Abstract
Objective
The aim of this study was to assess the effect on neonatal thyroid function of iodinated contrast media administered for CT pulmonary angiography (CTPA) in babies whose mothers were investigated for suspected pulmonary embolism during pregnancy.
Methods
Retrospective review of 115 pregnant patients investigated for suspected pulmonary embolism. The patient cohort consisted of two groups: Group A consisted of 73 pregnant females who received iodinated contrast agent for CTPA, and Group B (control group) consisted of 42 pregnant females who were investigated by perfusion imaging only. The results of the neonatal thyroid function tests for the babies of the mothers in Groups A and B were compared.
Results
All of the neonatal thyroid function tests for both groups were normal with no statistical difference between the two groups.
Conclusion
No adverse effect on thyroid function was demonstrated in neonates exposed to in utero iodinated contrast media. However, as our study involves a small patient group, the results should be interpreted with caution.
Pulmonary embolism is a major cause of maternal mortality in developed countries [1,2]. Cohort studies have established the incidence of pulmonary embolism in pregnancy to be 10–12 per 100 000 woman-years [3]. Pregnant females are at a greater risk of developing venous thromboembolism due to a combination of factors such as venous stasis, hypercoagulability and vascular damage that occurs with pregnancy. A clinical diagnosis of pulmonary embolism in pregnancy is not straightforward, as some of the symptoms associated with pulmonary embolism can be regarded as normal symptoms of pregnancy, particularly at term. However, accurate diagnosis of acute pulmonary embolism is essential as management of thromboembolism in pregnancy can be challenging. A failure to diagnose and treat thromboembolism could be fatal for the mother and diagnosis based on clinical grounds only will lead to over diagnosis, unnecessary treatment and possibly unnecessary interventions in future pregnancies.
CT pulmonary angiography (CTPA) is a well-validated investigation for pulmonary embolism in pregnancy. Besides the risk of exposing the foetus and the mother to the potential harmful effects of radiation, harmful effects related to intravenous iodinated contrast agent that is administrated for CTPA should be considered. The risk of foetal hypothyroidism due to long-term maternal ingestion of iodides is well documented [4-6]. Subsequently, there is a theoretical risk of contrast-induced hypothyroidism in neonates following antenatal exposure to iodinated contrast agent; however, this has not been confirmed in practice. The guidelines issued by the Members of Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR) [7] state that thyroid function should be checked in neonates during the first week of life, following administration of iodinated contrast agents to the mother during pregnancy.
This study aims to assess the effect of iodinated contrast agent used for CTPA in pregnant females on neonatal thyroid function.
Methodology
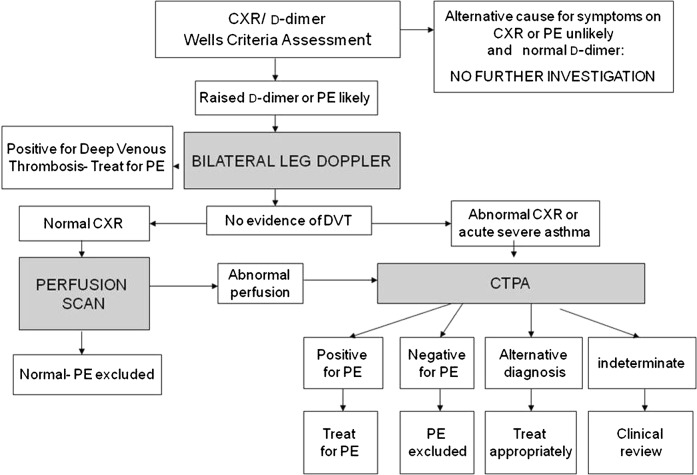
A retrospective study of all pregnant females investigated for suspected pulmonary embolism who were admitted to our trust hospitals from April 2004 to April 2009 was performed. Our trust protocol for imaging pregnant females with suspected acute pulmonary embolism is outlined in Figure 1. To summarise, all patients initially undergo bilateral leg Doppler examination. If this reveals deep venous thrombosis, the patient is treated as for pulmonary embolism. All patients with a normal or indeterminate leg Doppler ultrasound with a normal chest radiograph are referred for a lung perfusion scan. All remaining patients and those with a non-diagnostic perfusion scan undergo CTPA. The radiation risk to the female and her foetus is explained and written consent obtained prior to entering the imaging pathway. The pregnant patients with suspected pulmonary embolism were divided into two groups: those who had CTPA, and hence received intravenous iodinated contrast media (Group A), and those that had perfusion imaging only and did not receive contrast (Group B).
Figure 1.
Local trust protocol for imaging pregnant females with suspected acute pulmonary embolism (PE). CTPA, CT pulmonary angiography; CXR, chest radiograph; DVT, deep venous thrombosis.
As per the neonatal screening programme in the United Kingdom, all newborn infants were screened for congenital hypothyroidism (CHT) by measuring thyroid-stimulating hormone (TSH) levels. The blood samples for TSH levels were obtained from newborns by heel puncture test at the age of 5–8 days. TSH levels were measured by fluoroimmunoassay on an AutoDELFIA instrument (PerkinElmer Inc., Waltham, MA). The normal reference range of the TSH value was 0–10 μIU ml–1, with a higher value indicating possible hypothyroidism. Abnormal results required confirmation by means of repeat assay and, possibly, measurement of thyroxine levels. Statistical analyses were performed with SPSS software (SPSS Inc., Chicago, IL) and a p-value ≤0.05 was considered to indicate a statistically significant difference. The two groups were compared using the Mann–Whitney non-parametric test.
Ethics committee and research and development committee approval was obtained from the primary trust at which the mother was investigated and from the trust performing the neonatal thyroid function testing.
Results
115 pregnant females were admitted with suspected pulmonary embolism during the study period. 73 of the females were investigated by CTPA and 42 females had perfusion imaging only, and hence did not receive iodinated contrast. A maximum dose of 100 ml of non-ionic iodinated low-molecular-weight agent containing 300 mg I ml–1 Ultravist 300 (Schering AG, Berlin, Germany) was used as a standard contrast agent.
For Group A (females who received intravenous iodinated contrast agent), the mean age was 31.9 years (age range 21–46 years). The gestational age of the foetus ranged from 12 to 40 weeks, with a mean of 28 weeks. 39 of the 73 patients in this group were in the third trimester of pregnancy. The neonates in this group underwent TSH screening between 5 and 11 days of age, with an average age at TSH screening of 6 days. The average TSH value for this group was 1.1 μIU ml–1. One newborn had a TSH value of 10 μIU ml–1when screened at day 5; a repeat TSH performed after 10 days was 0.49 μIU ml–1. This neonate was exposed to iodinated contrast media at 25 weeks of gestation. 7 of the mothers (9.6%) in this group were diagnosed with pulmonary embolism on CTPA.
For Group B (females who did not receive contrast agent), the mean age was 29.5 years; with a range of 17–40 years. The mean gestational age was 29 weeks (range 7–38 weeks). 26 of the 42 patients were in the third trimester of pregnancy. The neonates were screened between 4 and 14 days of age, with an average age at TSH screening of 5.9 days. The average TSH value for this group was 1.07 μIU ml–1 with a maximum value of 3.1 μIU ml–1. 1 mother (2.4%) in this group was diagnosed with pulmonary embolism following perfusion scan. The overall results are summarised in Table 1.
Table 1. Summary of results for patients who received iv contrast media (Group A) and patients who did not receive iv contrast media (Group B).
| Age/TSH level | Group A (received iv contrast media) | Group B (did not receive iv contrast media) | p-value |
| Total number of patients | 73 | 42 | |
| Maternal age (years) | |||
| Mean | 31.9 | 29.5 | 0.1 |
| Range | 21–46 | 17–40 | |
| Gestational agea (weeks) | |||
| Mean | 28 | 29 | 0.3 |
| Range | 12–40 | 7–38 | |
| Neonatal TSH value (μIU ml–1) | |||
| Median | 0.74 | 0.95 | 0.67 |
| Range | 0.02–10 | 0–3.1 | |
| Neonatal age at screening (days) | |||
| Mean | 6 | 5.9 | 0.6 |
| Range | 5–11 | 4–14 | |
| Neonatal median TSH by trimester at time contrast exposure (μIU ml–1)b | |||
| 1st | 0.35 (0.27–3.01) | 0.25 (0.2–1.6) | 0.27 |
| 2nd | 0.70 (0.37–1.83) | 0.95 (0.6–3.1) | 0.08 |
| 3rd | 0.82 (0.02–10) | 1.05 (0–2.8) | 0.53 |
TSH, thyroid stimulating hormone; normal reference 0–10 μIU ml–1.
p≤0.05 was considered to indicate a statistically significant difference. The two groups were compared using the Mann–Whitney non-parametric test.
aGestational age refers to gestational age at the time of iodinated contrast exposure.
bTSH values refer to TSH at term for each trimester.
The serum TSH levels were normal in all the neonates. The Mann–Whitney U-test demonstrated no statistical difference between the two groups for maternal gestational age at time of investigation, age of neonate at time of screening or serum TSH levels. In addition, this test showed that there was no statistically difference in the TSH values when the antenatal contrast exposure occurred in different trimesters.
Discussion
There are controversies related to the optimal imaging pathway for diagnosing pulmonary embolism in pregnancy. Compared with ventilation perfusion scintigraphy, CTPA can offer an alternative diagnosis of the patient's symptoms. CTPA has a high positive predictive value for lobar pulmonary embolism, up to 97% in one study [8]. Depending on the imaging protocol and type of scanner, the radiation dose to the foetus is much lower than ventilation perfusion (V/Q) scintigraphy. Estimated foetal radiation exposure for CTPA varies from 3.3 mGy to 130.0 mGy. However, the foetal radiation dose for V/Q scanning is estimated around 100–370 mGy, which is three times higher than CTPA [9-13].
A potential adverse effect on the foetal thyroid gland from maternal intravenous administration of iodinated contrast agent resulting in neonatal hypothyroidism has been postulated but has not been systematically studied. It should be borne in mind that the studies in the literature referring to neonatal hypothyroidism following maternal ingestion of iodine, however administered, generally refer to chronic ingestion of much higher doses of iodine than those received during a single maternal contrast-enhanced CT scan [14].
Thyroid function during the intra-uterine period is critical for neurological development and metabolism. The thyroid gland of the foetus starts synthesising thyroxine under the influence of TSH by 12 weeks of gestation.
At the time of parturition, there is an acute TSH surge, which results in a transient increase of the TSH value in neonates [15]. Owing to this phenomenon, it is routine practice to repeat abnormal TSH levels in the neonatal period. This effect is most likely to be the cause of the transient high TSH level found in the single newborn that demonstrated a high TSH level in the group of neonates that were given contrast in utero.
The hypothalamus–pituitary–thyroid axis is highly sensitive to variations in iodide concentration. Secondary to elevated levels of circulating iodide, an autoregulatory phenomenon inhibits the formation of thyroid hormones through blockade of the enzyme peroxidase system (Wolff–Chaikoff effect). This response lasts for approximately 2 days following the administration of large amounts of iodide in adults. It is hypothesised that this response is exaggerated in the foetus secondary to an immature thyroid gland and an exaggerated Wolff–Chaikoff effect [16,17]. It is postulated that hypothyroidism may arise in some neonates due to a failure to escape this mechanism [18]. There is delayed excretion of the contrast agent from the maternal circulation with impaired maternal renal function and, therefore, the harmful effect of the iodides on the foetal thyroid is accentuated in maternal renal failure [19]. Hence, excess iodine exposure with use of iodinated maternal antiseptics, iodinated medications, topical iodine solutions and iodine-containing contrast media are generally contraindicated in pregnancy due to the risk of neonatal hypothyroidism [20,21]. In our study, all the mothers had normal renal function, hence the influence of maternal renal impairment on neonatal TSH levels is not reflected upon. The neonatal renal function was not assessed.
Studies in animal models and case reports in humans have shown that iodinated contrast agents can cross the placenta and enter the foetus [22-27]. It is postulated that the iodinated contrast that enters the foetal circulation is excreted by the kidneys into the amniotic fluid. Alternatively, it could pass into the amniotic fluid directly from maternal blood and then be swallowed by the foetus into the gut [27]. The free iodide in the iodinated contrast media is the harmful component, and this forms either due to de-iodination of the contrast agent or as a contamination product [28].
According to quality control regulations, the amount of free iodine per millilitre in a bottle of 300 mgI ml–1 contrast should be less than 50 μg ml–1 immediately after production and less than 90 μg ml–1 after 3–5 years of shelf life [19]. In most cases the actual iodine content is less than one-tenth of these levels. Thus, in 100 ml of contrast medium (300 mgI ml–1) used in our study, the maximum dose of free iodide would be no more than 9000 μg and possibly as little as 500 μg. In a rabbit model looking at the transplacental passage of iohexol and iobitrol, it was estimated that as little as 0.003% of the injected dose reached the foetal blood [29]. If this study could be extrapolated to humans, the amount of free iodide entering the foetus could be a low as 0.21 μg and would therefore appear insignificant. However, to our knowledge, no such study has been performed in humans to confirm this. There is no experimental data to indicate how much free iodide crosses the placenta, how long it remains in the foetus or what its effects on the foetal thyroid function might be.
Atwell et al [30] analysed 23 maternal patients who underwent contrast-enhanced CT during pregnancy for a variety of indications, and their results showed no abnormality in the neonatal thyroid function test as indicated by TSH levels. Bourjeily et al [31] showed similar results in a larger cohort of patients. However, in their study, thyroid function in neonates was evaluated with T4 level in accordance with US guidelines (Rhode Island Department of Health). Ours was a UK-based study and we used the British guidelines for screening hypothyroidism in neonates. Our study has the benefit of a control group of pregnant females investigated for the same clinical indication as the study group, which controls for unknown confounding factors related to the aetiology of maternal symptoms leading to investigation for pulmonary embolism that could theoretically impact later on neonatal thyroid function. Also, we have used TSH levels for screening for neonatal hypothyroidism as T4 does not detect subclinical hypothyroidism.
In a systematic review of neonatal exposure to iodinated contrast media, a tendency towards raised TSH and reduced free T4 was detected in term and premature infants [32]. Between 8.3% and 18.2% of neonates included in the review were treated for hypothyroidism. However, the studies included in the review had heterogeneous methodology and small numbers within the study groups. These were also biased by numerous factors such as neonatal ill health and focused on the administration of intravenous contrast directly to the neonate after birth. These infants received a much higher dose of iodine than the neonates included in this study that were exposed in utero. In addition, the direct effect of the neonatal comorbidities on thyroid function was not controlled for. Therefore, caution should be used in extrapolating these findings to neonates exposed to in utero contrast media.
Congenital hypothyroidism is rare (worldwide incidence of 1 in 3500–4000 live births), and larger studies are required in order to thoroughly investigate the effects of iodine on the neonatal thyroid. Furthermore, geographical location and patient race can influence iodine reserve. In addition, different contrast agents may have different influences on the thyroid. The above-mentioned factors stress the need for further studies in diverse populations.
The results of our study show that the single maternal exposure to iodinated contrast agent does not appear to affect the neonatal thyroid function as indicated by normal TSH level. The newborn screening TSH level following antenatal iodinated contrast agent administration was not significantly elevated compared with the control group, who did not receive contrast agent.
However, these results should be interpreted with caution. The Guthrie test used to screen neonates for hypothyroidism is not perfect, and both false-negative and false-positive results arise [33]. The neonatal hypothyroidism may be transient and resolve spontaneously; hypothyroidism may develop after the first few weeks of life and therefore may not be detected by the screening test. Screening has been shown to double the number of cases of congenital hypothyroidism compared with countries where neonates are not screened secondary to the detection of asymptomatic and very mild cases. In this study only a small group of patients were analysed. As the rate of congenital hypothyroidism in the UK is approximately 1 in 4000, it may be necessary to investigate a much larger cohort of patients to firmly ascertain that thyroid function is unaffected by iodinated contrast media administered to the mother prior to delivery. In addition, if the mother was exposed to iodinated contrast agent earlier in pregnancy, the elevation of the TSH value could be transient and might result in normal newborn TSH values at the time of newborn screening.
So far, only the short-term effects of iodine-containing contrast media exposure, by assessing the TSH level, have been examined. The long-term effects are unclear. Studies have shown that transient hypothyroidism developing in response to an iodine load is associated with an increased risk of hypothyroidism later in life, although this effect may relate to early unmasking of the disease rather than the increased iodine load acting as the causative agent [18]. It is possible that long-term monitoring of the few neonates that show transient hypothyroidism at birth following maternal intravenous contrast administration will be required.
In the summary document of the Contrast Media Safety Committee of ESUR, it was concluded that foetal exposure to iodinated contrast media and iodide is likely to be short-lived and that all infants born to mothers who received iodinated contrast during pregnancy should have their thyroid function checked in the first week of life [7]. There is currently very little information on the results of such tests in this group. Our study, although small, has shown no short-term adverse effect on the neonatal thyroid function of babies born to mothers who received antenatal iodinated contrast agent administered for the investigation of suspected acute pulmonary embolism.
Acknowledgment
The work was carried out at Sheffield Teaching Hospital NHS trust (Royal Hallamshire Hospital, Sheffield, UK and Northern General Hospital, Sheffield, UK).
References
- 1.Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367:1066–74 [DOI] [PubMed] [Google Scholar]
- 2.Panting-Kemp A, Geller SE, Nguyen T, Simonson L, Nuwayhid B, Castro L. Maternal deaths in an urban perinatal network, 1992–1998. Am J Obstet Gynecol 2000;183:1207–12 [DOI] [PubMed] [Google Scholar]
- 3.Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ., 3rd Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med 2005;143:697–706 [DOI] [PubMed] [Google Scholar]
- 4.Wolff J. Iodide goiter and the pharmacologic effects of excess iodide. Am J Med 1969;47:101–24 [DOI] [PubMed] [Google Scholar]
- 5.Galina MP, Avnet ML, Einhorn A. Iodides during pregnancy: an apparent cause of neonatal death. N Engl J Med 1962;267:1124–7 [DOI] [PubMed] [Google Scholar]
- 6.Anderson GS, Bird T. Congenital iodide goitre in twins. Lancet 1961;2:742–3 [DOI] [PubMed] [Google Scholar]
- 7.Webb JA, Thomsen HS, Morcos SK. The use of iodinated and gadolinium contrast media during pregnancy and lactation. Eur Radiol 2005;15:1234–40 [DOI] [PubMed] [Google Scholar]
- 8.Stein PD, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006;354:2317–27 [DOI] [PubMed] [Google Scholar]
- 9.Winer-Muram HT, Boone JM, Brown HL, Jennings SG, Mabie WC, Lombardo GT. Pulmonary embolism in pregnant patients: fetal radiation dose with helical CT. Radiology 2002;224:487–92 [DOI] [PubMed] [Google Scholar]
- 10.Matthews S. Short communication: imaging pulmonary embolism in pregnancy: what is the most appropriate imaging protocol? Br J Radiol 2006;79:441–4 [DOI] [PubMed] [Google Scholar]
- 11.Doshi SK, Negus IS, Oduko JM. Fetal radiation dose from CT pulmonary angiography in late pregnancy: a phantom study. Br J Radiol 2008;81:653–8 [DOI] [PubMed] [Google Scholar]
- 12.Chan WS, Ray JG, Murray S, Coady GE, Coates G, Ginsberg JS. Suspected pulmonary embolism in pregnancy: clinical presentation, results of lung scanning, and subsequent maternal and pediatric outcomes. Arch Intern Med 2002;162:1170–5 [DOI] [PubMed] [Google Scholar]
- 13.Cook JV, Kyriou J. Radiation from CT and perfusion scanning in pregnancy. BMJ 2005;331:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raymond J, LaFranchi SH. Fetal and neonatal thyroid function: review and summary of significant new findings. Curr Opin Endocrinol Diabetes Obes 2010;17:1–7 [DOI] [PubMed] [Google Scholar]
- 15.Fisher DA, Odell WD. Acute release of thyrotropin in the newborn. J Clin Invest 1969;48:1670–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher DA, Klein AH. Thyroid development and disorders of thyroid function in the newborn. N Engl J Med 1981;304:702–12 [DOI] [PubMed] [Google Scholar]
- 17.Wolff J, Chaikoff IL. The inhibitory action of iodide upon organic binding of iodine by the normal thyroid gland. J Biol Chem 1948;172:855. [PubMed] [Google Scholar]
- 18.Markou K, Georgopoulos N, Kyriazopoulou V, Vagenakis AG. Iodine-Induced hypothyroidism. Thyroid 2001;11:501–10 [DOI] [PubMed] [Google Scholar]
- 19.van derMolen AJ, Thomsen HS, Morcos SK. Effect of iodinated contrast media on thyroid function in adults. Eur Radiol 2004;14:902–7 [DOI] [PubMed] [Google Scholar]
- 20.Etling N, Gehin-Fouque F, Vielh JP, Gautray JP. The iodine content of amniotic fluid and placental transfer of iodinated drugs. Obstet Gynecol 1979;53:376–80 [PubMed] [Google Scholar]
- 21.Weber G, Vigone MC, Rapa A, Bona G, Chiumello G. Neonatal transient hypothyroidism: aetiological study. Italian collaborative study on transient hypothyroidism. Arch Dis Child Fetal Neonatal Ed 1998;79:F70–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon AJ, Katzberg RW, Sherman MP. Transplacental passage of iohexol. J Pediatr 2000;136:548–9 [DOI] [PubMed] [Google Scholar]
- 23.Kelleher J, Feczko PJ, Radkowski MA, Griscom NT. Neonatal intestinal opacification secondary to transplacental passage of urographic contrast medium. AJR Am J Roentgenol 1979;132:63–5 [DOI] [PubMed] [Google Scholar]
- 24.Theodoropoulos T, Braverman LE, Vagenakis AG. Iodide-induced hypothyroidism: a potential hazard during perinatal life. Science 1979;205:502–3 [DOI] [PubMed] [Google Scholar]
- 25.Robuschi G, Montermini M, Alboni A, Borciani E, Cersosimo G, Negrotti L, et al. Cord blood iodothyronine and thyrotropin concentrations in newborns of mothers exposed to povidone iodine in the last trimester. J Endocrinol Invest 1987;10:183–6 [DOI] [PubMed] [Google Scholar]
- 26.Rodesch F, Camus M, Ermans AM, Dodion J, Delange F. Adverse effect of amniofetography on fetal thyroid function. Am J Obstet Gynecol 1976;126:723–6 [DOI] [PubMed] [Google Scholar]
- 27.Morrison JC, Boyd M, Friedman BI, Bucovaz ET, Whybrew WD, Koury DN, et al. The effects of Renografin-60 on the fetal thyroid. Obstet Gynecol 1973;42:99–103 [PubMed] [Google Scholar]
- 28.Laurie AJ, Lyon SG, Lasser EC. Contrast material iodides: potential effects on radioactive iodine thyroid uptake. J Nucl Med 1992;33:237–8 [PubMed] [Google Scholar]
- 29.Bourrinet P, Dencausse A, Havard P, Violas X, Bonnemain B. Transplacental passage and milk excretion of iobitridol. Invest Radiol 1995;30:156–8 [DOI] [PubMed] [Google Scholar]
- 30.Atwell TD, Lteif AN, Brown DL, McCann M, Townsend JE, Leroy AJ. Neonatal thyroid function after administration of IV iodinated contrast agent to 21 pregnant patients. AJR Am J Roentgenol 2008;191:268–71 [DOI] [PubMed] [Google Scholar]
- 31.Bourjeily G, Chalhoub M, Phornphutkul C, Alleyne TC, Woodfield CA, Chen KK. Neonatal thyroid function: effect of a single exposure to iodinated contrast medium in utero. Radiology 2010;256:744–50 [DOI] [PubMed] [Google Scholar]
- 32.Ahmet A, Lawson ML, Babyn P, Tricco AC. Hypothyroidism in neonates post-iodinated contrast media: a systematic review. Acta Paediatr 2009;98:1568–74 [DOI] [PubMed] [Google Scholar]
- 33.Why arenewbornbabiesscreenedforcongenitalhypothyroidism? UK Newborn Screening Programme Centre Available from: www.newbornbloodspot.screening.nhs.uk.