Abstract
Objectives
The aims of this study are to assess the extent of ovarian movement on consecutive MRI examinations in patients with gynaecological malignancies and to define potential safety volumes around the ovaries that may avoid ovarian ablation during pelvic irradiation.
Methods
Patients with cervical, vaginal and endometrial cancer who underwent MRI examinations of the pelvis before and during radiotherapy were included in the study. The position of the ovaries was retrospectively determined on two consecutive axial and sagittal T2 weighted MRI examinations of the pelvis. Ovarian movement was determined in craniocaudal, anteroposterior and mediolateral directions. Safety volumes were calculated by computing elliptical volumes based on the derived 95% and 99% reference intervals.
Results
30 patients with a gynaecological malignancy were included. Both ovaries could be identified on the MRI examinations in all cases. The safety volumes around the ovaries encompassing 95% and 99% of ovarian movement were 11 and 25 cm3 (95%), and 24 and 54 cm3 (99%), for the left and right ovary, respectively.
Conclusion
Adding a safety volume around the ovaries may reduce the high radiation dose to the ovaries. This could potentially avoid ovarian ablation, reducing significant fertility morbidity.
Radiotherapy damages the ovaries, and when young females undergo radical pelvic radiotherapy for cancer, there is a risk of premature menopause and infertility due to the high radiation dose received by the ovaries, also known as ovarian ablation [1]. The radiation dose required for this to occur depends on patient age and previous exposure to chemotherapy; immediate ovarian failure results from a dose of 16.5 Gy in females younger than 20 years, while 14 Gy is sufficient in 30-year-old females [1,2]. In current clinical practice, this is either accepted as a known complication of the procedure or the ovaries are surgically transposed out of the pelvis to reduce the received dose below the ablation threshold positioning [3]. In both cases, future pregnancies would require either donor ova or in vitro fertilisation. Avoiding these complications is especially relevant in young females with unilateral (bony or soft tissue) pelvic sarcoma and unilateral pelvic lymphoma as in these patients the tumour and the ovary are located on contralateral sides in the pelvis, and the safety volumes will not overlap the treatment areas.
With recent technical improvements in radiotherapy such as intensity-modulated radiotherapy (IMRT), which allows reduction of the radiation dose to critical structures adjacent to the high-dose area and the ability to plan treatment on MRI examinations fused to CT images [4], the position of the ovaries can be delineated prior to the procedure and the radiation dose to the ovaries may be improved. If the ovaries are within the target volume, as in the treatment of gynaecological cancers, then transposing the ovaries out of the pelvis is the only possibility to maintain ovarian function. For sarcoma of the pelvic bones, soft tissue or unilateral lymph nodes within the pelvis or upper thigh, the ovarian dose can be minimised by the use of adequate shielding, or a non-divergent border adjacent to the ovary [5]. However, the ovaries are known to move within the pelvis every day, probably in relation to bladder and rectal distension [6-8]. Therefore a safety margin (planning organ-at-risk volume, PRV) has to be added around the ovaries to encompass the daily movement of the ovaries during radiotherapy to ensure that the ovaries are not within the high-radiation-dose area.
Previous studies have determined the position of the ovaries within the pelvis on CT and MRI [7,8]. However, to date, no information is available on the extent of ovarian movement over time to deduce what margins should be added to the position of the ovary to account for ovarian movement and avoid ovarian ablation. Therefore the aims of our study were to assess the extent of ovarian movement on consecutive MRI examinations in patients with gynaecological malignancies and to define potential safety volumes around the ovaries in order to avoid ovarian ablation during pelvic radiotherapy.
Methods and materials
Patients
The study was approved by the Cambridge University Hospitals ethics committee, which waived the requirement for informed consent owing to the retrospective nature of the study design. Patients with advanced cervical, endometrial and vaginal cancer who underwent external beam radiotherapy followed by brachytherapy in our institution and had consecutive MRI examinations of the pelvis before and 4 weeks after the start of radiotherapy between November 2008 and June 2010 were eligible for inclusion in the study. Although the ovaries in this patient group are included in the radiation field, this particular group of patients was chosen because they routinely undergo consecutive MRI examinations as part of their standard clinical care. Patients younger than 18 years or older than 80 years and patients with ovarian cancer, a history of a hysterectomy and/or unilateral or bilateral oophorectomy were excluded.
Image analysis
MRI examinations included axial and sagittal T2 weighted images with the ovaries included in the field of view on both series and the L5–S1 intervertebral disc included on the sagittal images. No further constraints on image parameters, such as echo time, repetition time, image-matrix, field of view or magnetic field strength, were defined. Patients were asked to empty the bladder prior to the MRI examination.
The position of the ovaries was determined by a radiologist with 8 years of experience in pelvic MRI by retrospectively reviewing the MRI examinations. The slice numbers of the axial and sagittal images in which the left and right ovaries were positioned were identified on a picture archiving and communication system (PACS; GE Healthcare, Waukesha, WI) and recorded.
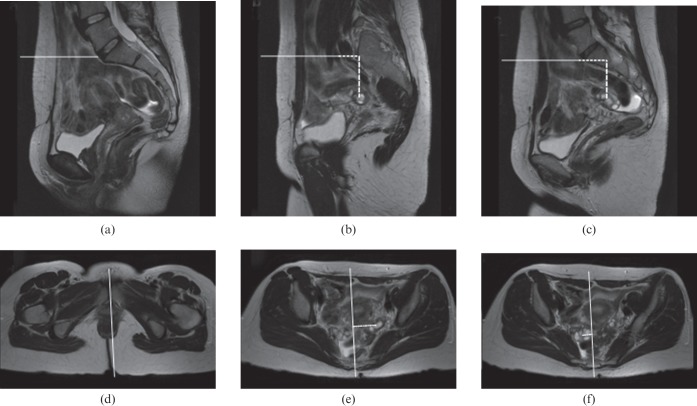
The position of the ovaries was determined relative to a bony reference point. Axial and sagittal images were shown on adjacent monitors on a dedicated workstation (Advanced Workstation; GE Healthcare). Two reference lines were superimposed onto the images. A horizontal reference line was positioned on the sagittal images perpendicular to the centre of the anterior margin of the L5–S1 intervertebral disc and copied to all slices. The second reference line was positioned on the axial images, from the pubic symphysis anteriorly and through the centre of the sacrum posteriorly, and copied to all slices. The craniocaudal and anteroposterior position of the ovaries was determined on the sagittal images (Figure 1). Positive values were assigned if the ovaries were positioned below and posterior to the reference line for the craniocaudal and anteroposterior direction, respectively. If the ovaries were located above or anterior to the reference line, negative values were assigned to the ovarian position. The mediolateral position of both ovaries was determined on the axial images. Positive values were assigned to the position if the left ovary was positioned on the left side of the reference line and the right ovary on the right side; in all other cases medio-lateral ovarian position was assigned a negative value (Figure 1). All measurements were repeated on the second MRI examination of the same patient using the same method. If patients underwent more than two MRI examinations, the first two available MRI studies were used in the analyses.
Figure 1.
Typical example of the measurement of the ovarian position on (a–c) sagittal and (d–f) axial T2 weighted MR images in (a) a 38-year-old patient with Stage IIb cervical cancer. The position of the ovaries relative to the L5–S1 intervertebral disc were (b) 43 mm and (c) 43 mm in craniocaudal direction, (b) 20 mm and (c) 32 mm in anteroposterior direction, and (e) 35 mm and (f) 13 mm in mediolateral direction for the (b, e) left and (c, f) right ovary, respectively.
Data collection and statistical analysis
Data on patient age (mean and range), menopausal status (pre-menopausal or post-menopausal), number of days between consecutive MRI examinations (mean and range) and tumour type were collected.
Ovarian movement in each direction (craniocaudal, anteroposterior and mediolateral) was defined as the position of the ovary determined on the second MRI examination minus the position as determined on the first MRI. Ovarian movement was reported in millimetres. Movement in cranial, anterior and medial direction was defined as positive movement, and movement in caudal, posterior and lateral direction was defined as negative movement. For the purpose of graphical representation Bland–Altman plots were composed to illustrate the extent of left and right ovarian movement. The normality assumption was formally assessed from the resulting distributions using a Shapiro–Wilk test.
The combined craniocaudal, anteroposterior and mediolateral movement was summarised as the magnitude of the vector defining gross ovarian movement between the consecutive MRI examinations. This was formally quantified as m=√(x2+y2+z2), where x is mediolateral, y is craniocaudal and z is anteroposterior movement. The magnitude of gross movement (m) between left and right ovaries was compared using a paired Student's t-test. Difference in m between pre- and post-menopause was formally assessed using an unpaired Student's t-test for both left and right ovary. Absolute values of the individual movement (mediolateral, craniocaudial and anteriorposterior) were also assessed by comparing the absolute differences using a paired Wilcoxon signed-rank test.
A two-way analysis of variance model was used to compute the within- and between-subject variations, and the model was performed for each separate direction in both the left and right ovaries [the 95th and 99th reference intervals (RIs) were defined as mean ±1.96 standard deviation (SD) and mean ±2.58 SD, respectively, with SD defined as the square-root of the within-subject variation]. Overall safety volumes in craniocaudal, antero-posterior and mediolateral direction were estimated by using the 95% and 99% RIs for both ovaries and volumes bound within a three-dimensional ellipse, which was defined as v=(4π/3)xyz. The analyses were performed using the statistical programming language R (v. 2.5.1; The R Foundation for Statistical Computing, Vienna, Austria).
Results
30 patients with advanced cervical, endometrial and vaginal cancer underwent successive MRI examinations of the pelvis before and during radiotherapy treatment. The mean age of patients was 53 years (range 29–79 years). The mean time period between consecutive MRI scans was 79 days (range 23–331 days). Patients' baseline characteristics are summarised in Table 1.
Table 1. Baseline characteristics.
| Parameter | Value |
| Mean age (range) | 53 years (29–79 years) |
| Menopausal status | |
| Pre-menopausal | 15 |
| Post-menopausal | 15 |
| Mean number of days between MRI scans (range) | 79 days (23–331 days) |
| Cancer type | |
| Cervical cancer | 28 |
| Vaginal cancer | 1 |
| Endometrial cancer | 1 |
Both ovaries could be identified on the axial and sagittal MR images in all cases. A typical example of the assessment of ovarian movement is shown in Figure 2. The movement of both ovaries in all three directions is graphically illustrated in Figure 3.
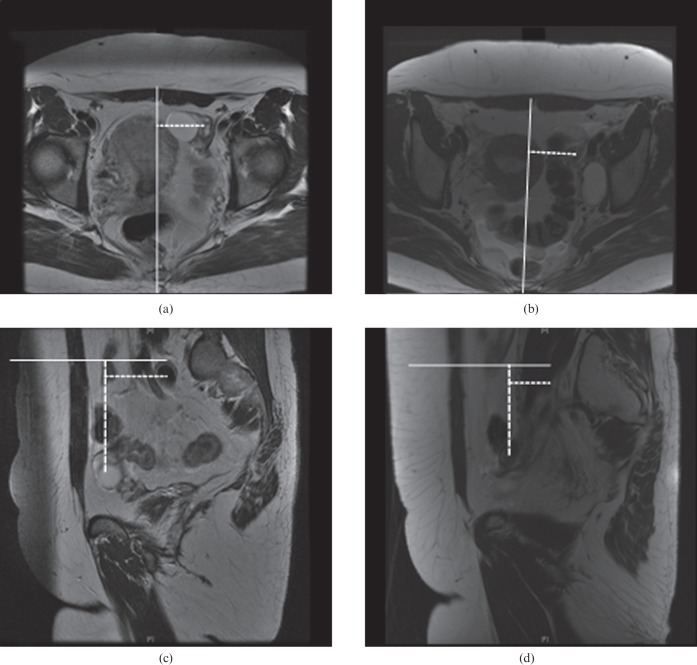
Figure 2.
Typical example of the assessment of movement of the left ovary in a 37-year-old patient with node-positive Stage IB1 cervical cancer (not shown) on (a, b) axial and (c, d) sagittal T2 weighted MR images. (b) A left-sided lymphocele was noted on the second MRI examination. The left ovary moved 13, −10 and 0 mm in craniocaudal, anteroposterior and mediolateral directions, respectively, between the (a, c) first and (b, d) second MRI examinations. The continuous lines represent the reference lines and the dotted lines represent the measurements from the reference line to the ovaries.
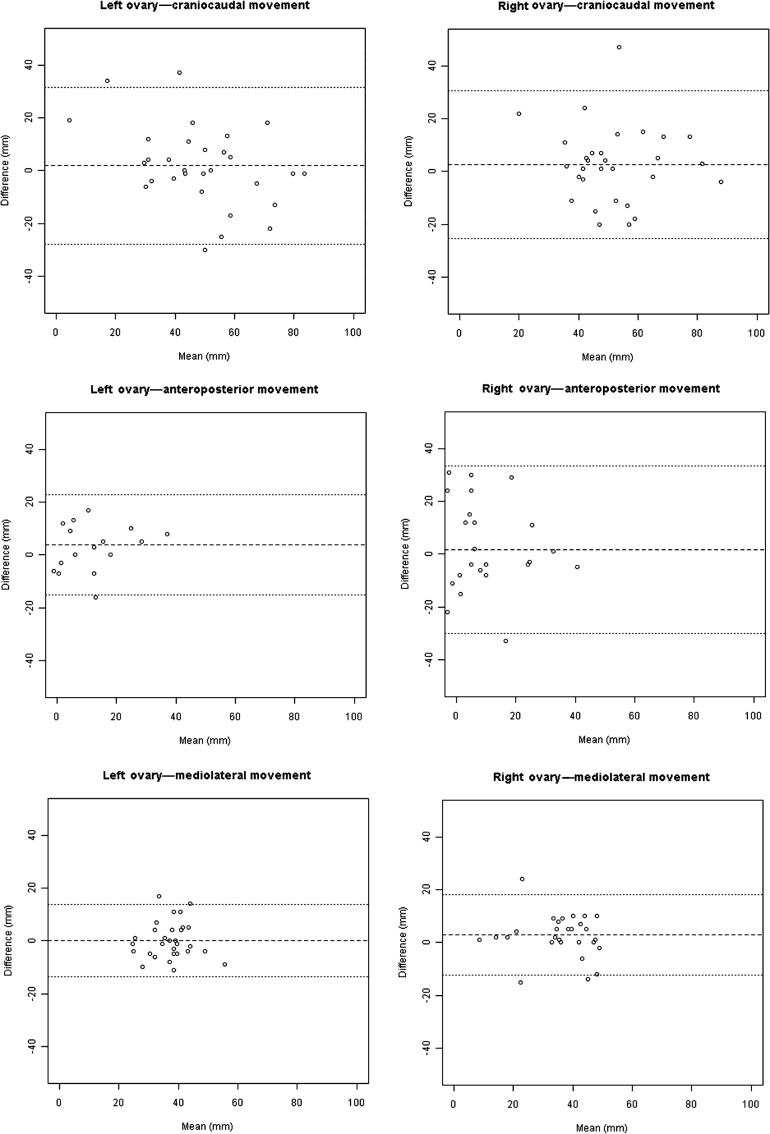
Figure 3.
Bland–Altman plots showing craniocaudal, anteroposterior and mediolateral movement in both the left and right ovaries.
No statistically significant difference was found in the gross movement (m) between the left (17.0±9.67 mm) and right ovary (19.7±11.95 mm; p=0.314), nor between pre-menopausal (left ovary 19.1±10.09 mm; right ovary 16.1±9.19 mm) and post-menopausal females (left ovary 14.9±9.08 mm; right ovary 23.3±13.56 mm; p=0.24). A paired comparison of movement between left and right ovary in all three directions is reported in Table 2; none of the individual components of movement was statistically significant. However, there is some evidence to suggest that there could be a difference in the antero-posterior direction (p=0.084).
Table 2. Paired comparison of movement between the left and right ovaries.
| Parameter | Left ovary (mm) | Right ovary (mm) | p-value |
| Craniocaudal movement | 7.5 (3–18) | 9 (3–15) | 0.718a |
| Anteroposterior movement | 7.5 (3–12) | 11 (4–19) | 0.084a |
| Mediolateral movement | 4.5 (2–8) | 5 (2–9) | 0.895a |
Data are medians and first and third inter-quartiles.
aPaired Wilcoxon signed-rank test.
Based on derived RIs (Table 3), safety volumes for the 95th and 99th RIs would encompass 11 and 25 cm3 for the left ovary and 24 and 54 cm3 for the right ovary, respectively. Figure 4 shows the safety volumes (95% and 99%) superimposed onto the pre-treatment planning CT scan. An example of defining a safety volume for the left ovary based on the 95% RI therefore involves extending axes from the centre of the ovary and prescribing locations from the boundary of the ovary at −16 and +12 mm in caudal and cranial directions, −34 and +26 mm in posterior and anterior directions, and −7 and +6 mm in lateral and medial directions, respectively (Table 3).
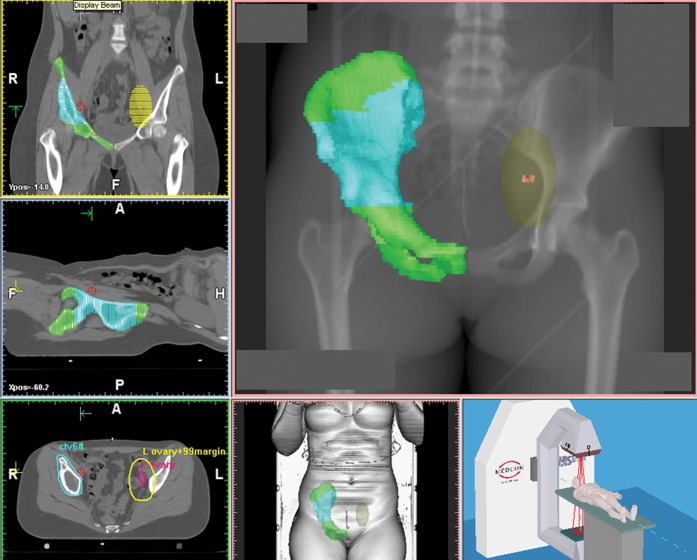
Figure 4.
Radiotherapy planning CT scan of the pelvis of a 34-year-old female with pelvic Ewing sarcoma of the right iliac wing. The left ovary is shown in pink, the 99% safety margins is superimposed onto the images in yellow. The gross tumour volume is marked in blue and the treatment volume in green. In this patient, both ovaries were transposed out of pelvis as there was no evidence available in the literature on what planning organ-at-risk volume to use.
Table 3. 95% and 99% reference intervals and safety volumes for both ovaries.
| Parameter | Left ovary | Right ovary |
| Craniocaudal movement (mm) | ||
| 95% RI | 28 (−16 to +12) | 41 (−23 to +18) |
| 99% RI | 37 (−21 to +17) | 53 (−29 to +24) |
| Anteroposterior movement (mm) | ||
| 95% RI | 59 (−34 to +26) | 25 (−14 to +11) |
| 99% RI | 78 (−43 to +35) | 33 (−18 to +15) |
| Mediolateral movement (mm) | ||
| 95% RI | 13 (−7 to +6) | 44 (−25 to +19) |
| 99% RI | 17 (−9 to +8) | 58 (−32 to +26) |
| Safety volumes (cm3) | ||
| 95% safety volume | 11 | 24 |
| 99% safety volume | 25 | 54 |
RI, reference interval.
Discussion
Irradiation produces severe dose-related gonadal damage to both the germ cell and endocrine components of ovarian tissue. It may cause immediate permanent sterility or temporary cessation of menses, or lead to premature menopause. The probability of infertility from a given dose of radiotherapy increases with age and concurrent use of chemotherapy: immediate ovarian failure will be produced by 16.5 Gy in females 20 years of age, while 14 Gy is sufficient in 30-year-olds [1,2]. If the ovaries are adjacent to irradiation beams but not within the target volume, the dose can be minimised by the use of adequate shielding, by angulation of the treatment head or by a non-divergent border adjacent to the ovary in question. However, the level of scattered irradiation may still be sufficient to destroy function in this ovary and methods of oophoropexy (surgical relocation of the ovary) should be considered [1,3].
With recent technical advances in radiotherapy such as IMRT and the fusion of planning CT images with MRI examinations using commercially available Conformité Européenne marked software, the ovaries can now be reliably delineated for radiotherapy planning purposes. ICRU report 62 suggests drawing margins around the organs at risk (PRV) to account for geometric uncertainty in the radiotherapy treatment process [9]. The ovaries can be clearly depicted on the MRI examination during treatment planning and an integrated margin (PRV) could be added to take ovarian movement during treatment into account.
To our knowledge, no information is available in literature on the extent of ovarian movement. Our study showed that the ovaries move substantially within the pelvis over time. Based on our measurements, defining safety volumes of 11 and 25 cm3 (based on the 95% RI) or 24 and 54 cm3 (based on the 99% RI) around the ovaries during treatment planning to account for ovarian movement could possibly avoid ovarian ablation and premature menopause and infertility. Our results are especially relevant for young females with unilateral bony or soft tissue pelvic sarcoma and unilateral pelvic lymphoma as in these patients the tumour and the ovary are located on contralateral sides and the safety volumes do not overlap the treatment areas. Furthermore, in patients with unilateral sarcoma or lymphoma the uterus is not in the high-dose radiation field, which would be the case in patients with a gynaecological malignancy and could further reduce the probability of a successful future pregnancy.
It seems that no other studies have assessed the extent of ovarian movement over time. Counsell et al [7] reported the position of the ovaries on CT examinations and found 83% of the ovaries (19 patients) to be located in the upper two-thirds of a ring defined by the sacro–iliac joints, the bony pelvic side wall and the pubic symphysis. The position of the ovaries was not found to be influenced by parity, uterine orientation, the degree of bladder filling or faecal loading in the rectum [7]. Nicholson et al [8] assessed the effect of bladder filling on the position of the ovary and found the ovaries to move 2.1 cm inferiorly and 0.32 cm posteriorly after bladder emptying by determining ovarian position on MRI examinations acquired before and after micturition. Since the purpose of their study was to assess the effect of micturition on the ovarian position, the successive MRI examinations were performed within a very short period of time, and this probably did not allow for the full extent of ovarian movement to take place, and therefore the degree of ovarian movement could have been underestimated. Furthermore, both studies were conducted more than 10 years ago and the ovaries could not be identified in 13% (3 of 23 patients) of patients on CT and in 42% (5 of 12 volunteers) of patients on MRI [7,8]. With the increasing technical developments in MRI scanners and MRI acquisition protocols, the quality and spatial resolution of MRI examinations have improved substantially over the last decade. In our study, we were able to locate the ovaries in all patients on both axial and sagittal imaging.
We found no significant difference in the extent of ovarian movement between the left and right ovary (p=0.314). However, there was a substantial size difference between both safety volumes, the safety volume for the right ovary being twice the size of the safety volume for the left ovary. We hypothesise that the lesser extent of movement of the left ovary could be explained by movement of the left ovary being restricted by the overlying sigmoid colon, while the right ovary has no anatomical restrictions. No comparable observations were reported in previous studies on any asymmetry in ovarian position or ovarian movement [7,8].
Our study has some limitations. First, our study has a relatively small patient population, which impairs the precision of the RI calculations. This study is a proof of principle, which presents a novel combination of image and statistical analysis techniques and reports an approach to quantify safety margins. Prescribing safety margins around the ovaries seems timely because of the advent of more targeted radiotherapy treatment planning systems and commercially available Conformité Européenne marked software to fuse MRI to CT, making delineation of the ovaries during radiotherapy planning feasible. Design of future prospective studies to quantify safety margins should ideally include an MRI examination at an immediate short-term follow-up, as well as a longer-term follow-up scan within 1–2 months. Second, we only used two MRI examinations per patient with a wide variability in the time interval between the two MRI studies. Third, in our study we included females with cervical, endometrial and vaginal cancer who underwent radical pelvic chemoradiotherapy, although it would be impossible to reduce the high radiation dose to the ovaries in these patients as the ovaries are located too close to the primary tumour. Finally, we studied patients with a broad age range of 29–79 years; however, the application of ovarian safety margins is probably suited to younger females with higher likelihood of subsequent conception and in future studies it might be advisable to specifically target this population. The patients included in our study are the only patients that routinely undergo consecutive pelvic MRI examinations as part of their standard clinical care, and are therefore a suitable patient group in which ovarian movement over time can be studied on MRI. Young female patients with sarcoma in the pelvis or unilateral lymph nodes within the pelvis or in the upper thigh could benefit from our results as these tumours are generally located at a larger distance from the ovaries.
In conclusion, with the recent development of being able to plan radiotherapy treatment on MRI examinations fused with a planning CT examination, the ovaries can be clearly delineated during radiotherapy treatment planning. The results of our measurements suggest a safety volume of 11 and 25 cm3 (based on the 95% RI) or 24 and 54 cm3 (based on the 99% RI) should be added to the left and right ovary, respectively, to account for ovarian movement in order to reduce the radiation dose to the ovaries. If our results were to be implemented into clinical practice during radiotherapy planning in young females with unilateral pelvic sarcoma or lymphoma, perhaps the incidence of premature menopause and infertility could potentially be reduced in these patients during radiotherapy of the pelvis.
Acknowledgments
We would like to thank Wendy Philips for her help with the ethics application and Prof. A K Dixon for his advice on the paper.
Footnotes
Funding support from NIHR: Cambridge Biomedical Research Centre, the University of Cambridge, Hutchison Whampoa Limited, the Cambridge Experimental Cancer Medicine Centre, ACT and Cancer Research UK.
References
- 1.Wallace WH, Thomson AB, Saran F, Kelsey TW. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys 2005;62:738–44 [DOI] [PubMed] [Google Scholar]
- 2.Wallace WH, Shalet SM, Crowne EC, Morris-Jones PH, Gattamaneni HR. Ovarian failure following abdominal irradiation in childhood: natural history and prognosis. Clin Oncol (R Coll Radiol) 1989;1:75–9 [DOI] [PubMed] [Google Scholar]
- 3.Mazonakis M, Damilakis J, Varveris H, Gourtsoyiannis N. Radiation dose to laterally transposed ovaries during external beam radiotherapy for cervical cancer. Acta Oncol 2006;45:702–7 [DOI] [PubMed] [Google Scholar]
- 4.Schlemmer HP. Imaging for new radiotherapy techniques. Cancer Imaging 2010;10:S73 [Google Scholar]
- 5.Royal Colleges of Physicians, Radiologists, and Obstetricians and Gynaecologists. The effects of cancer treatment on reproductive functions. Guidance on management. 2007 [cited 30 November 2010]. Available from: http://www.rcr.ac.uk/index.asp?PageID=149&PublicationID=269. [Google Scholar]
- 6.Williams P, Warwick R. Gray's anatomy. 36th edn. London, UK: Churchill Livingstone; 1980. p. 1423 [Google Scholar]
- 7.Counsell R, Bain G, Williams MV, Dixon AK. Artificial radiation menopause: Where are the ovaries? Clin Oncol 1996;8:250–53 [DOI] [PubMed] [Google Scholar]
- 8.Nicholson R, Coucher J, Thornton A, Connor F. Effect of a full and empty bladder on radiation dose to the uterus, ovaries and bladder from lumbar spine CT and X-ray examinations. Br J Radiol 2000;73:1290–6 [DOI] [PubMed] [Google Scholar]
- 9.International Commission on Radiation Units and Measurements Prescribing, recording and reporting photon beam therapy (supplement to ICRU Report 50). ICRU Report 62. Bethesda, MD: International Commission on Radiation Units and Measurements; 1999 [Google Scholar]