Abstract
Objectives
To assess the safety and feasibility of high-intensity focused ultrasound (HIFU) ablation of liver tumours and to determine whether post-operative MRI correlates with intra-operative imaging.
Methods
31 patients were recruited into two ethically approved clinical trials (median age 64; mean BMI 26 kg m−2). Patients with liver tumours (primary or metastatic) underwent a single HIFU treatment monitored using intra-operative B-mode ultrasound. Follow-up consisted of radiology and histology (surgical trial) or radiology alone (radiology trial). Radiological follow-up was digital subtraction contrast-enhanced MRI.
Results
Treatment according to protocol was possible in 30 of 31 patients. One treatment was abandoned because of equipment failure. Transient pain and superficial skin burns were seen in 81% (25/31) and 39% (12/31) of patients, respectively. One moderate skin burn occurred. One patient died prior to radiological follow-up. Radiological evidence of ablation was seen in 93% (27/29) of patients. Ablation accuracy was good in 89% (24/27) of patients. In three patients the zone of ablation lay ≤2 mm outside the tumour. The median cross-sectional area (CSA) of the zone of ablation was 5.0 and 5.1 cm2 using intra-operative and post-operative imaging, respectively. The mean MRI:B-mode CSA ratio was 1.57 [95% confidence interval (CI)=0.57–2.71]. There was positive correlation between MRI and B-mode CSA (Spearman's r=0.48; 95% CI 0.11–0.73; p=0.011) and the slope of linear regression was significantly non-zero (1.23; 95% CI=0.68–1.77; p<0.0001).
Conclusions
HIFU ablation of liver tumours is safe and feasible. HIFU treatment is accurate, and intra-operative assessment of treatment provides an accurate measure of the zone of ablation and correlates well with MRI follow-up.
Colorectal cancer is one of the leading causes of mortality in Europe and the USA [1]. Of those diagnosed with colorectal cancer, 20–30% will have metastases in the liver at the time of diagnosis, and overall 50% will develop liver metastases during the course of their disease [2,3]. Despite this, at post-mortem, approximately one-quarter of patients are found to have metastases confined to the liver, and so the possibility of cure in such patients is available if these metastases can be treated effectively. Hepatic resection is feasible in only 10–25%, and although it is associated with an operative mortality of up to 5%, it can achieve 5-year survival rates in the region of 40% [4]. The favoured current alternative to surgery is combination chemotherapy, but this is associated with an objective response rate of just 20–50%, and relatively short median overall survival of approximately 12 months [5]. As a result there have been considerable efforts to provide minimally invasive alternatives to surgery for these patients. These alternatives include transarterial chemo-embolisation (TACE), direct percutaneous ethanol injection (PEI), and energy-based ablative techniques such as radiofrequency ablation (RFA), cryoablation, microwave thermotherapy and high-intensity focused ultrasound (HIFU).
The only entirely non-invasive local therapy to be proposed to date is HIFU, which was proposed as a technique for extracorporeal ablation some years ago [6]. Its attractiveness stems from its ability to create homogeneous coagulative necrosis in an accurately targeted area without entering the abdominal cavity, and without damage to surrounding normal tissue. Serious complications occur rarely, and discharge from hospital is invariably possible within 24 h. Wu et al [7] used the extracorporeal Model JC tumour therapy device (Chongqing Haifu, Chongqing, China) to treat 68 patients with liver malignancies. They reported 30 cases for which surgical excision followed HIFU ablation; in all cases the tumour was completely ablated [7]. The group went on to report a series of 474 patients with hepatocellular carcinoma treated using the same Model JC device, although HIFU was often used in combination with other treatments such as TACE [8,9]. HIFU ablation has also been used for symptom palliation in patients with advanced-stage liver cancer. Li et al [10] reported a series of 100 patients with liver cancer who were treated with HIFU, including 62 patients with primary liver cancer and 38 with metastatic liver cancer. Following treatment, symptoms (such as pain and lethargy) were relieved in 87% of the patients. Leslie et al [11] have previously published data from a small pilot trial in Oxford, UK. Six patients with liver tumours were recruited into a surgical trial in which formal tumour resection followed HIFU treatment. Contrast-enhanced MRI successfully predicted complete ablation in three cases. In each case, histological analysis confirmed complete ablation. In one case, the region of ablation observed on MRI appeared smaller than predicted at the time of HIFU treatment, but histology revealed complete ablation of the target region. In the other two cases there was evidence of incomplete ablation. The predominant appearance of HIFU-ablated tissue was of coagulative necrosis, but heat fixation was evident in some areas. Heat-fixed cells appeared normal under haematoxylin and eosin (H&E) staining, indicating that this is unreliable as an indicator of HIFU-induced cell death.
Herein we report the radiological outcomes of liver HIFU from all patients recruited to the above trials. This includes the first six cases reported in the aforementioned analysis [11]. The primary aim of the study was to assess the safety and feasibility of HIFU ablation of liver tumours and to determine whether post-operative MRI correlates with intra-operative imaging.
Methods and materials
Two prospective non-randomised Phase II study protocols were designed to evaluate the use of HIFU in liver ablation in a Western population. In the radiological trial, patients with liver tumours who had not responded to conventional therapies were recruited. In the histological trial, patients with liver tumours that were suitable for resection were recruited. All patients attended for a single HIFU treatment and were followed up for 30 days afterwards for radiological assessment of response. In the radiological trial, follow-up finished after this response assessment. In the surgical trial, patients then underwent surgical resection of the treated tumour to allow further histological assessment [11]. During the intervening period, patients did not receive other anticancer treatments.
Approval for the clinical investigation was obtained from the Oxford Local Research Ethics Committee. Written informed consent was obtained from all patients prior to enrolment. Follow-up was standardised according to the International Working Group on Image-guided Tumor Ablation, which categorises study goals into: (a) technical success, (b) technique effectiveness, (c) patient morbidity and (d) oncological outcomes [12,13]. Technical success was judged at the time of the procedure and was defined as complete treatment of the entire pre-defined tumour area. This area did not always represent the entire tumour area. Technique effectiveness was determined by cross-sectional imaging after treatment and measured using the method described below. Given the short duration of follow-up in these Phase I/II trials, an assessment of long-term oncological outcome could not be made.
Between November 2002 and May 2007, 31 patients (20 male, 11 female) were recruited (median age 62 years; range 45–84 years). Initially both trials recruited simultaneously; owing to slow recruitment to the surgical trial, the investigators decided to close this trial early. Seven patients were recruited into the surgical trial, and the remaining 24 into the radiological trial. 30 patients had liver metastases, the primary sites being colorectal (22), stomach (1), breast (1), lung (1), ovary (1), oesophagus (1) and pancreas (1) and adenocarcinoma of unknown primary (2). One patient had a primary HCC. All patients were treated with HIFU and were evaluable for adverse events.
Inclusion and exclusion criteria
All patients were aged over 18 years. Previous surgery, chemo- and biological therapy were permitted provided they had recovered from any related side effects. No patients had received radiotherapy to the target region in the preceding 12 months. The inclusion and exclusion criteria are summarised in Tables 1 and 2.
Table 1. Inclusion criteria for trial recruitment.
| Parameter | Criterion |
| Bone marrow function | |
| Haemoglobin | ≥10 g dl–1 |
| Neutrophil count | ≥1500 mm−3 |
| Platelet count | ≥100 000 mm−3 |
| Renal function | |
| Urea | ≤2.5×ULN |
| Creatinine | ≤2.5×ULN |
| Hepatic function | |
| Prothrombin time | ≤1.5×ULN |
| APPT time | ≤1.5×ULN |
| Total bilirubin | ≤1.5×ULN |
| AST | ≤3×ULN |
| Alkaline phosphatase | ≤2×ULN |
| Anaesthetic risk | |
| ASA score | ≤2 |
| WHO performance status | ≤1 |
APTT, activated partial thromboplastin time; ASA, American Society of Anesthesiologists; AST, aspartate transaminase; ULN, upper limit of normal; WHO, World Health Organization.
Table 2. Exclusion criteria for trial recruitment.
| Criterion |
| Pregnancy/breastfeeding |
| Tumours <5 mm from vital structures |
| Immunosuppressive medication |
| Brain metastases |
| Anti-arrhythmic/anticoagulant drugs |
| Permanent implanted pacemakers |
| Documented severe intra-abdominal adhesions |
Clinical, laboratory and radiological assessments
All patients were evaluated at baseline, on the day of HIFU treatment (day 1), and on days 2 and 12 after treatment. Patients were asked to report any existing symptoms. These were recorded with an assessment of their severity, according to Common Toxicity Criteria [14]. Pre-procedure assessment required a formal diagnostic ultrasound examination, to ensure that a suitable index lesion could be visualised. Adequate visualisation required clear imaging of all tumour margins and surrounding vital structures. Intravenous (iv) microbubble ultrasound contrast (SonoVue; Bracco, Milan, Italy) was used to enable an assessment of tumour perfusion. A suitable acoustic window for the HIFU transducer—unimpeded by bone or air-filled structures, and avoiding major vessels—was also required.
MRI of the abdomen (with iv gadolinium) also took place prior to HIFU treatment. All patients underwent imaging on a 1.5-T MRI system (Twinspeed; GE Healthcare, Amersham, UK) with high performance gradients and a torso phased array coil. Prior to the study, an iv cannula was placed in an arm vein and attached to an MRI-compatible power injector (Medrad, Warrendale, PA).
All patients underwent MRI using breath-hold techniques and comprising coronal T2 weighted, T1 weighted in-phase and out-of-phase gradient-echo, fat-suppressed T2 weighted and fast short inversion time inversion recovery sequences, according to our routine protocol. All subsequently also underwent a breath-hold fat-saturated volumetric interpolated breath-hold examination using a three-dimensional spoiled gradient-echo acquisition (LAVA; liver acquisition volume acceleration) before, during and after injection of gadolinium (Prohance; Bracco, Amsterdam, Netherlands). The LAVA sequence allows thin-section imaging with sequential enhanced image acquisition and also allows isotropic spatial resolution to demonstrate arterial, venous and delayed contrast enhancement. Owing to the precise timing of data acquisition during selected periods of enhancement, detection and characterisation of all lesions was possible. Subsequent subtraction imaging was performed to demonstrate the enhancement in isolation.
At baseline, three-dimensional measurements of the tumour were recorded. At 12±3 days, the sizes of any zone of coagulation necrosis and of the tumour were measured. These were given both as short-axis diameters (the minimum requirements according to the International Working Group on Image Guided Tumour Ablation [13]) and as maximum cross-sectional area estimates derived from antero-posterior and transverse dimensional measurements. Where ablation was seen, the radiologist categorised the location of the zone of ablation as either good or poor, depending on whether it lay inside or outside the target tumour boundaries, respectively. It should be noted that the intended area of ablation did not necessarily equate to the patient's total tumour burden, as some patients had very large solitary or multiple tumours, not all of which were targeted.
High-intensity focused ultrasound treatment
The Model JC tumour therapy device used in all treatments has previously been described by Kennedy et al [15]. All treatments were performed prone under general anaesthetic (Figure 1). The target tumour was identified using the integrated diagnostic ultrasound transducer (3.5 MHz) and was then segmented into parallel imaging slices of 5 mm separation. Two therapeutic transducer heads were used:
Figure 1.
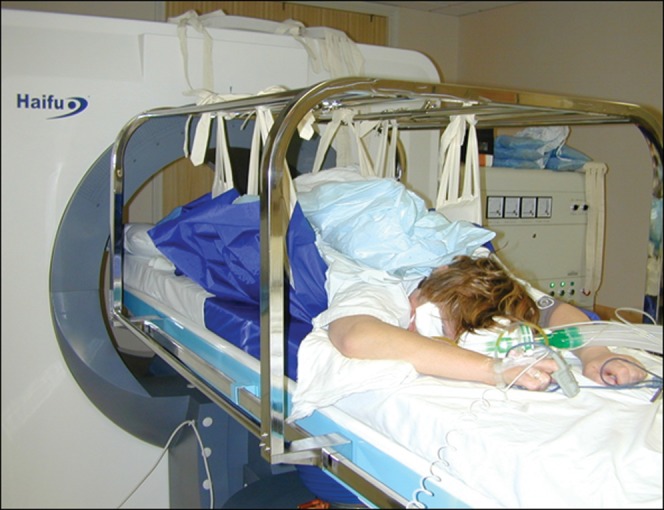
Patient under general anaesthesia positioned prone on high-intensity focused ultrasound treatment table. The therapeutic head is below the table. Padded slings are used to suspend the patient over the water reservoir.
2001048: 0.84 MHz, focal length 135 mm, focal zone (transverse×axial) 2.7×29 mm
20010A2: 1.8 MHz, focal length 122 mm, focal zone 0.9×8.9 mm.
Acoustic power outputs were determined by using sequential short HIFU pulses (2–3 s) of increasing power until hyperechoic regions became visible on the diagnostic image. The appearance of hyperecho in the diagnostic image following HIFU exposure can be used as an indicator of successful ablation. [16-18]. The target tumour was systematically exposed to HIFU slice by slice, using the greyscale diagnostic images changes to identify the extent of the treatment (output power 140–350 W). As specified in the trial protocol, HIFU treatment time was limited to 2 h. In the allotted time, an area of tumour was targeted, which was often less than the total tumour area. The quality of the diagnostic ultrasound image dictated the area of tumour that could be treated—this varied significantly between patients. At the end of the procedure the area of tumour treated by HIFU was measured using electronic callipers on the device imaging software. This was recorded as the estimated treament cross-sectional area.
Results
The mean body mass index (BMI) of patients treated was 26.1 kg m−2 (range 19.3–40 kg m−2).
Technical success
Table 3 summarises the treatment parameters. During the research period, treatment times were limited to approximately 2 h, as specified in the trial protocol. Treatment according to protocol was possible in 30 of the 31 patients, who were therefore designated as technical successes. One treatment session was abandoned when the water reservoir housing the treatment head leaked. This patient was not included in the analysis of efficacy. As a result, 30 of the 31 patients were evaluated for technique effectiveness.
Table 3. Treatment times for high-intensity focused ultrasound treatment.
| Parameter | Mean | Median | Range |
| Anaesthetic time (min) | 199 | 186 | 150–271 |
| Patient positioning (min) | 18 | 16 | 10–45 |
| Time to locate tumour and plan treatment (min) | 43 | 35 | 10–133 |
| Treatment duration (min) | 111 | 118 | 30–175 |
| Total exposure (min) | 21 | 21 | 0.2–44 |
Patient morbidity
All 31 patients were evaluable for device-related patient morbidity. These data are summarised according to Common Toxicity Criteria grade in Table 4. Three events were anaesthetic-related—one case of ecchymosis of the right eyelid, one episode of post-operative hypertension and one case of bruising of the right arm were seen. All three were managed conservatively and resolved fully. Seven patients reported symptoms related to disease progression during the follow-up period, including shortness of breath, lethargy and weight loss. Two patients died during the follow-up period, one as a consequence of small bowel obstruction secondary to progression of the primary tumour, and the other from disease progression. In neither case was the HIFU treatment implicated as a contributing factor.
Table 4. Adverse events possibly/probably related to high-intensity focused ultrasound treatment, showing number of events by Common Toxicity Criteria grade [14].
| Event | Common Toxicity Criteria grade |
|||
| 0 | 1 | 2 | 3 | |
| Discomfort at treatment site | 5 | 17 | 7 | 2 |
| Skin toxicity at treatment site | 18 | 11 | 1 | 0 |
| Oedema at treatment site | 23 | 4 | 1 | 2 |
| Fever | 27 | 1 | 2 | 0 |
| Other | 28 | 3 | 0 | 0 |
Transient pain over the treatment site was described by 25/31 (83%) of patients. This was mild or moderate in 23 cases, required no more than simple oral analgesia and had resolved within 24 h. Two cases of severe pain occurred. These required treatment with opioid analgesia in the immediate post-operative period. Both cases resolved quickly, requiring only simple oral analgesia after 24 h.
Superficial skin burns were seen in 12/31 (39%) of patients treated. This was mild in 11 cases, causing no symptoms and requiring no direct intervention. One moderate skin burn was noted (a 2×3 cm partial thickness burn requiring topical treatment for symptomatic relief). It resolved fully after 2 weeks.
Overall, HIFU treatment was well tolerated with an acceptable side-effect profile in this patient cohort. There was a transient and clinically insignificant drop in haemoglobin immediately after HIFU (mean decrease of 1.0 g dl–1, range 0.9–3.2). This is likely to be a dilutional artefact following iv fluid administration during anaesthesia. A transient rise was also seen in the white blood cell count immediately after treatment (mean difference of +1.71×10−9 l–1, range −8.10 to +9.94), and CRP values on day 2 were also raised (median difference of +9.8 mg l–1, range −52 to 70). These generally small and transient rises from baseline imply a mild, non-specific inflammatory process.
No changes were seen in biochemical markers of renal function. Those patients who had deterioration in liver function were all part of the non-surgical group who had inoperable liver disease. Clinical disease progression was seen in seven patients; biochemical progression was also noted in a further seven patients. There were transient rises in bilirubin in two patients, both of which had settled by day 12. One patient had a slow rise in bilirubin (not associated with an immediate post-treatment spike), probably related to cancer progression. Aspartate transaminase (AST) rose transiently after treatment in 18/31 patients (mean rise of 22.6 IU l–1 on day 2 post HIFU) as expected following cellular destruction. Alkaline phosphatase (ALP) levels rose gradually in six patients, and lactate dehydrogenase (LDH) in five patients, but in these instances there was no transient rise immediately after HIFU.
Technique effectiveness
Of the 31 patients treated, 30 were evaluable in terms of response to treatment. One patient had clinical disease progression and died before follow-up imaging. A subsequent post-mortem examination provided histological assessment of ablation; this patient was therefore included in the analysis. Overall, evidence of ablation was seen (radiological or histological) in 28 patients (93%). Of the 29 patients who had a day 12 radiological evaluation, 27 had clear zones of ablation on post-HIFU MRI. Accuracy was assessed as “good” in 24 (89%). Two patients had the closest zones of ablation, lying 2 mm in front of the target tumour, and one patient had a zone of ablation lying 2 mm beyond the target.
The data for overall radiological and histological evaluation are given in Table 5—here the median and mean of both the overall estimated and actual cross-sectional area (CSA) of the zone of ablation is given. A comprehensive breakdown of individual patient characteristics, CSA and treatment outcomes is presented in Table 6. The appearance of any intra-operative greyscale changes on B-mode ultrasound is recorded in this table together with a subjective assessment (by the same radiologist, RP) of whether any MRI zone of ablation was accurately located in the target region. A percentage of the size of the MRI zone of ablation to the size of the targeted area is also given. To analyse the effectiveness of intra-operative ultrasound imaging in assessment of the size of the zone of ablation, a scatter plot of estimated and actual cross-sectional area of ablation is presented in Figure 2. The data cannot be assumed to come from a parametric distribution; therefore a correlation coefficient using Spearman's correlation has been used, with the null hypothesis that there is no correlation. The analysis reveals correlation between these two variables (Spearman's r=0.48; 95% CI=0.11–0.73; p=0.011). A slope of best fit has also been determined using linear regression to estimate the relationship between intra-operative and MRI assessments of the size of the zone of ablation. Using a null hypothesis that there is no demonstrable relationship between the two variables (zero slope), analysis reveals a slope of 1.23 (95% CI=0.68–1.77), which is significantly non-zero (p<0.0001). Examples of MRI evidence of radiological ablation are shown in Figures 3–5. Figure 6 demonstrates microbubble-enhanced ultrasound images of a tumour showing a lack of contrast uptake in the post-treatment images indicating successful ablation.
Table 5. Comparison between the estimated and measured cross-sectional area of ablation assessed during and after treatment, respectively.
| Radiological evidence of ablation | Estimated CSA of ablation (cm2) (assessed during treatment) |
Measured CSA of ablation (cm2) (assessed on MRI) |
||||
| Median | Mean | 95% CI of mean | Median | Mean | 95% CI of mean | |
| 27/29 (93%) | 5.0 | 5.5 | 4.2–6.8 | 5.1 | 7.9 | 4.2–11.6 |
CI, confidence interval; CSA, cross-sectional area.
Table 6. Patient demographics and tumour characteristics, with intra-operative and post-operative imaging data.
| Trial | Trial no. | Age (years) | BMI (kg m–2) | Primary tumour | Liver segment | Tumour CSA (cm2) | Estimated treatment CSA (cm2) | Ablation seen on MRI? | Accurate ablation on MRI? | MRI ablation CSA (cm2) | MRI ablation vs estimated (%) |
| R | 101 | 47 | 28.2 | Breast | 4a | 15.2 | 9.0 | Yes | Yes | 10.8 | 120 |
| R | 102 | 65 | 19.3 | Unknown | 5 | 9.0 | 4.0 | Yes | Yes | 0.9 | 23 |
| R | 103 | 58 | 24.4 | Colorectal | 5 | 9.0 | 6.3 | Yes | Yes | 0.3 | 5 |
| R | 104 | 54 | 19.7 | Unknown | 5 | 9.0 | 3.8 | No | N/A | N/A | N/A |
| R | 105 | 60 | 27.5 | Colorectal | 6 | 6.2 | 2.3 | Yes | No | 2.4 | 107 |
| R | 106 | 71 | 34.0 | Colorectal | 6 | 3.4 | 0.8 | Yes | Yes | 1.2 | 161 |
| R | 107 | 79 | 30.0 | Colorectal | 5 | 4.8 | 0.5 | N/A | N/A | N/A | N/A |
| R | 108 | 77 | 26.3 | Colorectal | 8 | 6.7 | 4.0 | Yes | Yes | 1.2 | 30 |
| R | 109 | 57 | 23.8 | Colorectal | 4a | 3.8 | 2.3 | Yes | Yes | 0.8 | 36 |
| R | 110 | 65 | 29.1 | Colorectal | 5 | 135.0 | 4.5 | Yes | Yes | 5.3 | 117 |
| R | 111 | 60 | 25.8 | Colorectal | 1 | 96.0 | 3.0 | Yes | Yes | 42.9 | 1431 |
| R | 112 | 84 | 24.9 | Colorectal | 5 | 16.0 | 7.5 | Yes | Yes | 9.6 | 128 |
| R | 113 | 58 | 27.0 | Colorectal | 8 | 14.1 | 6.0 | Yes | Yes | 7.8 | 129 |
| R | 114 | 52 | 26.4 | Colorectal | 3 | 2.9 | 2.3 | Yes | No | 0.1 | 4 |
| R | 115 | 74 | 28.2 | Lung | 8 | 18.0 | 4.5 | Yes | Yes | 6.3 | 140 |
| R | 116 | 61 | 21.1 | Oesophagus | 5 | 9.9 | 16.0 | Yes | Yes | 12.7 | 79 |
| R | 117 | 65 | 23.2 | Pancreas | 5 | 13.8 | 5.0 | Yes | Yes | 8.7 | 175 |
| R | 118 | 69 | 30.8 | Ovarian | 4, 5, 8 | 31.2 | 4.0 | No | N/A | N/A | N/A |
| R | 119 | 60 | 26.3 | HCC | 5 | 48.1 | 5.0 | Yes | Yes | 21.1 | 421 |
| R | 120 | 59 | 25.2 | Colorectal | 8 | 14.2 | 8.8 | Yes | Yes | 11.6 | 133 |
| R | 121 | 65 | 28.0 | Stomach | 5 | 3.1 | 3.1 | Yes | Yes | 3.1 | 100 |
| R | 122 | 62 | 23.0 | Colorectal | 5 | 10.5 | 9.0 | Yes | Yes | 4.8 | 54 |
| R | 123 | 66 | 26.0 | Colorectal | 8 | 16.0 | 16.0 | Yes | Yes | 22.4 | 140 |
| S | 124 | 60 | 27.1 | Colorectal | 5 | 2.7 | 4.0 | No | N/A | 0.0 | 0 |
| S | 201 | 72 | 22.8 | Colorectal | 5 | 3.1 | 4.0 | Yes | Yes | 1.5 | 39 |
| S | 203 | 45 | 25.2 | Colorectal | 4a | 2.7 | 5.0 | Yes | Yes | 0.3 | 6 |
| S | 204 | 75 | 30.3 | Colorectal | 8 | 18.9 | 7.5 | Yes | Yes | 10.2 | 135 |
| S | 205 | 54 | 40.0 | Colorectal | 7 | 6.3 | 6.3 | Yes | No | 1.0 | 16 |
| S | 206 | 73 | 23.6 | Colorectal | 5 | 26.0 | 5.0 | Yes | Yes | 8.7 | 174 |
| S | 207 | 55 | 20.3 | Colorectal | 5 | 30.0 | 5.0 | Yes | Yes | 21.4 | 428 |
| S | 208 | 73 | 23.0 | Colorectal | 8 | 7.5 | 6.0 | Yes | Yes | 4.2 | 70 |
| Mean | 64 | 26.1 | 19.1 | 5.5 | 7.9 | 157 | |||||
| Median | 62 | 26.0 | 9.9 | 5.0 | 5.1 | 112 |
BMI, body mass index; CSA, cross-sectional area; HCC, hepatocellular carcinoma; N/A, not applicable; R, radiology; S, surgery.
Figure 2.
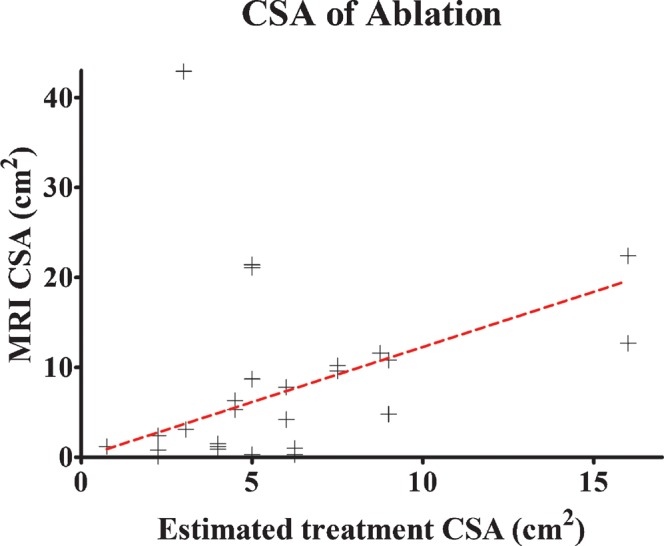
Scatter plot of estimated cross-sectional area (CSA) of ablation zone against MRI-calculated CSA of ablation zone (Spearman's coefficient=0.48; slope of linear regression 1.27, 95% confidence interval 0.68–1.77).
Figure 3.
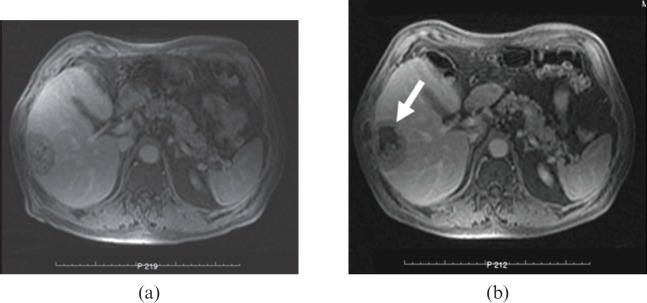
Axial T1 weighted contrast-enhanced MRI scans taken 1 min after intravenous infusion of gadolinium-containing contrast medium. Metastatic carcinoma of the lung to the right lobe of the liver (a) before high-intensity focused ultrasound (HIFU) and (b) 12 days after HIFU, showing lack of contrast uptake within the targeted tumour (arrow) at the site of ablation
Figure 5.
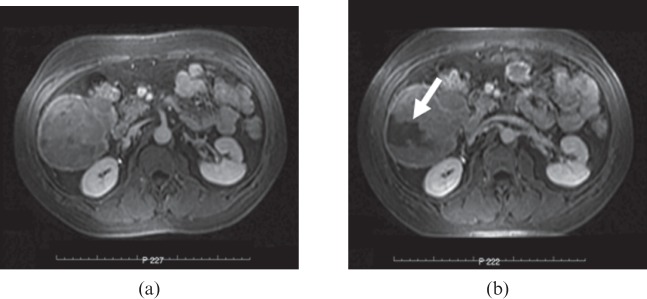
Hepatocellular carcinoma within the right lobe of the liver (a) before high-intensity focused ultrasound (HIFV) treatment and (b) 12 days after HIFV. Arrow demonstrates the zone of ablation on MRI.
Figure 6.
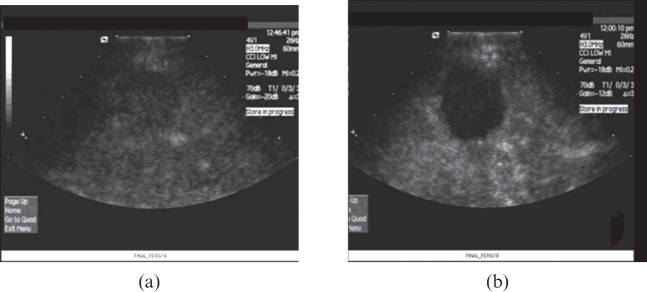
Positive technical success of high-intensity focused ultrasound (HIFU) liver treatment indicated by comparison between (a) pre-HIFU and (b) immediately post-HIFU microbubble contrast-enhanced ultrasound showing area of ablated tumour.
Figure 4.
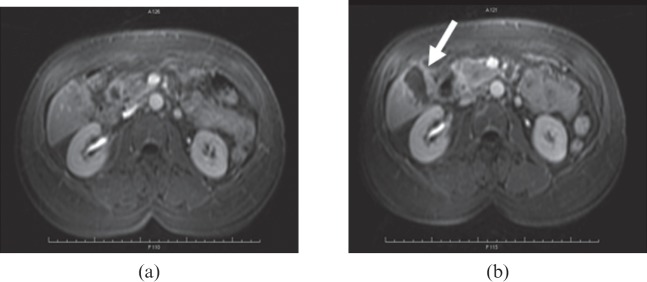
Metastatic carcinoma of the pancreas to the right lobe of the liver (segment V) (a) before high-intensity focused ultrasound (HIFU) and (b) 12 days after HIFU. Scanning protocol as in Figure 3.
Discussion
Previous work in China suggests that this modality has great potential for the treatment of solid tumour deposits in the liver [19]. One of the objectives of this study was to assess treatment efficacy in patients typically seen in the Western population who have more body fat, particularly in the abdominal wall. The mean BMI in our patient group is similar to the UK mean (26.1 vs 27.4 kg m−2; p=0.11) [20]. This typical UK population sample differs significantly from the mean BMI of the Chinese population, on which this device was originally tested and from which most published data arise (our group vs Chinese population: 26.1 vs 21.7 kg m−2; p<0.0001). Despite these inherent demographic variations, we have shown that extracorporeal HIFU is a safe and feasible option for Western patients with this type of cancer, with a favourable side-effect profile. All adverse events were local to the treatment site and self-limiting. Skin toxicity was the only consistently observed adverse event in patients undergoing HIFU treatment. It occurs as a result of the significant change in impedance as the ultrasound beam propagates from the water bath into the skin and subcutaneous fat. In the majority of cases the skin burns were reassuringly mild and without long-term consequence. However, moderate skin burns did occur and careful intra-operative skin assessment is required to enable early detection of this problem. Full-thickness skin burns are entirely avoidable.
The working party on image-guided tumour ablation identifies “post-ablation syndrome” consisting of a self-limiting symptom complex of low-grade fever and general malaise [12]. 10% of those treated experienced a low-grade fever, consistent with post-ablation syndrome. This occurred within the first 12 h and had settled within 24 h. There are other potential complications of HIFU, including damage to adjacent viscera (such as bowel or gallbladder) and secondary infection of the resulting necrotic volume, but these were not observed in our patients.
Changes in laboratory values demonstrate that the physiological consequences of HIFU upon both hepatic and renal function are minimal, even when those organs are the target. Creatinine levels were unaffected—an important consideration if HIFU is to be administered to those with poor renal reserve. Likewise, hepatic function remained stable after HIFU, with the exception of those patients with gross metastatic infiltration of the liver, where a decline in liver function was expected owing to the burden of their disease. The transient elevations in white cell count and C-reactive protein seen immediately after HIFU are not clinically significant, and are likely to reflect a systemic inflammatory response to the tumour ablation. We have demonstrated that HIFU exposure results in the creation of a discrete and accurate zone of ablation in the majority of cases. Occasionally, the boundaries of the zone lay outside (<5 mm) the targeted zone—this is a potential concern for safe treatment. However, it is important to put this into clinical context, as during any cancer surgery or ablation, tumours are excised or ablated along with a surrounding margin of normal tissue, usually no less than 1 cm. On the assumption that similar principles would apply to HIFU treatment, all observed zones of ablation occurred within these widely accepted “surgical” margins. The radiological analysis of HIFU-treated liver tumours shows that the median area of ablation seen on MRI is similar to that predicted at the time of treatment. There is a clear correlation between intra-operative estimates of ablation and post-operative MRI estimates. Therefore the integrated ultrasound imaging software enables safe prediction of the treatment zone size. More importantly, it gives the operator confidence that the targeted area is indeed ablated.
It is noteworthy that a small number of outliers exist where the radiological zone of ablation is far greater in size than that predicted during the treatment. In these cases, it is likely that a feeding vessel has been coagulated during treatment, leading to a zone of ischaemic necrosis in the distribution of the vessel that may be significantly larger than the region targeted.
Technical difficulties have been encountered in a number of areas, however. First, there has been a high dropout rate between screening and recruitment in the context of these trials. Approximately 50% of patients deemed suitable for HIFU treatment based on their demographics and baseline imaging were subsequently unable to be treated. The primary cause for these drop-outs has been the anatomical location of tumours. Tumours have frequently been situated at depths beyond the effective range of the HIFU beam for this device (maximum distance 135 mm from transducer to distal tumour) or in the dome of the liver, or adjacent to the bowel or gallbladder. Treatment times are longer than is desirable. A treatment session lasting for 2 h for a superficial 2–3 cm tumour may be acceptable when compared with the alternative of surgical resection, but it compares less favourably with other minimally invasive techniques such as radiofrequency ablation. For the treatment of large tumours, for which there is no minimally invasive alternative option, the longer treatment times may be justified on the grounds of a lower morbidity and mortality than conventional surgery. Despite this, it is likely that treatment times will reduce with development of the technology, experience and in combination with methods to reduce tumour perfusion, such as TACE [9]. In our series, HIFU was performed under general anaesthetic to ensure patient comfort and immobility. This is generally regarded as a limitation, but the use of a dual lumen endotracheal tube allows single lung ventilation, and thus provides a means of controlling respiratory excursion of the liver during treatment. Movement of these organs during HIFU exposure could compromise treatment efficacy, and preventing this motion overcomes what would otherwise be a further limitation of HIFU.
Conclusions
This work indicates that HIFU for the treatment of solid tumour deposits in the liver is both safe and feasible when using the Chongqing Haifu Model JC tumour therapy system. There are several potential limitations worthy of further investigation, including tumour depth and the obstruction posed by ribs, but the feasibility of HIFU as a possible alternative to surgery has been demonstrated in this clinical setting. A limiting factor has been the nature of the patient group that was (by necessity) recruited for this trial; the majority had multiple liver deposits that had become unresponsive to systemic therapy. This meant that longer-term follow-up was unrealistic and means that a further study will be required to assess the longer-term outcomes of liver treatment. Following on from these studies, larger-scale and more specific investigations will be necessary to determine clinical efficacy of the treatment. From these early cases, however, it is clear that treatment times will have to be reduced if HIFU is to compare favourably with other currently available minimally invasive techniques for small-volume tumours.
Footnotes
T Leslie and R Ritchie are joint first authors on this paper.
Funding received from Cancer Research UK, Oxford Biomedical Research Centre and UCARE.
References
- 1.Nicum S, Midgley R, Kerr DJ. Colorectal cancer. Acta Oncol 2003;42:263–75 [DOI] [PubMed] [Google Scholar]
- 2.Geoghegan JG, Scheele J. Treatment of colorectal liver metastases. Br J Surg 1999;86:158–69 [DOI] [PubMed] [Google Scholar]
- 3.August DA, Ottow RT, Sugarbaker PH. Clinical perspective of human colorectal cancer metastasis. Cancer Metastasis Rev 1984;3:303–24 [DOI] [PubMed] [Google Scholar]
- 4.Sasson AR, Sigurdson ER. Surgical treatment of liver metastases. Semin Oncol 2002;29:107–18 [DOI] [PubMed] [Google Scholar]
- 5.Simmonds PC. Palliative chemotherapy for advanced colorectal cancer: systematic review and meta-analysis. Colorectal Cancer Collaborative Group. BMJ 2000;321:531–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Illing RO, Kennedy JE, Wu F, ter Haar GR, Protheroe AS, Friend PJ, et al. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer 2005;93:890–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu F, Chen WZ, Bai J, Zou JZ, Wang ZL, Zhu H, et al. Pathological changes in human malignant carcinoma treated with high-intensity focused ultrasound. Ultrasound Med Biol 2001;27:1099–106 [DOI] [PubMed] [Google Scholar]
- 8.Wu F, Wang ZB, Chen WZ, Wang W, Gui Y, Zhang M, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: an overview. Ultrason Sonochem 2004;11:149–54 [DOI] [PubMed] [Google Scholar]
- 9.Wu F, Wang ZB, Chen WZ, Zou JZ, Bai J, Zhu H, et al. Advanced hepatocellular carcinoma: treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology 2005;235:659–67 [DOI] [PubMed] [Google Scholar]
- 10.Li CX, Xu GL, Jiang ZY, Li JJ, Luo GY, Shan HB, et al. Analysis of clinical effect of high-intensity focused ultrasound on liver cancer. World J Gastroenterol 2004;10:2201–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leslie TA, Kennedy JE, Illing RO, Ter Haar GR, Wu F, Phillips RR, et al. High-intensity focused ultrasound ablation of liver tumours: can radiological assessment predict the histological response? Br J Radiol 2008;81:564–71 [DOI] [PubMed] [Google Scholar]
- 12.Goldberg S, Charboneau J, Dodd Gr , Dupuy D, Gervais D, Gillams A, et al. Image-guided tumor ablation: proposal for standardization of terms and reporting criteria. Radiology 2003;228:335–45 [DOI] [PubMed] [Google Scholar]
- 13.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol 2005;16:765–78 [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute. Common Toxicity Criteria, version 2. [Cited 30 April 1999; cited 24 May 2012]. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. [Google Scholar]
- 15.Kennedy JE, Wu F, ter Haar GR, Gleeson FV, Phillips RR, Middleton MR, et al. High-intensity focused ultrasound for the treatment of liver tumours. Ultrasonics 2004;42:931–5 [DOI] [PubMed] [Google Scholar]
- 16.Cheng S, Zhou X, Tang Z, Yu Y, Wang H, Bao S, et al. High-intensity focused ultrasound in the treatment of experimental liver tumour. J Cancer Res Clin Oncol 1997;123:219–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaezy S, Shi X, Martin R, Chi E, Nelson P, Bailey M, et al. Real-time visualization of high-intensity focused ultrasound treatment using ultrasound imaging. Ultrasound Med Biol 2001;27:33–42 [DOI] [PubMed] [Google Scholar]
- 18.ter Haar G, Sinnett D, Rivens I. High intensity focused ultrasound—a surgical technique for the treatment of discrete liver tumours. Phys Med Biol 1989;34:1743–50 [DOI] [PubMed] [Google Scholar]
- 19.Wu F, Chen W, Bai J, Zou J, Wang Z, Zhu H, et al. Pathological changes in human malignant carcinoma treated with high-intensity focused ultrasound. Ultrasound Med Biol 2001;27:1099–106 [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Global infobase. [Cited 5 July 2011]. Available from: https://apps.who.int/infobase. [Google Scholar]