Abstract
Objectives
To assess the value of contrast-enhanced ultrasound (CEUS) in differentiating hepatocellular carcinoma (HCC) from non-neoplastic lesion in cirrhotic liver in comparison with baseline ultrasound.
Methods
A total of 147 nodules (diameter ≤5.0 cm) in 133 cirrhotic patients (mean age±standard deviation: 52±13 years, range 20–82 years; gender: 111 males and 22 females) were examined with CEUS. There were 116 HCCs, 26 macroregenerative nodules and 5 high-grade dysplastic nodules. CEUS was performed with a real-time contrast-specific mode and a sulphur hexafluoride-filled microbubble contrast agent.
Results
Hypervascularity was observed in 94.8% (110/116) HCCs, 3.8% (1/26) macroregenerative nodules and 60.0% (3/5) high-grade dysplastic nodules during arterial phase on CEUS. Detection rates of typical vascular pattern (i.e. hypervascularity during arterial phase and subsequent washout) in HCCs with a diameter of ≤2.0 cm, 2.1–3.0 cm and 3.1–5.0 cm were 69.2% (27/39), 97.1% (33/34) and 100.0% (43/43), respectively. CEUS significantly improved the sensitivity [88.8% (103/116) vs 37.1% (43/116), p<0.001], negative predictive value [70.5% (31/44) vs 31.5% (29/92), p<0.001], and accuracy [91.2% (134/147) vs 49.0% (72/147), p<0.001] in differentiating HCCs from non-neoplastic lesions when compared with baseline ultrasound. However, the sensitivity and accuracy of CEUS for HCCs ≤2.0 cm in diameter were significantly lower than those for HCCs of 2.1–3.0 cm and 3.1–5.0 cm in diameter.
Conclusions
CEUS improves diagnostic performance in differentiating HCCs from non-neoplastic nodules in cirrhotic patients compared with baseline ultrasound. Diagnosis of HCCs ≤2.0 cm diameter by CEUS is still a clinical concern, and thus needs further investigation.
Hepatocarcinogenesis in cirrhosis is supposed to be a multistage process, which includes a spectrum of lesions from macroregenerative nodule (MRN) to low-grade dysplastic nodule (LGDN), high-grade dysplastic nodule (HGDN) and hepatocellular carcinoma (HCC) [1,2]. Along with the popularisation of various diagnostic imaging modalities and the establishment of a follow-up system for the hepatitis-related cirrhotic patients, an increasing number of these lesions has been detected in clinical practice. Discrimination between neoplastic and non-neoplastic lesions, which is mandatory for treatment and follow-up planning, is the main concern for clinicians [3].
Arterial hypervascularity is regarded as a distinctive feature of HCC in cirrhotic liver, since most non-neoplastic lesions still have a prevalent portal vascularisation. This feature has been taken into account for diagnosis of HCC using various diagnostic modalities. The 2001 European Association for the Study of the Liver (EASL) conference recommended that the diagnosis of HCC can be made without biopsy in patients with cirrhosis who have a mass with a diameter >2 cm that shows characteristic arterial vascularisation on two imaging modalities (e.g. triphasic CT scan and MRI) [4]. Because of the simplicity of its execution, non-invasiveness, wide availability and relative inexpensiveness, periodic ultrasound (every 3–6 months) has been used as modality of choice to screen HCCs in cirrhotic patients in many countries, and conventional Doppler ultrasound has also been used with an attempt to depict the vascularity in HCC. However, its lower performance in assessing low-velocity flow and small blood vessels and its associated artefacts limit its diagnostic effectiveness [2,3].
Contrast-enhanced ultrasound (CEUS) has been developed to overcome the limitations of conventional Doppler ultrasound. Significant improvement has been made in this technique in the last few years such that real-time CEUS is possible using low acoustic power imaging in combination with second-generation ultrasound contrast agents [2-19]. Real-time CEUS is operated under a greyscale fashion and the scanning method is similar to conventional greyscale ultrasound [5]. Generally, arterial hypervascularity and subsequent washout are regarded as typical enhancement patterns of HCC on CEUS and the enhancement features have been served as the diagnostic criteria for HCC in several recent reports [5,6,8-14]. The 2005 American Association for the Study of Liver Diseases (AASLD) guidelines accepted CEUS as a reference imaging for diagnosis of HCC just like contrast-enhanced CT or MRI [20]. However, CEUS was not considered as a reference imaging technique for the HCC diagnosis according to the AASLD guidelines of 2010 because some cholangiocarcinomas can enhance like HCCs [21-24]. The presence of a background of liver cirrhosis can substantially narrow the spectrum of focal liver lesions (FLLs) and change the ultrasound and CEUS appearance of hepatocellular nodules, which may make the differential diagnosis of malignant and benign lesions much more difficult than in normal liver [15-17]. The aim of this study was to evaluate in a prospective study the diagnostic performance of real-time CEUS in differentiating HCCs from non-neoplastic lesions in cirrhotic patients.
Methods and patients
Patients
From March 2004 to March 2005, 225 consecutive hepatitis-related cirrhotic patients with FLLs underwent CEUS examination in our institution. The patients were referred for CEUS examination because of detection of FLLs in an ultrasound imaging surveillance programme or to seek further confirmation after detection of FLLs in a primary clinic. Finally, a total of 133 patients were enrolled into the study based on the following inclusion criteria:
maximal lesion ≤5.0 cm in diameter
lesion number ≤5
no procedures such as biopsy or percutaneous ablation therapies had been performed on the lesion
not simple cyst.
The patients comprised 111 males and 22 females with a mean±standard deviation (SD) age of 52±13 years (range 20–82 years). The underlying causes of liver cirrhosis were hepatitis B virus (HBV) infection in 126, hepatitis C virus (HCV) infection in 5, and HBV and HCV infection in 2. The patients with 1, 2, 3 and 4 nodules numbered 124, 5, 3 and 1, respectively. Therefore, a total of 147 nodules (diameter 1.0–5.0 cm, mean±SD 2.6±1.1 cm) were included in the study. The nodules sized 1.0–2.0, 2.1–3.0 and 3.1–5.0 cm numbered 65, 37 and 45, respectively (Table 1). No nodule <1.0 cm was found in this series. Informed consent was obtained from each patient and approval was obtained from the ethical committee of the hospital. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki [25].
Table 1. The greyscale ultrasound features of 147 nodules in 133 cirrhotic patients.
| Characteristic | Entity |
p-value | ||
| HCC (n=116) | MRN (n=26) | HGDN (n=5) | ||
| Diameter [mean±SD (cm)a] | 2.8±1.1 (1.0–5.0) | 1.7±0.5 (1.0–3.0) | 2.6±1.0 (1.7–4.1) | <0.001 |
| 1.0–2.0 cm | 39/116 (33.6%) | 24/26 (92.3%) | 2/5 (40.0%) | |
| 2.1–3.0 cm | 34/116 (29.3%) | 2/26 (7.7%) | 1/5 (20.0%) | |
| 3.1–5.0 cm | 43/116 (37.1%) | 0/26 | 2/5 (40.0%) | <0.001 |
| Echogenicity | ||||
| Hyper- | 15/116 (12.9%) | 12/26 (46.2%) | 0/5 | |
| Iso- | 23/116 (19.8%) | 7/26 (26.9%) | 2/5 (40.0%) | |
| Hypo- | 75/116 (64.7%) | 7/26 (26.9%) | 3/5 (60.0%) | |
| Mixed | 3/116 (2.6%) | 0/26 | 0/5 | 0.002 |
| Boundary | ||||
| Well circumscribed | 81/116 (69.8%) | 15/26 (57.7%) | 5/5 (100%) | |
| Poorly circumscribed | 35/116 (30.2%) | 11/26 (42.3%) | 0/5 | 0.159 |
| Peripheral hypo-echoic halo | ||||
| Present | 49/116 (42.2%) | 2/26 (7.7%) | 2/5 (40.0%) | |
| Absent | 67/116 (57.8%) | 24/26 (92.3%) | 3/5 (60.0%) | 0.001 |
HCC, hepatocellular carcinoma; HGDN, high-grade dysplastic nodule; MRN, macroregenerative nodule; SD, standard deviation.
aAnalysis of variation test: HCC vs MRN, p<0.001; HCC vs HGDN, p=0.305; MRN vs HGDN, p=0.002.
HCC was proved in 116 nodules detected in 108 patients by pathology (n=79) or clinical criteria (n=37). Pathology was achieved with specimens obtained from percutaneous ultrasound-guided biopsy (n=49) or surgery (n=30). The clinical diagnostic criteria for HCC in cirrhotic liver were according to a recent proposal: arterial hypervascularity and subsequent washout (i.e. typical vascular pattern) on one form of contrast-enhanced imaging such as CT or MRI, or serum alpha-protein level equal to or above 200 ng ml–1 for HCCs with a diameter >2.0 cm; typical vascular pattern on two forms of coincidental dynamic imaging for HCCs 1.0–2.0 cm in diameter [20]. 26 nodules in 21 patients were proved histopathologically to be MRNs and 5 nodules in 4 patients were HGDNs, with specimens obtained by means of ultrasound-guided biopsy and subsequent follow-up for more than 12 months. One of the HGDNs developed into HCC after an 8-month follow-up, which was also proved by percutaneous biopsy. The biopsy was performed using an 18-gauge automated side-cutting 2.2-cm core biopsy needle (Magnum; CR Bard, Inc., Covington, GA), and two to three biopsy procedures were applied for each nodule. All the pathological examination was performed by one experienced pathologist who specialised in liver pathology, according to the proposal of International Working Party consensus [1].
Baseline and contrast-enhanced ultrasound investigation
Baseline ultrasound and CEUS were performed using the same ultrasound scanner (Acuson Sequoia 512; Siemens Medical Solutions, Mountain View, CA) and a 4V1 vector transducer with frequency range of 1.0–4.0 MHz. A contrast-specific software operating at low acoustic power—contrast pulse sequencing (CPS; Siemens Medical Solutions)—was installed in the scanner.
The patients were studied after an 8-hour fast and in supine position. The liver was first examined with baseline ultrasound to locate the intrahepatic focal lesion. After the target lesion was determined, the parameter settings of greyscale ultrasound and Doppler ultrasound were optimised. The echogenic and intralesional blood flow features of the target lesion were evaluated and recorded.
CEUS was started by initiation of CPS function soon after baseline ultrasound. The settings for CPS were as follows: mechanical index; 0.15 to 0.21, power output; −21 dB; and CPS gain, −3 to −9. A sulphur hexafluoride (SF6)-filled microbubble contrast agent, SonoVue (BR1; Bracco SpA, Milan, Italy), was injected into the antecubital vein with a volume of 2.4 ml by a bolus fashion, and a flush of 5 ml of 0.9% normal saline was followed. The blood pool contrast agent can pass through pulmonary circulation several times. The arrival of contrast agent in the lesion and the subsequent dynamic perfusion were continuously observed for the first 120 s and intermittently observed thereafter until 360 s after contrast agent administration.
The whole imaging process was stored digitally for subsequent analysis. The images of baseline ultrasound and CEUS were reviewed by two independent radiologists with consensus, who were not involved in ultrasound scanning and blinded to other relevant information. For baseline ultrasound, the lesion was recognised as hypervascularity when Doppler ultrasound depicted a pulsatile arterial flow signal in the lesion. On CEUS, the imaging process was divided into arterial (i.e. 8–30 s after contrast agent administration), portal (31–120 s) and late (121–360 s) phases [8-12]. When a lesion exhibited hyperenhancement compared with surrounding liver during arterial phase, it was recognised as hypervascularity. Washout was determined when a lesion showed hyper- or iso-enhancement during arterial phase and hypo-enhancement during late phase. After evaluating the intralesional vascularity, the two radiologists were asked to determine the nature of the observed lesion (i.e. HCC or not). The diagnostic criteria for HCC on baseline ultrasound and CEUS were based on the previous studies [5-18]. Hypo-echoic rim and intralesional arterial blood flow on baseline ultrasound were regarded as diagnostic clues for HCC, otherwise benign lesion was considered. Hypervascularity during arterial phase and subsequent washout on CEUS were used as diagnostic criteria for HCC, otherwise benign lesion was considered. The confidence level was categorised as: 1, definitely HCC; 2, indeterminate; 3, definitely benign.
Statistical analysis
The quantitative data were expressed as mean±SD. Independent t-test was used to compare quantitative data. A lesion that was assigned a diagnostic scale of 1 was defined as a positive result, and a lesion assigned a scale of 2 or 3 was defined as a negative result. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and overall accuracy of baseline ultrasound or CEUS were computed. McNemar test was used to compare the sensitivity, specificity and accuracy between baseline ultrasound and CEUS. The χ2 test was used to compare PPV and NPV between baseline ultrasound and CEUS. For comparisons between independent qualitative data, χ2 test or Fisher's exact probability test was used. A two-tailed p-value <0.05 was considered statistically significant. All statistics were computed using SPSS software (v. 11.0; SPSS Inc., Chicago, IL).
Results
Baseline ultrasound
The appearances of the FLLs in cirrhotic livers are shown in Table 1. 49 (42.2%) HCC nodules showed peripheral hypo-echoic halo, which appeared in 9 (23.1%) of 39 nodules ≤2.0 cm in diameter, 15 (44.1%) of 34 nodules 2.1–3.0 cm and 25 (58.1%) of 43 nodules 3.1–5.0 cm.
On Doppler ultrasound, intralesional pulsatile arterial flow signals were depicted in 62.1% (72/116) HCC nodules, 11.5% (3/26) MRN nodules and 60.0% (3/5) HGDN nodules, respectively (Table 2).
Table 2. Hypervascularity before and after contrast agent administration.
| Entity | Diameter |
Total |
||||||
| 1.0–2.0 cm |
2.1–3.0 cm |
3.1–5.0 cm |
||||||
| Before | After | Before | After | Before | After | Before | After | |
| HCC (n=116) | 16/39 (41.0%)a,b | 34/39 (87.2%) | 23/34 (67.6%)a | 33/34 (97.1%) | 33/43 (76.7%)b | 43/43 (100.0%) | 72/116 (62.1%) | 110/116 (94.8%) |
| MRN (n=26) | 3/24 (12.5%) | 1/24 (4.2%) | 0/2 | 0/2 | 0/0 | 0/0 | 3/26 (11.5%) | 1/26 (3.8%) |
| HGDN (n=5) | 1/2 (50.0%) | 2/2 (100.0%) | 0/1 | 1/1 (100.0%) | 2/2 (100.0%) | 0/2 | 3/5 (60.0%) | 3/5 (60.0%) |
HCC, hepatocellular carcinoma; HGDN, high grade dysplastic nodule; MRN, macroregenerative nodule.
The values with the same superscript are compared:
ap =0.023.
bp =0.001.
Contrast-enhanced ultrasound
During the arterial phase, 110 (94.8%) out of 116 HCC nodules showed hyperenhancement compared with adjacent liver tissue, and the remaining 6 (5.2%) showed iso-enhancement (Table 2). 91 (78.4%) nodules appeared homogeneously enhanced and 25 (21.6%) heterogeneously enhanced, and there was a significant difference in diameter between them (2.5±1.0 vs 3.8±1.0 cm, p<0.001). In late phase, the HCC nodules became hyperenhanced in 1 (0.9%), iso-enhanced in 9 (7.7%) and hypo-enhanced in 106 (91.4%), respectively. The typical vascular pattern for HCC, hyper-enhancement during the arterial phase and hypo-enhancement (washout) during the late phase, was present in 103 (88.8%) of 116 HCCs (Figure 1). When considering the relationship with tumour size, the detection rates of arterial hyperenhancement with subsequent washout were 69.2% (27/39) for HCCs 1.0–2.0 cm in diameter, 97.1% (33/34) for HCCs 2.1–3.0 cm (p=0.002 in comparison with those 1.0–2.0 cm) and 100% (43/43) for HCCs 3.1–5.0 cm (p<0.001 in comparison with those 1.0–2.0 cm). The 13 HCC nodules that showed atypical vascular pattern were mainly found in nodules ≤2.0 cm, and none was found in nodules 3.1–5.0 cm.
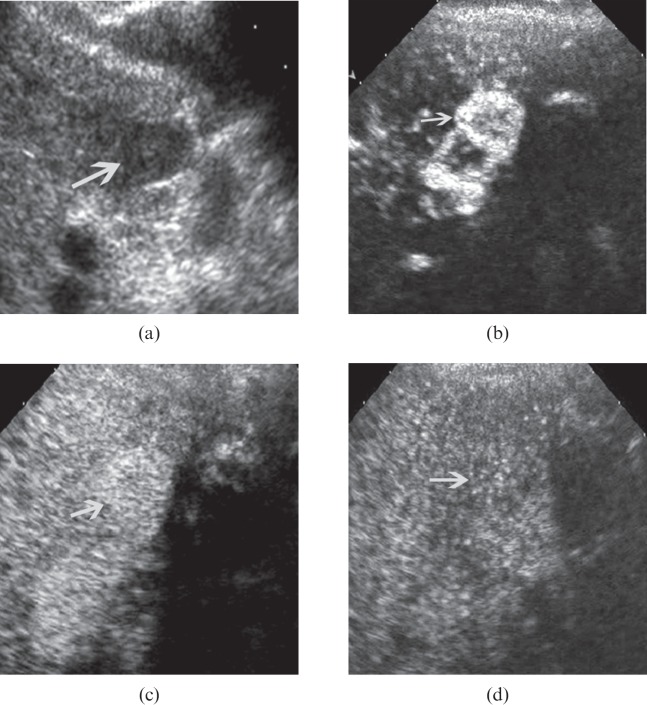
Figure 1.
Hepatocellular carcinoma in a 48-year-old male with hepatitis B-related cirrhosis. (a) Baseline ultrasound shows a 2.3 cm well-defined hypo-echoic nodule (arrow) in segment 8 of the liver. (b) During the arterial phase (17 s after contrast agent administration) the nodule (arrow) shows homogeneous hyperenhancement. (c) During the portal phase (108 s after contrast agent administration) the nodule (arrow) shows washout and becomes slightly hypo-enhanced.
During the arterial phase, the 26 MRN nodules exhibited hyperenhancement in 1 (3.8%), iso-enhancement in 22 (84.6%) and hypo-enhancement in 3 (11.6%). Until late phase, all 26 (100.0%) nodules showed iso-enhancement.
3 (60.0%) of 5 HGDN nodules showed hyper-enhancement and 2 (40.0%) showed hypo-enhancement during the arterial phase. All of them showed iso-enhancement during the late phase (Figures 2 and 3). One HGDN showing hypo-enhancement during arterial phase and iso-enhancement during late phase developed into an HCC after an 8-month follow-up, and a typical vascular pattern of HCC was detected at that time.
Figure 2.
High-grade dysplastic nodule in a 72-year-old male with hepatitis B-related cirrhosis. (a) Baseline ultrasound shows a 1.6 cm hypo-echoic nodule (arrow) in segment 5 of the liver. (b) During the arterial phase (13 s after contrast agent administration) the nodule (arrow) shows homogeneous hyper-enhancement. (c) During the portal phase (51 s after contrast agent administration) the nodule (arrow) shows iso-enhancement. (d) During the late phase (225 s after contrast agent administration) the nodule (arrow) continues to be iso-enhanced.
Figure 3.
High-grade dysplastic nodule in a 70-year-old female with hepatitis B-related cirrhosis. (a) Baseline ultrasound shows a 2.7 cm hypo-echoic nodule (arrow) in segment 7 of the liver. (b) During the arterial phase (22 s after contrast agent administration) the nodule (arrow) shows hypo-enhancement. (c) During the portal phase (54 s after contrast agent administration) the nodule (arrow) shows iso-enhancement. (d) During the late phase (215 s after contrast agent administration) the nodule (arrow) shows iso-enhancement.
Discriminating hepatocellular carcinoma from non-neoplastic lesion
Baseline ultrasound correctly diagnosed 43 (37.1%) of 116 HCC nodules, whereas CEUS made correct diagnoses in 103 (88.8%) (p<0.001). All of the correctly diagnosed HCCs showed hyperenhancement during the arterial phase and hypoenhancement during the late phase on CEUS. The NPV and overall accuracy also increased significantly after adding CEUS for analysis (Table 3). The confidence levels in 116 HCCs were 1 in 43, 2 in 72 and 3 in 1 on baseline ultrasound vs 1 in 103, 2 in 12 and 3 in 1 on CEUS. The indeterminate lesions decreased from 72 to 12 after contrast agent administration. On CEUS, the incorrectly diagnosed 13 HCC nodules were the 13 that showed atypical vascular pattern, and 12 (92.3%) of them were ≤2.0 cm in diameter. The 13 nodules showed hyper-enhancement during both the arterial and late phases in 1, hyper-enhancement during the arterial phase and iso-enhancement in 6, iso-enhancement during both the arterial and late phases in 3, and iso-enhancement during the arterial phase and hypo-enhancement during the late phase in 3. 1 HCC nodule that showed iso-enhancement during both the arterial and late phases was misdiagnosed as benign with CEUS, and the other 12 were categorised as indeterminate.
Table 3. Diagnostic performance of baseline and contrast-enhanced ultrasound in discriminating hepatocellular carcinomas from non-neoplastic lesions.
| Parameter | Baseline ultrasound | CEUS | p-value |
| Sensitivity | 37.1% (43/116) | 88.8% (103/116) | <0.001a |
| Specificity | 93.5% (29/31) | 100.0% (31/31) | NA |
| PPV | 95.6% (43/45) | 100.0% (103/103) | 0.091 |
| NPV | 31.5% (29/92) | 70.5% (31/44) | <0.001a |
| Accuracy | 49.0% (72/147) | 91.2% (134/147) | <0.001a |
CEUS, contrast-enhanced ultrasound; NA, not applicable; NPV, negative predictive value; PPV, positive predictive value.
aStatistically significant.
2 (40%) of 5 HGDNs were misdiagnosed as HCCs with baseline ultrasound, both of which showed abundant arterial flow in the nodules, and the remaining 3 (60%) were categorised as indeterminate. With CEUS, no HGDN was misdiagnosed as HCC; instead, all HGDNs were categorised as indeterminate.
On CEUS, 25 (96.2%) of 26 MRNs were determined to be benign nodules, 1 (3.8%) to be indeterminate and none to be HCC.
The diagnostic performances of CEUS were recalculated after dividing the 147 nodules into three groups (1.0–2.0, 2.1–3.0 and 3.1–5.0 cm) in terms of the diameter, and the results are presented in Table 4. The sensitivity and accuracy of CEUS for HCCs ≤2.0 cm were significantly lower than those for HCCs 2.1–3.0 and 3.1–5.0 cm.
Table 4. Diagnostic performance of contrast-enhanced ultrasound in discriminating hepatocellular carcinomas from non-neoplastic lesions in terms of nodules size.
| Parameter | Nodule diameter |
||
| 1.0–2.0 cm (n=65) | 2.1–3.0 cm (n=37) | 3.1–5.0 cm (n=45) | |
| Sensitivity | 69.2% (27/39)a,b | 97.1% (33/34)a | 100.0% (43/43)b |
| Specificity | 100.0% (26/26) | 100.0% (3/3) | 100.0% (2/2) |
| PPV | 100.0% (27/27) | 100.0% (33/33) | 100.0% (43/43) |
| NPV | 68.4% (26/38) | 75.0% (3/4) | 100.0% (2/2) |
| Accuracy | 81.5% (53/65)c,d | 97.3% (36/37)c | 100.0% (45/45)d |
NPV, negative predictive value; PPV, positive predictive value.
The values with the same superscript are compared:
ap=0.002.
bp<0.001.
cp=0.002.
dp=0.001.
Discussion
Differential diagnosis between malignant and benign lesions in the cirrhotic liver, which is a prerequisite for treatment planning and establishment of a follow-up scheme for the patient, is important. Along with the popularisation and refinement of various imaging modalities, the diagnosis performance for the lesions >2.0 cm in diameter has been greatly improved. On the other hand, increasing attention has been paid to enhancement of diagnostic ability in lesions of ≤2.0 cm and the precancerous lesions, which may finally improve the patient prognosis substantially [20,23].
Pathologically, in the hepatocellular nodules in cirrhotic liver, LGDN is a nodule in which atypia is mild, the nuclear to cytoplasmic ratio is normal or slightly increased, and portal tracts are present. Although the significance of LGDN remains unclear in clinical practice, this nodule can be included in the group of regenerative nodules with unusual histological changes [1,3,26]. HGDN is a nodule in which atypia is at least moderate but insufficient for the diagnosis of malignancy [3,26,27]. In general, HGDN is regarded as a premalignant lesion or a precursor of HCC, whereas MRN and LGDN seem to be only marginally implicated in hepatocarcinogenesis [3,28].
The stepwise progression from cirrhosis to hepatocellular nodules to HCC is characterised by an abnormal process of vascularisation, and particularly by a shift from a venous to an arterial supply [29,30]. Pathologically, portal tracts, including the portal vein and normal hepatic artery, were decreased in accordance with increasing grade of malignancy and were virtually absent in HCCs. On the other hand, abnormal arteries due to tumour angiogenesis developed in HGDNs during the course of hepatocarcinogenesis, and were markedly increased in moderately differentiated HCCs [30]. Roncalli et al [31] showed that MRNs and LGDNs showed an arterial and capillary supply similar to that detected in the adjacent cirrhotic nodules, whereas HGDNs and HCCs showed an abnormally increased arteriolar and capillary supply.
The above-mentioned feature of intranodular vascularisation shift has been regarded as the basis of various imaging modalities that were used for diagnosing HCC, such as angiography, contrast-enhanced CT, MRI, CT during arteriography and CT during arterial portography [2,3,32]. Although ultrasound has been regarded as the first-line imaging modality for diagnosis of HCC, conventional Doppler ultrasound has low ability to depict the intranodular vascularity. The role of ultrasound had not changed until the advent of CEUS, particularly real-time CEUS. Real-time CEUS is operated under low acoustic power, which allows real-time evaluation of dynamic blood flow perfusion in the nodules [5-19]. In this study, CEUS greatly increased the capability in depicting hypervascularity in HCC in comparison with conventional Doppler ultrasound, being 95% vs 62%, in agreement with a previous report [2]. Previous studies also showed that the detectability of vascularity in HCC by real-time CEUS was nearly the same as by contrast-enhanced CT, or even better [2,3].
In common with other contrast-enhanced imaging modalities, the pattern of hypervascularity during arterial phase and subsequent contrast washout has been revealed to be an important feature of HCC on CEUS, which has served as the diagnostic criterion for HCC in several reports [5-18,33-35]. The criterion has a satisfactory diagnostic capability that in this study the sensitivity, NPV and accuracy all increased significantly in comparison with baseline ultrasound.
In the present study, although CEUS possessed extremely high diagnostic performances in evaluating the HCC nodules 2.1–3.0 and 3.1–5.0 cm in diameter, the values decreased significantly in the HCC nodules of ≤2.0 cm. The sensitivities in nodules 1.0–2.0, 2.1–3.0 and 3.1–5.0 cm were 69%, 97% and 100%, respectively, and the accuracies were 82%, 97% and 100%, respectively. Diagnosis of HCC <2.0 cm in diameter is also difficult for other imaging modalities. Ikeda et al [36] found that only 56% of HCCs of ≤2.0 cm were hypervascular on angiography, and the smaller the nodule, the lower detection rate of hypervascularity. In another series by Chen et al [37], the finding was 64% for the same statistic. In Japan, HCCs <2.0 cm were divided into two categories on pathology: distinctly nodular type and vaguely nodular type (i.e. early HCC) [38]. In most of the distinctly nodular type HCCs, the arterial tumour vessels (unpaired arteries) are well developed, sinusoidal blood spaces are well vascularised and the tumours are encapsulated, and therefore most of the them were depicted as hypervascularity on contrast imaging, despite the small tumour size. Conversely, the vaguely nodular type HCCs have insufficient unpaired arteries and incomplete vascularisation of the sinusoidal blood spaces of the tumour, and the sinusoidal blood spaces and the sinusoids of the surrounding liver tissue are continuous at the boundary of the tumour since the tumours have not been encapsulated. Consequently, many vaguely nodular HCCs are hypo-vascular on contrast imaging [38]. On the other hand, although 87% of HCCs <2.0 cm in diameter showed hypervascularity on CEUS in this study, only 69% presented washout during portal or late phase, which may have been due to the fact that portal veins were still present in some small HCCs despite prominent abnormal arteries. The two reasons mentioned above may account for the lower sensitivity and accuracy of CEUS for HCCs <2.0 cm. Giorgio et al [17] found that only 53.6% of HCCs of ≤2.0 cm appeared as hypervascular on CEUS and 42.9% on CECT, as compared with 91.3% on CEUS and 76.1% on CECT for HCCs >2.0 cm. Quaia et al [16] also argue that CEUS has the same limitations as CECT and contrast-enhanced MRI in the non-invasive characterisation of HCCs ≤2.0 cm in diameter. Differentiation is often not possible even at histological examination because pathologists disagree about the dividing line between HGDN and well-differentiated HCC. However, Jang et al [15] believed that arterial phase hypervascularity on CEUS without a haemangioma pattern alone may be sufficient for diagnosis of HCC ≤2.0 cm, and the sensitivity, specificity and accuracy were 86.7%, 100% and 93.2%. The debate on this issue will continue and further prospective study is mandatory.
The high diagnostic performance of CEUS for HCC nodules >2.0 cm in diameter added evidence to an international consensus about diagnosis of HCC, in which the authors suggested that if nodules >2.0 cm show hypervascularity on arterial phase and subsequent washout on only one contrast imaging modality, the non-invasive diagnosis of HCC is available, and the contrast imaging modalities included CEUS [20]. Unfortunately, CEUS was not considered as a reference imaging technique for the HCC diagnosis according to the AASLD guidelines of 2010, mainly because some intrahepatic cholangiocarcinomas may behave like HCCs and cholangiocarcinomas can also be found in cirrhotic livers [21].
Typical vascular pattern on CEUS was valuable for diagnosis of HCC in cirrhotic patients for whom the specificity and PPV were both 100% in this study. However, there were a few hypervascular nodules that did not show washout on CEUS. How to treat these nodules is a clinically interesting problem. Should these nodules just be regarded as negative results, so that we can then leave them alone? This is difficult to accept if we consider that the hypervascular nodules without washout on CEUS included 6% HCCs, 4% MRNs and 60% HGDNs in this study.
HGDN has a high possibility to transform to HCC in that the transformation rate is about 35–63% [28,39] and about one-third of HGDNs even contain foci of well-differentiated HCC [31]. There has been increasing agreement that the HGDN in cirrhotic liver should also be treated aggressively, just like HCC, rather than leaving these patients in a follow-up programme, especially when curative techniques carrying a low risk of complications, such as percutaneous thermal ablations, are available [3,40-44]. Bolondi et al [3] reported six HGDNs, which exhibited hypovascularity in four and hypervascularity in two during arterial phase on CEUS. Of the five HGDNs in the present study, three were hypervascular and two were hypovascular during arterial phase, and all were iso-enhanced during late phase. The various vascular manifestations may reflect the complex process of vascular supply shift in these nodules. When the number of increased abnormal arteries due to tumour angiogenesis in the HGDN nodule is at a low level and portal tracts remain intact, the nodule may demonstrate hypovascularity. When the increased abnormal arteries compensate the decreased portal tracts, the nodule may show isovascularity. If the increased abnormal arteries become predominant enough, the nodule becomes hypervascular [26].
In this study, hypervascular HCCs without washout accounted for 6% (7/116) of HCCs, whereas in a series of Quaia et al [5] the rate was as high as 39% (89/226), and in other series the rates ranged from 31% to 50% [2-3]. The difference may be related to the fact that different imaging modes or different ultrasound scanners are used. The other factor may be that the HCCs had a different histological differentiation [45,46]. However, this finding further increases the concern about how to handle the hypervascular nodules without washout on CEUS.
In terms of the increasing belief that HGDN should also be treated aggressively, just like HCC, it is reasonable to pay close attention to the hypervascular nodules without washout, which included seven HCCs, three HGDNs and only one MRN. From the clinical viewpoint, ultrasound-guided biopsy is necessary to obtain the information of pathological characteristics of such nodules; however, even when the biopsy results are negative it is still advisable to take an aggressive approach to these nodules, considering the possible sample errors. The same opinion was also expressed by other investigators [3]. Of course, other hypervascular lesions such as haemangioma, liver metastasis, intrahepatic cholangiocarcinoma, focal nodular hyperplasia and liver abscess should be ruled out in advance, even though these lesions are less commonly found in cirrhotic livers.
In this study, no LGDNs were found in this series. Further studies including more HGDNs and LGDNs are mandatory to verify the results presented in this study. The other limitation of this study was that no other benign lesions (such as small haemangiomas) or malignant lesions (such as cholangiocarcinomas) were included in the study. Although most haemangiomas in cirrhotic liver have a typical enhancement pattern and it is easy to make a distinction between them and HCCs, some small haemangiomas may have a pattern similar to small HCCs and can be difficult to differentiate from HCC. Intrahepatic cholangiocarcinomas can also found in cirrhotic liver, and sometimes it can be difficult to differentiate them from HCCs [23,24], even though some authors concluded that CEUS is useful in this regard [22].
Conclusion
CEUS greatly improved the detectability of hypervascularity in HCC in comparison with baseline ultrasound. Using the hypervascularity and subsequent washout as diagnostic criteria, CEUS discriminated HCCs from non-HCCs reliably. However, improving the diagnostic performance in HCCs of ≤2.0 cm in diameter is still a clinical challenge. For the hypervascular nodules without washout on CEUS, an aggressive policy might be considered if the possibility of haemangioma is excluded.
Footnotes
This work was supported in part by grant NCET-06-0723 from Chinese Ministry of Education, grant 2008-2-10 of Public Welfare Research Special Project from Chinese Ministry of Health, and grant 2011003 of Key Project from Shanghai Health Bureau.
References
- 1.International Working Party Terminology of nodular hepatocellular lesions. Hepatology 1995;22:983–9 [DOI] [PubMed] [Google Scholar]
- 2.Gaiani S, Celli N, Piscaglia F, Cecilioni L, Losinno F, Giangregorio F, et al. Usefulness of contrast-enhanced perfusional sonography in the assessment of hepatocellular carcinoma hyper-vascular at spiral computed tomography. J Hepatol 2004;41:421–6 [DOI] [PubMed] [Google Scholar]
- 3.Bolondi L, Gaiani S, Celli N, Golfieri R, Grigioni WF, Leoni S, et al. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypo-vascular hepatocellular carcinoma. Hepatology 2005;42:27–34 [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma: conclusions of the Barcelona–2000 EASL Conference. J Hepatol 2001;35:421–30 [DOI] [PubMed] [Google Scholar]
- 5.Quaia E, Calliada F, Bertolotto M, Rossi S, Garioni L, Rosa L, et al. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology 2004;232:420–30 [DOI] [PubMed] [Google Scholar]
- 6.Brannigan M, Burns PN, Wilson SR. Blood flow patterns in focal liver lesions at microbubble-enhanced US. Radiographics 2004;24:921–35 [DOI] [PubMed] [Google Scholar]
- 7.Nicolau C, Vilana R, Catalá V, Bianchi L, Gilabert R, García A, et al. Importance of evaluating all vascular phases on contrast-enhanced sonography in the differentiation of benign from malignant focal liver lesions. AJR Am J Roentgenol 2006;186:158–67 [DOI] [PubMed] [Google Scholar]
- 8.Xu HX, Lu MD, Liu GJ, Xie XY, Xu ZF, Zheng YL, et al. Imaging of peripheral cholangiocarcinoma using low mechanical index contrast-enhanced sonography and SonoVue: initial experience. J Ultrasound Med 2006;25:23–33 [DOI] [PubMed] [Google Scholar]
- 9.Albrecht T, Blomley M, Bolondi L, Claudon M, Correas JM, Cosgrove D, et al. Guidelines for the use of contrast agents in ultrasound. January 2004. Ultraschall Med 2004;25:249–56 [DOI] [PubMed] [Google Scholar]
- 10.Nicolau C, Brú C. Focal liver lesions: evaluation with contrast-enhanced ultrasonography. Abdom Imaging 2004;29:348–59 [DOI] [PubMed] [Google Scholar]
- 11.Dietrich CF. Characterization of focal liver lesions with contrast enhanced ultrasonography. Eur J Radiol 2004;51Suppl.:S9–17 [DOI] [PubMed] [Google Scholar]
- 12.Xu HX, Liu GJ, Lu MD, Xie XY, Xu ZF, Zheng YL, et al. Characterization of small focal liver lesions using real-time contrast-enhanced ultrasound: diagnostic performance analysis in 200 patients. J Ultrasound Med 2006;25:349–61 [DOI] [PubMed] [Google Scholar]
- 13.Catalano O, Lobianco R, Cusati B, Siani A. Hepatocellular carcinoma: spectrum of contrast-enhanced gray-scale harmonic sonography findings. Abdom Imaging 2004;29:341–7 [DOI] [PubMed] [Google Scholar]
- 14.Ding H, Wang WP, Huang BJ, Wei RX, He NA, Qi Q, et al. Imaging of focal liver lesions: low-mechanical-index real-time ultrasonography with SonoVue. J Ultrasound Med 2005;24:285–97 [DOI] [PubMed] [Google Scholar]
- 15.Jang HJ, Kim TK, Wilson SR. Small nodules (1–2 cm) in liver cirrhosis: characterization with contrast-enhanced ultrasound. Eur J Radiol 2009;72:418–24 [DOI] [PubMed] [Google Scholar]
- 16.Quaia E, D'Onofrio M, Cabassa P, Vecchiato F, Caffarri S, Pittiani F, et al. The diagnostic value of hepatocellular nodule vascularity after sulfur hexafluoride-filled microbubble injection in patients with liver cirrhosis: analysis of diagnostic performance and confidence in malignancy characterization. AJR Am Journal Roentgenology 2007;189:1474–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giorgio A, Ferraioli G, Tarantino L, de Stefano G, Scala V, Scarano F, et al. Contrast-enhanced sonographic appearance of hepatocellular carcinoma in patients with cirrhosis: comparison with contrast-enhanced CT appearance. AJR Am J Roentgenol 2004;183:1319–26 [DOI] [PubMed] [Google Scholar]
- 18.von Herbay A, Vogt C, Willers R, Häussinger D. Real-time imaging with the sonographic contrast agent SonoVue: differentiation between benign and malignant hepatic lesions. J Ultrasound Med 2004;23:1557–68 [DOI] [PubMed] [Google Scholar]
- 19.Celik H, Ozdemir H, Yucel C, Gultekin S, Oktar SO, Arac M. Characterization of hyper-echoic focal liver lesions: quantitative evaluation with pulse inversion harmonic imaging in the late phase of Levovist. J Ultrasound Med 2005;24:39–47 [DOI] [PubMed] [Google Scholar]
- 20.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42:1208–36 [DOI] [PubMed] [Google Scholar]
- 21.Bruix J, Sherman M;American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen LD, Xu HX, Xie XY, Xie XH, Xu ZF, Liu GJ, et al. Intrahepatic cholangiocarcinoma and hepatocellular carcinoma: differential diagnosis with contrast-enhanced ultrasound. Eur Radiol 2010;20:743–53 [DOI] [PubMed] [Google Scholar]
- 23.Forner A, Vilana R, Ayuso C, Bianchi L, Solé M, Ayuso JR, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008;47:97–104 [DOI] [PubMed] [Google Scholar]
- 24.Vilana R, Forner A, Bianchi L, García-Criado A, Rimola J, de Lope CR, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology 2010;51:2020–9 [DOI] [PubMed] [Google Scholar]
- 25.Shephard DA. The 1975 Declaration of Helsinki and consent. Can Med Assoc J 1976;115:1191–2 [PMC free article] [PubMed] [Google Scholar]
- 26.Matsui O. Imaging of multistep human hepatocarcinogenesis by CT during intra-arterial contrast injection. Intervirology 2004;47:271–6 [DOI] [PubMed] [Google Scholar]
- 27.Kondo F, Kondo Y, Nagato Y, Tomizawa M, Wada K. Interstitial tumour cell invasion in small hepatocellular carcinoma. Evaluation in microscopic and low magnification views. J Gastroenterol Hepatol 1994;9:604–12 [DOI] [PubMed] [Google Scholar]
- 28.Borzio M, Fargion S, Borzio F, Fracanzani AL, Croce AM, Stroffolini T, et al. Impact of large regenerative, low grade and high grade dysplastic nodules in hepatocellular carcinoma development. J Hepatol 2003;39:208–14 [DOI] [PubMed] [Google Scholar]
- 29.Arakawa M, Kage M, Sugihara S, Nakashima T, Suenaga M, Okuda K. Emergence of malignant lesions within an adenomatous hyperplastic nodule in a cirrhotic liver. Observations in five cases. Gastroenterology 1986;91:198–208 [DOI] [PubMed] [Google Scholar]
- 30.Ueda K, Terada T, Nakanuma Y, Matsui O. Vascular supply in adenomatous hyperplasia of the liver and hepatocellular carcinoma: a morphometric study. Hum Pathol 1992;23:619–26 [DOI] [PubMed] [Google Scholar]
- 31.Roncalli M, Roz E, Coggi G, Di Rocco MG, Bossi P, Minola E. The vascular profile of regenerative and dysplastic nodules of the cirrhotic liver: implications for diagnosis and classification. Hepatology 1999;30:1174–8 [DOI] [PubMed] [Google Scholar]
- 32.Hayashi M, Matsui O, Ueda K, Kawamori Y, Gabata T, Kadoya M. Progression to hyper-vascular hepatocellular carcinoma: correlation with intranodular blood supply evaluated with CT during intraarterial injection of contrast material. Radiology 2002;225:143–9 [DOI] [PubMed] [Google Scholar]
- 33.Bhayana D, Kim TK, Jang HJ, Burns PN, Wilson SR. Hyper-vascular liver masses on contrast-enhanced ultrasound: the importance of washout. AJR Am J Roentgenol 2010;194:977–83 [DOI] [PubMed] [Google Scholar]
- 34.Maruyama H, Takahashi M, Ishibashi H, Yoshikawa M, Yokosuka O. Contrast-enhanced ultrasound for characterisation of hepatic lesions appearing non-hypervascular on CT in chronic liver diseases. Br J Radiol 2012;85:351–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quaia E, Alaimo V, Baratella E, Medeot A, Midiri M, Cova MA. The added diagnostic value of 64-row multidetector CT combined with contrast-enhanced US in the evaluation of hepatocellular nodule vascularity: implications in the diagnosis of malignancy in patients with liver cirrhosis. Eur Radiol 2009;19:651–63 [DOI] [PubMed] [Google Scholar]
- 36.Ikeda K, Saitoh S, Koida I, Tsubota A, Arase Y, Chayama K, et al. Diagnosis and follow-up of small hepatocellular carcinoma with selective intraarterial digital subtraction angiography. Hepatology 1993;17:1003–7 [PubMed] [Google Scholar]
- 37.Chen RC, Wang CK, Wang CS, Chen WT, Shih LS, Chiang LC, et al. Depiction of vasculature in small hepatocellular carcinoma, and dysplastic nodules: evaluation with carbondioxide ultrasonography and angiography. Acta Radiol 2002;43:66–70 [DOI] [PubMed] [Google Scholar]
- 38.Kojiro M. Focus on dysplastic nodules and early hepatocellular carcinoma: an Eastern point of view. Liver Transpl 2004;10Suppl. 1:S3–8 [DOI] [PubMed] [Google Scholar]
- 39.Takayama T, Makuuchi M, Hirohashi S, Sakamoto M, Okazaki N, Takayasu K, et al. Malignant transformation of adenomatous hyper-plasia to hepatocellular carcinoma. Lancet 1990;336:1150–3 [DOI] [PubMed] [Google Scholar]
- 40.Lencioni R, Cioni D, Crocetti L, Bartolozzi C. Percutaneous ablatioin of hepatocellular carcinoma: state-of-the-art. Liver Transpl 2004;10:S91–7 [DOI] [PubMed] [Google Scholar]
- 41.Lu MD, Yin XY, Xie XY, Xu HX, Xu ZF, Liu GJ, et al. Percutaneous thermal ablation for recurrent hepatocellular carcinoma after hepatectomy. Br J Surg 2005;92:1393–8 [DOI] [PubMed] [Google Scholar]
- 42.Xu HX, Lu MD, Xie XY, Yin XY, Kuang M, Chen JW, et al. Prognostic factors for long term outcome after percutaneous thermal ablation for hepatocellular carcinoma: a survival analysis of 137 consecutive patients. Clin Radiol 2005;60:1018–25 [DOI] [PubMed] [Google Scholar]
- 43.Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005;129:122–30 [DOI] [PubMed] [Google Scholar]
- 44.Liang P, Dong B, Yu X, Wang Y, Sheng L, Yu D, et al. Sonography-guided percutaneous microwave ablation of high-grade dysplastic nodules in cirrhotic liver. AJR Am J Roentgenol 2005;184:1657–60 [DOI] [PubMed] [Google Scholar]
- 45.Nicolau C, Catalá V, Vilana R, Gilabert R, Bianchi L, Solé M, et al. Evaluation of hepatocellular carcinoma using SonoVue, a second generation ultrasound contrast agent: correlation with cellular differentiation. Eur Radiol 2004;14:1092–9 [DOI] [PubMed] [Google Scholar]
- 46.Jang HJ, Kim TK, Burns PN, Wilson SR. Enhancement patterns of hepatocellular carcinoma at contrast-enhanced US: comparison with histologic differentiation. Radiology 2007;244:898–906 [DOI] [PubMed] [Google Scholar]