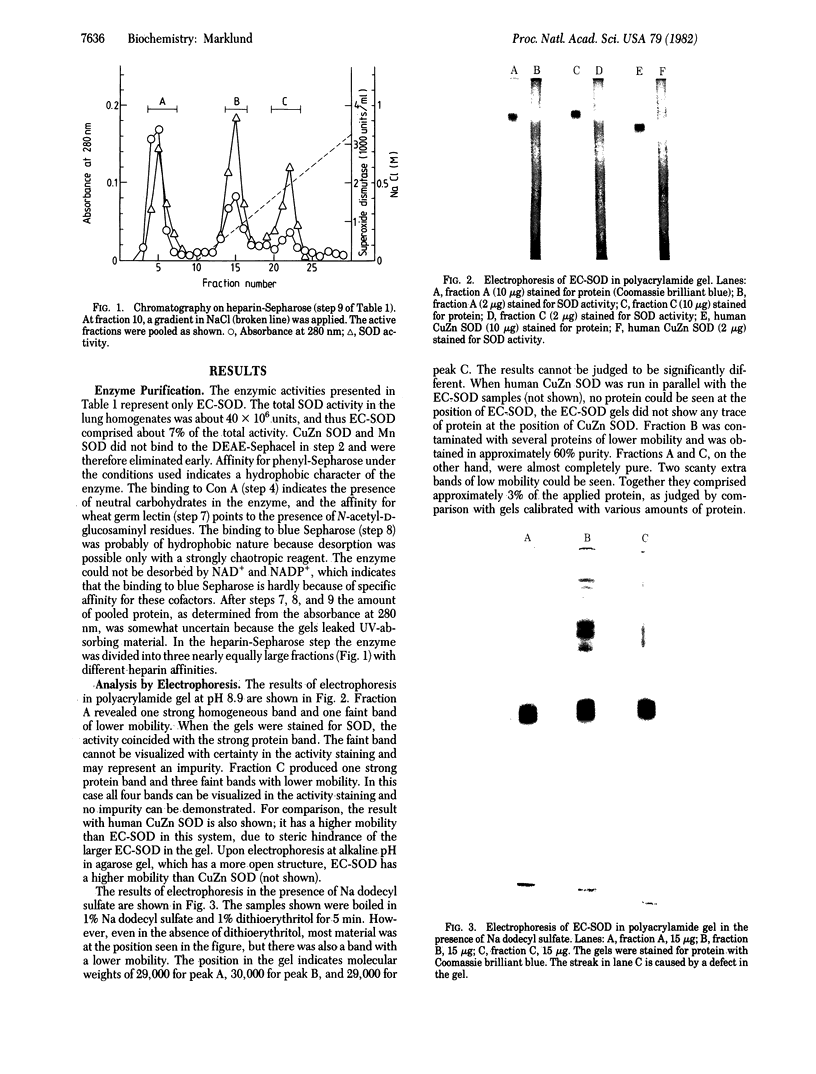
A superoxide dismutase (superoxide:superoxide oxidoreductase, EC 1.15.1.1), distinct from previously known superoxide dismutases, has been isolated from human lung tissue. It is probably of the same nature as a previously demonstrated high molecular weight superoxide dismutating factor in human extracellular fluids. The enzyme has a molecular weight around 135,000 and is composed of four equal noncovalently bound subunits. Each molecule appears to have four copper atoms. No iron or manganese was found in the enzyme. Cyanide inhibits the enzyme efficiently. The enzyme brings about a first-order dismutation of the superoxide radical, the rate constant for the catalyzed reaction being about 1 X 10(9) M-1 s-1 per copper atom. The enzyme has hydrophobic properties. Affinity for various lectins indicates the presence of carbohydrate. Upon chromatography on heparin-Sepharose it is divided into three fractions, one with no, one with weak, and one with strong affinity for heparin.
Full text
PDF

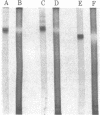
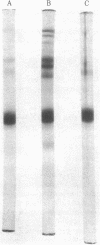
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bengtsson G., Olivecrona T., Hök M., Riesenfeld J., Lindahl U. Interaction of lipoprotein lipase with native and modified heparin-like polysaccharides. Biochem J. 1980 Sep 1;189(3):625–633. doi: 10.1042/bj1890625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervenka C. H. Long-column meniscus depletion sedimentation equilibrium technique for the analytical ultracentrifuge. Anal Biochem. 1970 Mar;34:24–29. doi: 10.1016/0003-2697(70)90082-5. [DOI] [PubMed] [Google Scholar]
- Keele B. B., Jr, McCord J. M., Fridovich I. Superoxide dismutase from escherichia coli B. A new manganese-containing enzyme. J Biol Chem. 1970 Nov 25;245(22):6176–6181. [PubMed] [Google Scholar]
- Marklund S., Beckman G., Stigbrand T. A comparison between the common type and a rare genetic variant of human cupro-zinc superoxide dismutase. Eur J Biochem. 1976 Jun 1;65(2):415–422. doi: 10.1111/j.1432-1033.1976.tb10356.x. [DOI] [PubMed] [Google Scholar]
- Marklund S. Distribution of CuZn superoxide dismutase and Mn superoxide dismutase in human tissues and extracellular fluids. Acta Physiol Scand Suppl. 1980;492:19–23. [PubMed] [Google Scholar]
- Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974 Sep 16;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Marklund S. Spectrophotometric study of spontaneous disproportionation of superoxide anion radical and sensitive direct assay for superoxide dismutase. J Biol Chem. 1976 Dec 10;251(23):7504–7507. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Rydén L., Björk I. Reinvestigation of some physicochemical and chemical properties of human ceruloplasmin (ferroxidase). Biochemistry. 1976 Aug 10;15(16):3411–3417. doi: 10.1021/bi00661a003. [DOI] [PubMed] [Google Scholar]
- Steinman H. M., Hill R. L. Sequence homologies among bacterial and mitochondrial superoxide dismutases. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3725–3729. doi: 10.1073/pnas.70.12.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Mitochondrial superoxide simutase. Site of synthesis and intramitochondrial localization. J Biol Chem. 1973 Jul 10;248(13):4793–4796. [PubMed] [Google Scholar]
- Yost F. J., Jr, Fridovich I. An iron-containing superoxide dismutase from Escherichia coli. J Biol Chem. 1973 Jul 25;248(14):4905–4908. [PubMed] [Google Scholar]