Abstract
The adrenal glands are an important site of both primary and secondary disease processes. Image-guided percutaneous biopsy of the adrenal gland is an accurate and safe alternative to surgical biopsy. This procedure is most often performed in patients with a suspicion of metastatic disease where an accurate pathological diagnosis plays an important role in disease staging and defining therapy. There are many different approaches to performing adrenal biopsy under CT guidance such as anterior transhepatic/transpancreatic, lateral transhepatic/transplenic or posterior transpulmonary/transpleural/paravertebral. We describe a technique in which the adrenal gland was biopsied using a CT-guided percutaneous paravertebral approach with the use of a hydrodissection manoeuver. 13 CT-guided adrenal gland percutaneous biopsies using this technique were performed at our institution between April 2009 and July 2010. All biopsies yielded sufficient material for pathological analysis and there were no complications reported after the procedure. Saline injection can expand the posterior paravertebral space and facilitate a posterior extrapleural approach with high accuracy and low complication rates, and we believe that this may be the best approach for adrenal gland biopsy.
The adrenal glands are an important site of both primary and secondary disease processes. Percutaneous adrenal biopsy has become a commonly performed interventional procedure since the 1970s. This procedure is most often performed in patients with a suspicion of metastatic disease, where an accurate pathological diagnosis plays an important role in disease staging and defining therapy. The usefulness of percutaneous adrenal biopsy is limited in other primary adrenal gland pathologies, such as non-functioning or functioning adenomas, infectious disease, adrenal haemorrhage or primary malignant tumours [1-3].
CT-guided biopsy has been widely accepted as an effective and safe procedure with high accuracy for diagnosis in many clinical settings [4]. Image-guided percutaneous biopsy of the adrenal gland is usually performed under CT guidance via an anterior transhepatic/transpancreatic approach, a lateral transhepatic/transsplenic approach or a posterior transpulmonary/transpleural approach [5]. Advances in histopathology, imaging equipment and biopsy devices have made this a subject of continuing interest in radiology.
We describe a technique in which the adrenal gland was biopsied using a CT-guided percutaneous paravertebral approach with the use of a hydrodissection manoeuver.
Methods and materials
13 CT-guided adrenal gland percutaneous biopsies using the hydrodissection technique were performed at our institution between April 2009 and July 2010.
All biopsies were performed on an outpatient basis unless the patient was hospitalised for another condition. Local anaesthesia was administered routinely and sedation was administered intravenously in a few cases at the discretion of the radiologist. Screening coagulation tests were ordered routinely. All biopsies were performed with a Tru cut needle (Angiotech, Vancouver, Canada) mounted on a biopsy gun using a coaxial system. The needles used were 10 or 15 cm in length depending on the distance between the skin and the lesion. Biopsy specimens were subjected to histological evaluation. All biopsies were performed by third-year trainee radiology residents under the supervision of a staff member. The patients were given standard post-biopsy care. At our institution, such care includes observation by a radiology nurse within our department for 1 h after the biopsy. If the patients remain asymptomatic, with stable vital signs, they are released after instructions about possible late complications and to return to hospital if they have any suspicious symptoms. In the presence of any symptoms during the observation period or if there is bleeding during the procedure, a CT scan control should be performed to rule out complications before discharge.
Biopsy technique
A single slice helicoidal CT (GE HiSPEED; GE Medical Systems, Milwaukee, WI) was used to perform all biopsies, with a slice thickness of 3 mm. The ideal body position to carry out biopsy varies from patient to patient. The preferred position is the one that makes the patient more comfortable during the procedure, which in the majority of cases is the prone position. However, in some cases the oblique or lateral position is required to move structures that are in the needle path, such as the lung and inferior vena cava. In the lateral or oblique position, the inferiorly located lung tends to expand less, thereby reducing the risk of pneumothorax. In cases of right adrenal biopsy, in which the inferior vena cava lies on the needle path, the left lateral or oblique position can help to move it and improve access for biopsy.
A lateral/oblique/prone decubitus spiral CT scan from the mid-thorax to the lower pole of the kidney was obtained before the procedure and a biopsy needle path was selected. A 21-gauge needle was introduced into the subcutaneous tissue and 5 ml of 2% lidocaine was instilled for local anaesthesia. A small skin incision was made and a 17-gauge coaxial needle was then introduced and placed in the subcutaneous tissue. A repeat CT was obtained at the level of the coaxial needle guide for further planning. The tip of the coaxial needle without the stylet was positioned in the fat plane between the vertebral body and the parietal pleura and 0.9% saline solution was injected to displace the parietal pleura, expanding the posterior paravertebral space while simultaneously advancing the coaxial needle carefully under serial CT control. We used the minimum volume of saline solution needed to allow proper passage of the needle through the space created by hydrodissection, which varied from 20 to 50 ml. During suspension of respiration, the coaxial needle tip traversed the diaphragm and was positioned next to or inside the adrenal gland. Then, several fragments were obtained using an 18-gauge needle. The number of fragments routinely collected is three and the needle is usually tilted in different angles for the removal of each fragment. The number of fragments can be increased if the first fragments are not appropriate (there is limited material) or reduced if there is significant bleeding or other complications. Procedures take approximately 30 min from positioning the patient on the CT scanner until the withdrawal of the needle (Figures 1 and 2).
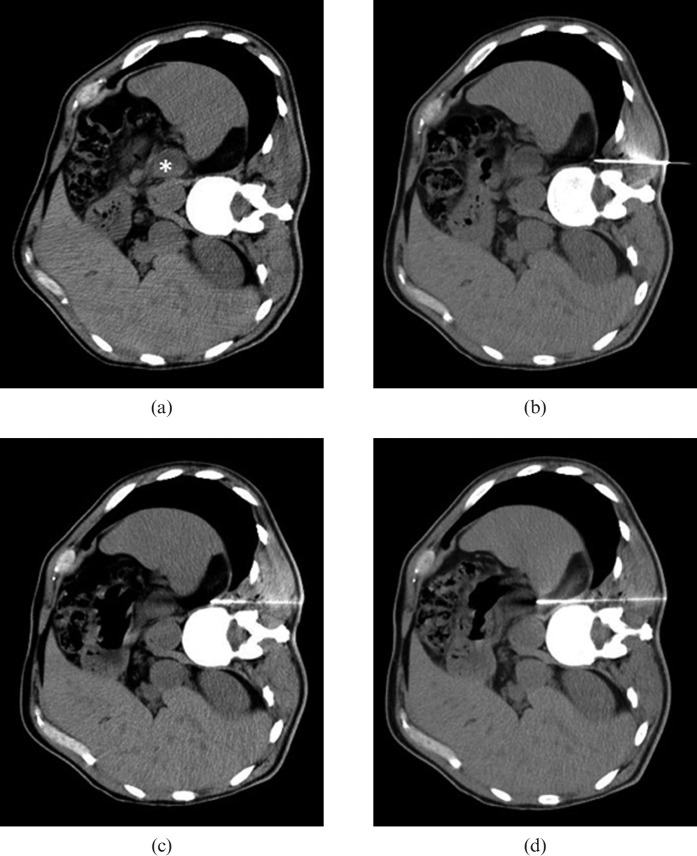
Figure 1.
Example of CT-guided paravertebral biopsy of the left adrenal gland using hydrodissection. (a) Axial CT of the abdomen with the patient in right lateral decubitus without the use of intravenous contrast, showing hypodense nodule in the left adrenal gland (*). (b) The tip of a 17-gauge coaxial needle was positioned in the fat plane between the vertebral body and the left parietal pleura. (c) After the injection of 20 ml of 0.9% saline solution, parietal pleura was displaced, expanding the posterior paravertebral space while simultaneously advancing the coaxial needle carefully under serial CT control. (d) During suspension of respiration, the coaxial needle tip traversed the diaphragm and was positioned next to the left adrenal gland. Five fragments were then obtained using an 18-gauge needle.
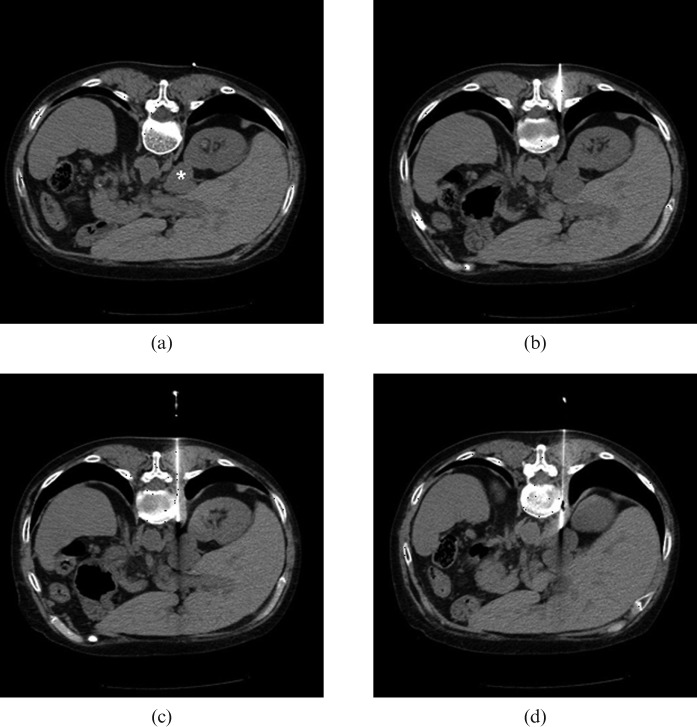
Figure 2.
Example of CT-guided paravertebral biopsy of the right adrenal gland using hydrodissection. (a) Axial CT of the abdomen with the patient in ventral decubitus without the use of intravenous contrast, showing a nodule in the right adrenal gland (*). (b) The tip of a 19-gauge coaxial needle was positioned in the fat plane between the vertebral body and the right parietal pleura. (c) After the injection of 50 ml of 0.9% saline solution, parietal pleura was displaced, expanding the posterior paravertebral space while simultaneously advancing the coaxial needle carefully under serial CT control. (d) During suspension of respiration, the coaxial needle tip traversed the diaphragm and was positioned next to the right adrenal gland. Seven fragments then were obtained using a 20-gauge needle.
Results
The majority of our study patients were male (84.6%) and the mean age of the cohort was 64±11 years (range, 48–84 years). The left adrenal gland was biopsied in 7 cases (54%) and the right was biopsied in 6 (46%). All lesions had a suspected secondary origin. The mean diameter of the adrenal lesions was 4.1±21 cm (range, 1.3–8.4 cm).
All biopsies yielded sufficient material for pathological analysis (Table 1). Nine cases (69%) had positive results, confirming a malignant origin, and only four cases (31%) had negative results, meaning that the adrenal gland tissue lacked evidence of malignancy. In all four cases where the biopsy result was negative for malignancy, patients performed follow-up imaging studies for at least 6 months, which showed no changes in adrenal lesions. None of the patients complained of severe pain, even during saline injection, and there were no complications reported after the procedure.
Table 1. Patient clinical data and pathological results.
| Case | Gender | Age (years) | Primary tumour site | Lesion dimensions | Pathological diagnosis |
| 1 | Male | 50 | Kidney | 69×36 mm | Metastasis of renal cell carcinoma |
| 2 | Male | 54 | Kidney | 29×16 mm | Metastasis of renal cell carcinoma |
| 3 | Male | 71 | Colon | 41×23 mm | Metastasis of colorectal cancer |
| 4 | Male | 77 | Colon | 21×13 mm | Benign lesion |
| 5 | Male | 67 | Larynx | 45×33 mm | Metastasis of epidermoid carcinoma |
| 6 | Male | 65 | Lung | 36×17 mm | Benign lesion |
| 7 | Female | 56 | Lung | 13×12 mm | Benign lesion |
| 8 | Female | 48 | Breast | 84×76 mm | Metastasis of adenocarcinoma |
| 9 | Male | 56 | Lung | 29×19 mm | Metastasis of epidermoid carcinoma |
| 10 | Male | 68 | Kidney | 28×20 mm | Metastasis of renal cell carcinoma |
| 11 | Male | 84 | Unknown | 32×26 mm | Metastasis of undifferentiated carcinoma |
| 12 | Male | 77 | Lung | 33×25 mm | Benign lesion |
| 13 | Male | 68 | Lung | 80×79 mm | Metastasis of small cell adenocarcinoma |
Discussion
Adrenal glands are one of the most common organs involved in metastatic disease, especially in lung cancer. The definite diagnosis of an adrenal lesion is of major importance, especially in oncology patients where it may be the only marker of metastatic disease and will have a significant impact on the management of these patients [1,5].
Advances in new imaging techniques with specific protocols such as multislice CT, MRI with chemical shift sequences and positron emission tomography have improved the non-invasive diagnosis of benign adrenal pathologies such as adenoma, avoiding the use of unnecessary procedures. After the introduction of these techniques on the clinical practice, the rate of biopsy of benign adrenal adenomas had declined from 40–57% to 12% in some series [6]. However, a biopsy of the adrenal gland is still useful in cases where diagnosis by non-invasive methods has been inconclusive. The procedure is also of use for diagnostic confirmation in cases of adrenal lesions in patients with more than one primary neoplasm site, where the primary site is unknown, and for culture in suspected cases of infectious process.
Image-guided percutaneous biopsy is an accurate and safe alternative to surgical biopsy for the diagnosis of pathological conditions of the adrenal gland [1-3]. In oncological patients even a negative result on CT-guided biopsy is reliable and can be regarded as a true negative evaluation with no need to repeat the biopsy [7].
Traditionally, the adrenal gland has been biopsied using the posterior approach with the patient prone but this expands the posterior lung and interferes with the proposed needle path. This situation must then be countered by entering the skin several centimetres below the mass and angling the needle in a cephalad orientation. The calculations can be difficult, pneumothorax is a possibility and it is time consuming [5].
The transhepatic and transplenic approach increases the difficulty of the procedure and may increase the risk of complications, specifically pain and haemorrhage. Small differences in respiration may make accurate needle positioning difficult, necessitating a greater number of punctures. The transpancreatic approach may increase the risks of traumatic pancreatitis and retroperitoneal haemorrhage [5].
A CT-guided percutaneous transpulmonary approach is feasible but the accuracy may worsen with the use of smaller needles and complications such as haemoptysis and pneumothorax are also possibilities [8].
Saline injection can displace the pleura laterally and expand the posterior paravertebral space, which facilitates a posterior extrapleural approach with high accuracy and low complication rates for either right or left adrenal glands and we believe that this may be the best approach for adrenal gland biopsy. This technique was previously used for pulmonary and mediastinal lesions [9,10], but the only author that described its use for adrenal lesions was Karampekios et al [11]. In our study, despite the reduced sample size, the method proved to be safe with no reported complications. The accuracy was excellent as the material obtained was sufficient for histological diagnosis in all cases.
References
- 1.Welch TJ, Sheedy PF, 2nd, Stephens DH, Johnson CM, Swensen SJ. Percutaneous adrenal biopsy: review of a 10-year experience. Radiology 1994;193:341–4 [DOI] [PubMed] [Google Scholar]
- 2.Bernardino ME, Walther MM, Phillips VM, Graham SD, Jr, Sewell CW, Gedgaudas-McClees K, et al. CT-guided adrenal biopsy: accuracy, safety and indications. AJR Am J Roentgenol 1985;144:67–9 [DOI] [PubMed] [Google Scholar]
- 3.Silverman SG, Mueller PR, Pinkney LP, Koenker RM, Seltzer SE. Predictive value of image-guided adrenal biopsy: analysis of results of 101 biopsies. Radiology 1993;187:715–18 [DOI] [PubMed] [Google Scholar]
- 4.Chojniak R, Isberner RK, Viana LM, Yu LS, Alta AA, Soares FA. Computed tomography guided needle biopsy: experience from 1300 procedures. Sao Paulo Med J 2006;124:10–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arellano RS, Boland GWL, Mueller PR. Adrenal biopsy in a patient with lung cancer: imaging algorithm and biopsy indications, technique, and complications. AJR Am J Roentgenol 2000;175:1613–17 [DOI] [PubMed] [Google Scholar]
- 6.Pausen SD, Nghiem HV, Korobkin M, Caoili EM, Higgins EJ. Changing of imaging-guided percutaneous biopsy of adrenal masses: evaluation of 50 adrenal biopsies. AJR Am J Roentgenol 2004;182:1033–7 [DOI] [PubMed] [Google Scholar]
- 7.Harisinghani MG, Maher MM, Hahn PF, Gervais DA, Jhaveri K, Varghese J, et al. Predictive value of benign percutaneous adrenal biopsies in oncology patients. Clin Radiol 2002;57:898–901 [DOI] [PubMed] [Google Scholar]
- 8.Krishnam M, Tomasian A, Davies L, Littler J, Curtis J. CT-guided percutaneous transpulmonary adrenal biopsy—a technical note. Br J Radiol 2008;81:e191–3 [DOI] [PubMed] [Google Scholar]
- 9.Klose KC. CT-guided large-bore biopsy: extrapleural injection of saline for safe transpleural access to pulmonary lesions. Cardiovasc Intervent Radiol 1993;16:259–61 [DOI] [PubMed] [Google Scholar]
- 10.Langen HJ, Klose KC, Keulers P, Adam G, Jochims M, Gunther RW. Artificial widening of the mediastinum to gain access for extrapleural biopsy: clinical results. Radiology 1995;196:703–6 [DOI] [PubMed] [Google Scholar]
- 11.Karampekios S, Hatjidakis AA, Drositis J, Gourtsoyiannis N. Technical note. Artificial paravertebral widening for percutaneous CT-guided adrenal biopsy. J Comput Assist Tomog 1998;22:308–10 [DOI] [PubMed] [Google Scholar]