Abstract
Endoanal ultrasound is now regarded as the gold standard for evaluating anal sphincter pathology in the investigation of anal incontinence. The advent of three-dimensional ultrasound has further improved our understanding of the two-dimensional technique. Endoanal ultrasound requires specialised equipment and its relative invasiveness has prompted clinicians to explore alternative imaging techniques. Transvaginal and transperineal ultrasound have been recently evaluated as alternative imaging modalities. However, the need for technique standardisation, validation and reporting is of paramount importance. We conducted a MEDLINE search (1950 to February 2010) and critically reviewed studies using the three imaging techniques in evaluating anal sphincter integrity.
Over the last three decades the anal sphincter complex has been the subject of increasing interest involving a variety of disciplines including obstetricians, colorectal surgeons, gastroenterologists, physiotherapists, paediatric surgeons, anatomists, radiologists and midwives. Obstetric trauma is the major cause of faecal incontinence. However, the precise mechanism of maintaining continence is complex, and our understanding of the major mechanism underlying the development of anal incontinence has evolved from that of progressive pudendal neuropathy [1,2] to that of unrecognised mechanical anal sphincter trauma at the time of vaginal delivery [3-5]. Although cadaveric dissections [6], physiological testing [7], ultrasound images [8] and MRI [9] have enabled progressive improvement in understanding the anatomy, function and pathophysiology of the anal sphincter, much remains to be understood.
The technique of anal endosonography was first described by Law and Bartram in 1989 [10] using a B&K type 1846 (Bruel & Kjaer, Naerum, Denmark) ultrasonographic scanner with a 7 MHz rotating endoprobe. The sonographic anatomy of five layers of the anal canal were described: mucosa, submucosa, internal anal sphincter (IAS), intersphincteric plane and external anal sphincter (EAS). In 1993 Sultan et al [6] correlated endosonographic findings with anatomical dissection and rectified the previous description. In 1994, they demonstrated the normal sonographic anal sphincter anatomy and highlighted differences between males and females [8]. Using histological confirmation as the “gold standard” they then validated the sonographic images of EAS defects and established a 100% accuracy of EAS defects when compared with clinical assessment by colorectal surgeons (50%), manometry (75%) and electromyography (75%) [11]. Sultan et al [12] then validated the appearance on internal sphincter defects by prospectively comparing images before and after lateral internal sphincterotomy. Anal endosonography is currently regarded as the diagnostic tool of choice in the investigation of anal incontinence. Recently, two-dimensional (2D) and three-dimensional (3D) volumetric endovaginal ultrasound (EVUS) and transperineal ultrasound (TPUS) have been proposed as alternative imaging modalities to describe anal sphincter integrity.
The aim of this review was, first, to critically evaluate the different ultrasound imaging modalities of the anal sphincter complex and, second, to analyse comparator studies between the three imaging modalities to determine the reproducibility of anal sphincter morphology and biometry among the three different methods (namely endoanal, endovaginal and transperineal). We conducted a MEDLINE search (1950 to February 2010) using the keywords “endoanal”, “endovaginal”, “transvaginal”, “transperineal”, “translabial” and “anal sphincter”. For the purpose of this article the term “transanal” is synonymous with the term “endoanal”; “transvaginal” with “endovaginal”; and “translabial” with “transperineal”.
Anal endosonography
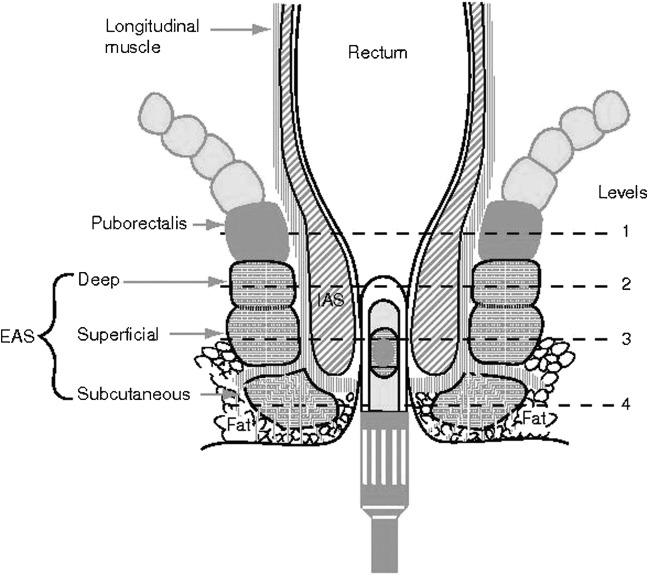
Traditionally, endoanal ultrasound (EAUS) is performed using a 2D ultrasound scanner with a 7 or 10 MHz rotating endoprobe (focal range 5–45 mm), providing a 360° axial view of the anal canal. The patient is usually scanned in the left lateral position, although the prone position may be preferred by others [13]. After the probe is inserted into the anal canal up to approximately 6 cm it is gently withdrawn down the anal canal, during which cross-sectional images of the puborectalis muscle, the longitudinal muscle, EAS, IAS and the anal epithelium are obtained (Figure 1) [14].
Figure 1.
Schematic representation of the anal canal with the probe in situ. Level 1, puborectalis. Level 2, deep (proximal) external anal sphincter (EAS). Level 3, superficial (mid) EAS. Level 4, subcutaneous (distal) EAS.
In earlier studies, anal sphincter defects were noted at three areas along the anal canal: the upper (proximal), middle and lower anal canal. Using these defined areas, Sultan et al [3] in 1993 determined that at 6 weeks after delivery 35% of primiparous females had defects of either the IAS or the EAS or both, and an increment of 4% in the multiparous females (from 40% to 44%). A B&K scanner with a rotating rectal probe fitted with a 7 MHz transducer was used.
In 1999, Gold et al [15] noted that the intra-observer and interobserver agreement for anal sphincter injury was influenced by the ease with which the IAS and EAS were visualised endosonographically. Using a B&K (type 3535) scanner with a 1850 axial endoprobe fitted with a 10 MHz transducer, the boundaries of the proximal, middle and distal anal canal were defined as the following:
proximal anal canal: at the most cranial level of the puborectalis
middle anal canal: level where the EAS forms a complete ring
distal anal canal: level below which the IAS terminates.
The hypoechogenic nature of the IAS made it more easily identifiable than the EAS since the echogenicity of the EAS was similar to that of the proximal structures (i.e. the longitudinal muscle medially and ischioanal fat laterally). In this study of 51 adults referred for investigation of possible sphincter injury, there was no disagreement with respect to IAS tears but some disagreement with assessing the radial and linear extent, as well as the sonographic boundaries of the EAS tears. The overall interobserver agreement with respect to diagnosis of IAS and EAS tears was found to be “very good” (weighted κ of 0.8). This investigator then performed 3D EAUS reconstructions on 24 consecutive patients with sphincter defects on EAUS, with specific attention to the radial and longitudinal extent of the defect. The shorter anterior part of the EAS (as compared with males) and the direct relationship between the radial and longitudinal extent of sphincter trauma was noted using volume imaging [16]. At 10 weeks post partum, Williams et al [17] found that the total incidence of sphincter trauma using EAUS was 29%, with 11% affecting the EAS (similar to the finding of Sultan et al [3]; 35% sphincter trauma at 6 weeks post partum). The author also found a significant decrease in the length of the anterior EAS in an group of 22 females after an atraumatic vaginal delivery and no endosonographic evidence of sphincter trauma after delivery (Table 1) [18].
Table 1. Anal endosonography studies.
| Aim | Cohort assessed | Probe characteristics | Technique | 2D/3D | Outcome |
| Gold et al [15] | 51 patients referred for possible anal sphincter abnormalities | Axial endoscopic probe, 10 MHz | Position: left lateral position | 2D | Overall interobserver agreement for diagnosis of EAS and IAS was found to be very good; κ=0.8 |
| Intra-observer and interobserver agreement of sonographic measurements of the anal structures | Probe: positioned at level of PR, probe withdrawn at increments of 1.25 mm until lower limit of anal canal | ||||
| Gold et al [16] | 20 controls and 24 patients with faecal incontinence | NS | NS | 3D | 3D multiplanar imaging revealed a direct relationship between the length of anal sphincter tear and radial extent |
| Relationship between radial and linear extent of anal sphincter tear | |||||
| Williams et al [17] | 55 females scanned at a median of 33 weeks′ gestation and 10 weeks post partum | B&K Sirius 3D system,a rotating transducer, 10 MHz | Position: left lateral position | 3D | Total incidence of obstetric sphincter trauma was 29% with 11% affecting the EAS |
| Incidence of obstetric trauma to the EAS and related structures | Probe: inserted into distal rectum and automated data acquisition | ||||
| Williams et al [18] | 22 females with no evidence of tears on post-delivery scans | B&K Sirius 3D system,a rotating transducer, 10 MHz | Automated dataset acquired while probe withdrawn from anal canal | 3D | Multiplanar anal endosonography allows longitudinal measurement of anal sphincter; after a vaginal delivery there are changes in the anal sphincter morphology |
| Assess morphological change in anal sphincter in absence of endosonographic evidence of trauma after vaginal delivery |
2D, two-dimensional; 3D, three-dimensional; EAS, external anal sphincter; NS, not stated; PR, puborectalis.
aBruel & Kjaer, Naerum, Denmark.
2D EAUS generates cross-sectional images in the axial plane only, and remains the mainstay of sphincter evaluation. As opposed to 2D static ultrasound, 3D imaging allows volume measurements which may be displayed as either multiplanar images (usually as three orthogonal planes, namely, coronal, sagittal and axial [16-18], and rendered images which display the entire volume in a single image) or tomographic slicing (which allows better visualisation of defects; Figures 2 and 3). Furthermore, the images can be rotated and sliced to enable visualisation from different angles. Offline analysis using proprietary software is also an advantage and has important research implications, as the image can be stored and reviewed for a second opinion, and also shortens the duration of procedure.
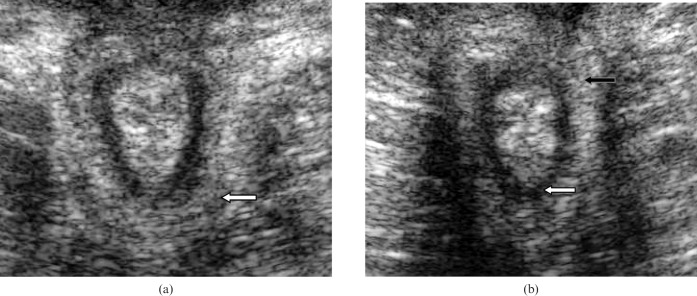
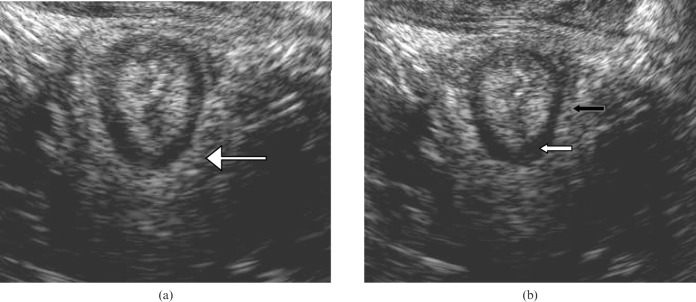
Figure 2.
(a) Transperineal scan demonstrating the puborectalis muscle. (b) Transperineal scan demonstrating the internal anal sphincter (white arrow) and the external anal sphincter (black arrow). Note that the external anal sphincter is circumferential at a more distal level to the puborectalis.
Figure 3.
(a) Third-degree sphincter tear with good repair as demonstrated on tomographic slicing (white arrow). (b) Third-degree tear with residual defect between 10 and 1 o'clock, as demonstrated on tomographic slicing (white arrow).
Investigators in the field have noted that most endoanal scanners are located in specialised radiological centres and also require specialised training, and thus transvaginal ultrasound (TVUS) and TPUS have been evaluated as alternative imaging modalities. It must be noted that images obtained with both these techniques might be complex, and thus require training as well. Transvaginal probes and the standard convex 5 MHz probe are available in almost all obstetric and gynaecological units. With this in mind several studies followed using the transvaginal and transperineal route to establish its place in the evaluation of the anal sphincter. The advantages and disadvantages of the two methods are mentioned in the conclusion.
Vaginal endosonography
In 1994 Sultan et al [19] described a new approach to imaging the anal canal at rest, using a B&K rotating endoprobe fitted with a 7 MHz transducer. Subjects included 20 females (10 healthy volunteers and 10 with faecal incontinence). With the patient lying in the left lateral position the probe was inserted 3 cm into the vagina. By gradually withdrawing the probe, the puborectalis muscle, EAS, IAS, anal submucosa and anal cushions were clearly imaged. The shorter EAS anteriorly in females as seen previously during endoanal endosonography [8] was also confirmed. When vaginal sonographic findings were correlated with anal endosonography it was found that anal endosonography consistently underestimated the thickness of the internal anal sphincter (2.3±0.5 vs 3.2±1.2 mm; mean±standard deviation), and this difference in thickness may be explained by the distension of the sphincter caused by the endoanal probe.
Sandridge et al [20] performed vaginal endosonography on 70 females as part of an indicated endovaginal scan. Patients with previous anorectal surgery and complaints of faecal or flatus incontinence were excluded. Using an Aloka 650 CL scanner (Aloka, Wallingford, CT) fitted with a 5 MHz phased array vaginal probe, an attempt was made to obtain at least three images per subject in a dorsal lithotomy position. The probe was placed vertically just inside the hymenal ring with the tip directed towards the floor.
The anal length and diameter, the thickness and angle of the puborectalis muscle, and the thickness of the IAS and EAS were measured. In this study it was found that 36% of subjects had occult IAS defects and 29% had occult EAS defects, and the sphincter measurements were similar to previously published data based on EAUS, MRI and cadaveric dissections. These findings were not directly compared with anal endosonography. Alexander et al [21] and Poen et al [22] demonstrated that, apart from detecting sphincter defects, TVUS was also useful in determining other causes of faecal incontinence such as rectal fistulae and abscesses. Although TVUS is more readily accessible in most units, is cheaper than the endoanal probe and eliminates distortion of anal epithelium, interpretation of images requires more expertise and clear images of the full length of the anal canal are not always obtainable [23]. This may be due to the utilisation of the endoanal probe for transvaginal scanning; the endoanal probe is approximately 55 cm long and obtaining optimum views of the anal canal may not be ergonomically possible, especially when the patient is in the supine position [16,22,24]. A summary of findings of relevant studies is shown in Tables 2 and 3. With TVUS, It is important to keep the transducer inserted into the vagina in a neutral position, since excessive pressure of the transducer on the perineum and inappropriate angle of incidence of the ultrasound beam to the anal sphincter may distort images and lead to erroneous results.
Table 2. Transvaginal ultrasound studies.
| Aim | Cohort assessed | Probe characteristics | Technique | 2D/3D | Outcome |
| Sandridge et al [20] | 70 females as part of an indicated endovaginal scan | Aloka 650 machine,a 5 MHz phased array vaginal probe | Position: dorsal lithotomy | 2D | 29% occult EAS defects and 36% occult IAS defects; anal sphincter measurements using vaginal ultrasound are comparable to endoanal sonography |
| To describe the anatomy of the anus and rectum with vaginal endosonography | Probe: held vertically just inside hymenal ring with the tip directed posteriorly | ||||
| Alexander et al [21] | 28 females complaining of faecal incontinence underwent transvaginal ultrasound | Acusonb (side-fire endorectal probe), 5–7 MHz; left lateral decubitus position | Position: left lateral decubitus position | 2D | Fistulas, peri-rectal abscesses (25%) and pudendal injuries (15%) account for other causes of faecal incontinence |
| Determine anatomic causes of faecal incontinence using transvaginal ultrasound | Probe: placed into the vagina at the expected level of the anal canal |
2D, two-dimensional; 3D, three-dimensional.
aAloka, Wallingford, CT.
bAcuson, Siemens Healthcare, Munich, Germany.
Transperineal ultrasound
In the quest for a less invasive, more user-friendly, more accessible and more patient-acceptable imaging modality, the transperineal approach was evaluated. Similar to the technological advancement of EAUS and TVUS, studies were performed with TPUS to determine the incidence of occult sphincter defects [25] and normal anal sphincter parameters [26-29], as well as its accuracy in detecting sphincter defects [25,30]. Another advantage of transperineal scanning is the ability to study the dynamic interaction between the pelvic floor and pelvic viscera without using an endocavity probe (endovaginal and endoanal) [31]. TPUS is usually performed with the patient placed in the dorsal lithotomy position, with the hips flexed and abducted, and the convex transducer positioned on the perineum between the mons pubis and the anal sphincter.
In a group of 139 primiparous females, Valsky et al [25] found using 3D TPUS that 7.9% had occult damage to the anal sphincter. In this study 91.4% of acquired volumes were adequate for interpretation. In the group that sustained third-degree tears (repaired by overlap technique) TPUS was possible as early as 48 h post-partum. These authors described the “half moon sign” as IAS thinning in the area of damage and opposite thickening, as well as an abnormal appearance of mucosal folds as signs indicative of sphincter damage. A 5–9 MHz vaginal probe (Olson 730; GE Healthcare, Waukesha, WI) was used. Suboptimal imaging of the EAS was noted in 15% in the 12 o'clock area. Hall et al [26] placed a 4–8 MHz curvilinear endovaginal probe (Phillips 1022; Philips Medical Systems, Bothell, WA) at the introitus of 60 Hispanic and Caucasian females presenting for a gynaecological ultrasound for symptoms other than pelvic organ prolapse and urinary or faecal incontinence. The aim was to determine normal values of IAS and EAS measurements at the proximal, middle and distal levels of the anal canal using clock-face terminology. This was possible for the IAS at all levels but not for the EAS, which was measured only at the distal level. In a subgroup of intact asymptomatic females (n=36), measurements were comparable with previously published endoanal data [15]. Peschers et al [27] applied a conventional 5 MHz convex transducer (Siemens SI 400; Siemens Healthcare, Munich, Germany) to the perineum (exoanal ultrasound) of a heterogenous group of 68 females (25 with faecal incontinence, 11 asymptomatic nulliparous and 32 asymptomatic parous females). In both axial and sagittal planes, all the layers of the anal sphincter complex as described by EAUS were visualised. The presence of sphincter defects were determined from video records by two independent examiners blinded to each other's results. There was 100% agreement for IAS defects, and one disagreement about an EAS defect. All defects detected by the transperineal method were verified at sphincter reconstructive surgery (five patients). Using a 5–9 MHz endovaginal transducer (Voluson 730; GE Healthcare) placed at the introitus and then directed posteriorly on the perineum, Lee et al [28] acquired 3D volumes to evaluate dynamic changes in anal sphincter measurements and the levator hiatus during rest and squeeze in 22 asymptomatic nulliparous females in the lithotomy position. While the IAS was easily defined, the EAS and intersphincteric space were not. There was no difference in IAS transverse thickness at the proximal level (puborectalis level) and distal level (middle of the EAS) at the 3 and 9 o'clock positions during rest and squeeze. Huang et al studied the biometry of the anal sphincter in 55 nulliparous Chinese females, and also demonstrated that all the levels of the EAS can be visualised using an endovaginal probe placed at the perineum, and that the EAS was thinner at 12 o'clock [29]. As can be seen in Table 4, many of the TPUS studies utilised vaginal transducers placed on the perineum with alteration of the axis to obtain optimal views. Since endocavity transducers have a higher resolution (4–8 MHz, 5–9 MHz) than transperineal transducers (5 MHz), these studies labelled as TPUS represent a different subset of the transperineal ultrasound imaging modality and are thus not “true transperineal scanning”.
Table 4. Transperineal ultrasound studies.
| Aim | Cohort assessed | Probe characteristics | Technique | 2D/3D | Outcome |
| Valsky et al [25] | 152 primiparous females | Vaginal transducer 5–9 MHz (Voluson 730 Expert, GEa) | Position: not stated | 3D | Scanning possible in 91.4% of cases |
| Role 3D TPUS in two groups of primiparous females – Group 1 without clinically recognised third- or fourth-degree tears | Group 1 included 139 females without clinically recognised third- or fourth-degree perineal tears who were examined 24–72 h following vaginal delivery; Group 2 included 13 females with clinically recognised third-degree perineal tears, who were examined from 48 h post-partum up to 4 months following surgical repair by the overlapping technique | Probe: placed on the fourchette and perineal body, and scanned in the transverse and sagittal planes | Occult sphincter damage in 7.9% (group) | ||
| Group 2 following surgical repair of third-degree tears by the overlapping technique | IAS in all cases and EAS in 84.6% determined reference data in post-partum females | ||||
| Hall et al [26] | 60 females presenting for gynaecological ultrasound for symptoms other than pelvic organ prolapse or urinary or anal incontinence | 4–8 MHz endovaginal transducer | Position: lithotomy | 2D | Anal sphincter measurements for intact asymptomatic and asymptomatic females were comparable with EAUS and MRI data |
| To determine normal values of the anal sphincter complex | Probe: directed posteriorly towards the anal sphincter complex and aligned nearly perpendicularly to the floor | ||||
| Peschers et al [27] | 68 patients (25 with faecal incontinence, 11 asymptomatic nulliparous and 32 asymptomatic parous females) | Conventional 5 MHz convex transducer (Siemens SI 400b) | Position: lithotomy | 2D | Anal sphincter anatomy can be visualised with TPUS; 100% agreement for IAS defects |
| Description of normal anal sphincter anatomy and sphincter defects using TPUS | Probe: placed on the perineal body and directed perpendicular to the longitudinal axis of the anal canal; angle adjusted until all layers of the anal canal visualised | One discordant result in EAS group | |||
| Lee et al [28] | 22 nulliparous healthy female volunteers | Endovaginal transducer, 5–9 MHz (Voluson 730, GEa) | Probe: placed on the perineum at the vaginal introitus and directed posteriorly on the perineum in a mid-sagittal orientation | 3D post processing with GE Kretz 4D View, version 5.0 software packagea | TPUS is useful in evaluating anal sphincter anatomy, and measurements are comparable with EAUS |
| Longitudinal muscle and outer border of EAS could not be measured in all subjects | |||||
| Dynamic evaluation of anal sphincter-at rest and contraction | |||||
| Description of normal anal sphincter anatomy using 3D TPUS | Position: lithotomy | Automated data acquisition | |||
| Huang et al [29] | 55 nulliparous Chinese females | Transvaginal transducer, 5–9 MHz (Voluson 730, GEa) | Position: supine | 3D post-processing with GE Kretz 4D View, version 5.0 software packagea | Morphology of anal sphincter clearly demonstrated on 3D TPUS and biometry is reproducible; however, EAS significantly anteriorly; longitudinal muscle not clearly visualised |
| Identify the morphological characteristics and normal biometry of the anal sphincter complex in nulliparous Chinese females | Probe: placed at the introitus in the mid-sagittal plane and then at the perineum after turning the probe 60–80○°downwardb | Multiplanar imaging allowing serial paramedian views, and post-processing can be repeated | |||
| Automated data acquisition |
Comparative studies
Frudinger et al reported that, when compared with EAUS, TVUS revealed a sensitivity of 44% and specificity of 96% for the detection of IAS defects, a sensitivity of 48% and specificity of 88% for EAS defects [30], and an interobserver agreement of 88.6% for identifying sphincter defects. Stewart et al [23] documented that their TVUS and EAUS sonographic findings were in agreement in a group of 40 out of 44 patients imaged prospectively (24 with intact sphincters and 20 with sphincter defects). Poen et al [22] and Ramirez et al [24] highlighted the added value of TVUS in identifying perianal pathology (e.g. perianal abscess and fistula) and the ability to clarify a “doubtful EAUS study”.
When compared with EAUS, difficulties encountered with TPUS include poor visualisation of the lateral border of the EAS, and the fact that the anal mucosa and submucosa cannot be viewed as separate entities [32]. In a study by Roche et al [32], TPUS was able to detect all cases of EAS defects identified on EAUS (six patients) and the IAS thickness obtained by TPUS was comparable with the EAUS findings. However, Lohse et al [33] found a significant difference in both the IAS and EAS thickness when comparing measurements obtained on TPUS and EAUS in 64 females attending a urogynaecological clinic complaining only of urinary incontinence. Two independent operators performed the scans using a 5 MHz linear probe (Aloka SSD) and a 7.5 MHz rectal endoprobe. In this study the sensitivity for the detection of anal sphincter defects using TPUS was 50%. However, the authors did not mention the technique of TPUS or the levels along the length of the sphincter used to detect lesions (Table 5). In both these studies the average thickness of the IAS was greater on TPUS than on EAUS, and the average thickness of the EAS was less on TPUS than on EAUS (Tables 3 and 5). Currently there are limited transvaginal and transperineal ultrasound studies that are directly compared with EAUS. Although the sensitivity for the detection of sphincter defects ranges from 44% for TVUS to 50% for TPUS, the higher resolution of vaginal probes and the larger field of view of transperineal probes maybe of added value.
Table 5. Comparative studies: transperineal ultrasound versus endoanal ultrasound.
| Aim | Cohort assessed | Probe characteristics | Technique | 2D/3D | Outcome | Difficulties noted/limitations |
| Roche et al [32]Describe biometry of anal sphincter | 20 healthy nulliparous females20 post-partum primiparous females | TPUS: Hitachi convex and linear probe, 3.5–7.5 MHz, EAUS: B&Ka 360° 7 MHz rotating probe | Position: dorsal lithotomyProbe: placed on the perineum between the anus and introitus and inclined until all levels visualised | 2D | TPUS demonstrated all EAS tears, and all IAS tears except one | Cannot clearly visualise the anal mucosa separate from the submucosa |
| Lohse et al [33]Comparison of TPUS and EAUS | 64 urogynaecological patients with urinary incontinence only | TPUS: Aloka SSD 2000,b 5 MHz linear probeEAUS: Aloka SSD 2000,b 7.5 MHz endoanal probe | Patient: supineProbe: not stated | 2D | Significant difference between EAS and IAS measurementsSensitivity of TPUS for the diagnosis of sphincter lesions using EAUS as gold standard is 50% |
EAS, external anal sphincter; EAUS, endoanal ultrasound; IAS internal anal sphincter; TPUS, transperineal ultrasound.
aBruel & Kjaer, Naerum, Denmark.
bAloka, Wallingford, CT.
Table 3. Comparative studies: transvaginal ultrasound versus endoanal ultrasound.
| Aim | Cohort assessed | Probe characteristics | Technique | 2D/3D | Outcome | Difficulties noted/limitations |
| Frudinger et al [30]Transvaginal versus anal endosonography for detecting damage to the anal sphincter | 47 parous and 1 nulliparous (75% complained of faecal incontinence) | Anal and vaginal ultrasound with B&K rectal endoprobe,a 10-MHz | Position: supine left lateral position? | 2D | TVUS: sensitivity and specificity for detection of IAS defects were 44% and 96%, and for EAS defects were 48% and 88%, respectively | Limited anatomical information on TVUS due to axial plane imaging only |
| Modified vaginal probe in 5 patients, B&K,a 10 MHz transducer | Probe: Inserted 3cm into vagina and gradually withdrawn | |||||
| Poen et al [22] | 56 females (36 patients with faecal incontinence, 20 patients with perianal sepsis) | Anal and vaginal ultrasound with B&K,a 7 MHz rotating endoprobe, probe inserted into vagina until rectum was visualised | Patient position not stated | 2D | TVUS increased the diagnostic yield in 25% (added important information—location of abscess and fistulae tracts) | Limited focal range of the vaginal probe in viewing the dorsolateral part of the EAS |
| Evaluate TVUS in the diagnosis of faecal incontinence and perianal sepsis | Probe: inserted into the vagina until the rectum was visualised and gradually withdrawn while images of the PR and anal sphincters were taken | |||||
| Stewart et al [23] | 50 patients of which 32 were referred for faecal incontinence and rest for other anorectal problems; 44 had both EAUS and TVUS | EAUS: B&K,a with 10 MHz rotating endoprobe | Position: EAUS—left lateral decubitus position | 2D | TVUS is accurate as EAUS for sphincter evaluation | TVUS and EAUS performed by same radiologist |
| TVUS: with 7.5 MHz biplane side-fire transrectal probe | TVUS—supine position | |||||
| Validate the use of TVUS for sphincter evaluation | Probe: For TVUS, special attention to depression of the probe towards the perineal body as the probe is withdrawn | |||||
| Ramirez et al [24] | 30 females with faecal incontinence (3 sepsis from episiotomy,4 previous anal surgery, 3 complained of rectal prolapse) | Both EAUS and TVUS; B&K,a 7 MHz endoprobe | Patient position not stated | 2D | TVUS more valuable in a group of patients with a “doubtful” EAUS study | TVUS is difficult to perform and 1 in 4 patients could be adequately scanned (reason not stated), but TVUS clarified doubts in 10% of cases arising from findings on EAUS |
| The value of TVUS as compared with EAUS | Probe: Inserted into the vagina until the rectum was visualised and gradually withdrawn while images of the PR and anal sphincters were taken |
2D, two-dimensional; 3D, three-dimensional; EAS, external anal sphincter; EAUS, endoanal ultrasound; IAS, internal anal sphincter; TVUS, transvaginal ultrasound.
aBruel & Kjaer, Naerum, Denmark.
Conclusion
The use of ultrasound in the evaluation of pelvic floor disorders has increased dramatically (Figures 2, 4 and 5). It has been shown to be useful, safe and well tolerated by patients. Imaging has evolved from static 2D imaging to dynamic 3D volumetric imaging, and recently even four-dimensional (4D) imaging.
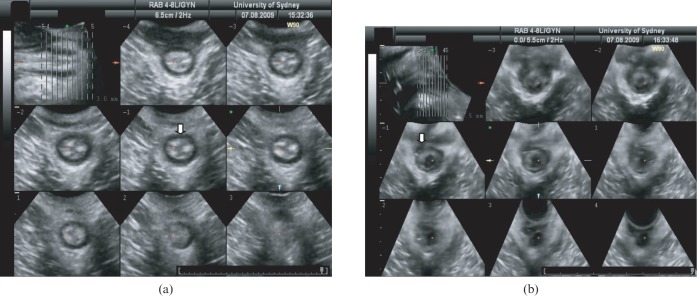
Figure 4.
(a) Endoanal scan demonstrating the “U”-shaped puborectalis muscle, which attaches to the pubic rami anteriorly. (b) Endoanal scan demonstrating the internal anal sphincter (white arrow) and the external anal sphincter (black arrow). (c) Three-dimensional endoanal ultrasound demonstrating the circumference/width as well as length of the anal sphincter defect.
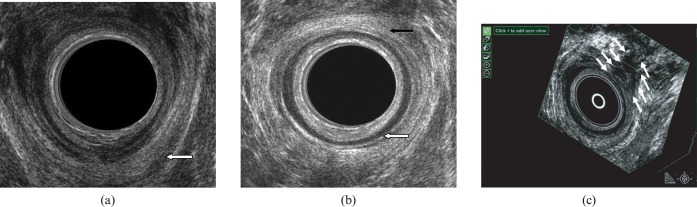
Figure 5.
(a) Endovaginal scan demonstrating the puborectalis muscle (white arrow). (b) Endovaginal scan demonstrating the internal anal sphincter (white arrow) and the external anal sphincter (black arrow).
This review highlights that normal anal sphincter morphology and anal sphincter measurements can be obtained using both transvaginal and transperineal routes. From the literature it is evident that the incidence of occult anal sphincter damage is comparable between EAUS and TVUS (29%), but is significantly lower with TPUS (7.9%; highlighted in Tables 1, 2 and 4); thus, more TPUS studies are necessary. Advantages of the transvaginal and transperineal route include availability of commonly used transducers, absence of distortion of the anal canal and better patient acceptability. The transvaginal route may be more valuable in patients with a short anal canal and wide introitus [24], and since the need for insertion of an endocavity probe is negated with TPUS, it may be more acceptable and less painful in patients with perianal pathology.
There is a need for further corroboration, technique standardisation (especially with TPUS) and reporting of defects, as current studies differ in methodology and include heterogeneous samples [34]. Currently, 3D EAUS is still the preferred method of sphincter defect evaluation. Future studies should focus on the predictive value of both TVUS and TPUS as compared with EAUS in the detection of sphincter defects.
References
- 1.Allen RE, Hosker GL, Smith AR, Warrell DW. Pelvic floor damage and childbirth: a neurophysiological study. Br J Obstet Gynaecol 1990;97:770–9 [DOI] [PubMed] [Google Scholar]
- 2.Snooks SJ, Setchell M, Swash M, Henry MM. Injury to innervation of pelvic floor sphincter musculature in childbirth. Lancet 1984;8:546–50 [DOI] [PubMed] [Google Scholar]
- 3.Sultan AH, Kamm MA, Hudson CN, Thomas JM, Bartram CI. Anal-sphincter disruption during vaginal delivery. N Engl J Med 1993;23:1905–11 [DOI] [PubMed] [Google Scholar]
- 4.Kamm MA. Obstetric damage and faecal incontinence. Lancet 1994;344:730–3 [DOI] [PubMed] [Google Scholar]
- 5.Andrews V, Sultan AH, Thakar R, Jones PW. Occult anal sphincter injuries—myth or reality? BJOG 2006;113:195–200 [DOI] [PubMed] [Google Scholar]
- 6.Sultan AH, Nicholls RJ, Kamm MA, Hudson CN, Beynon J, Bartram CI. Anal endosonography and correlation with in vitro and in vivo anatomy. Br J Surg 1993;80:508–11 [DOI] [PubMed] [Google Scholar]
- 7.Sultan AH, Kamm MA. Relationship between parity and anal manometry. Dis Colon Rectum 1993;36:783–4 [DOI] [PubMed] [Google Scholar]
- 8.Sultan AH, Kamm MA, Hudson CN, Nicholls JR, Bartram CI. Endosonography of the anal sphincters: normal anatomy and comparison with manometry. Clin Radiol 1994;49:368–74 [DOI] [PubMed] [Google Scholar]
- 9.Beets-Tan RG, Morren GL, Beets GL, Kessels AG, el Naggar K, Lemaire E, et al. Measurement of anal sphincter muscles: endoanal US, endoanal MR imaging, or phased-array MR imaging? A study with healthy volunteers. Radiology 2001;220:81–9 [DOI] [PubMed] [Google Scholar]
- 10.Law PJ, Bartram CI. Anal endosonography: technique and normal anatomy. Gastrointest Radiol 1989;14:349–53 [DOI] [PubMed] [Google Scholar]
- 11.Sultan AH, Kamm MA, Talbot IC, Nicholls RJ, Bartram CI. Anal endosonography for identifying external sphincter defects confirmed histologically. Br J Surg 1994;81:463–5 [DOI] [PubMed] [Google Scholar]
- 12.Sultan AH, Kamm MA, Nicholls RJ, Bartram CI. Prospective study of the extent of internal anal sphincter division during lateral sphincterotomy. Dis Colon Rectum 1994;37:1031–3 [DOI] [PubMed] [Google Scholar]
- 13.Frudinger A, Bartram CI, Halligan S, Kamm M. Examination techniques for endosonography of the anal canal. Abdom Imaging 1998;23:301–3 [DOI] [PubMed] [Google Scholar]
- 14.Thakar R, Sultan AH. Anal endosonography and its role in assessing the incontinent patient. Best Pract Res Clin Obstet Gynaecol 2004;18:157–73 [DOI] [PubMed] [Google Scholar]
- 15.Gold DM, Halligan S, Kmiot WA, Bartram CI. Intraobserver and interobserver agreement in anal endosonography. Br J Surg 1999;86:371–5 [DOI] [PubMed] [Google Scholar]
- 16.Gold DM, Bartram CI, Halligan S, Humphries KN, Kamm MA, Kmiot WA. Three-dimensional endoanal sonography in assessing anal canal injury. Br J Surg 1999;86:365–70 [DOI] [PubMed] [Google Scholar]
- 17.Williams AB, Bartram CI, Halligan S, Spencer JA, Nicholls RJ, Kmiot WA. Anal sphincter damage after vaginal delivery using three-dimensional endosonography. Obstet Gynecol 2001;97:770–5 [DOI] [PubMed] [Google Scholar]
- 18.Williams AB, Bartram CI, Halligan S, Marshall MM, Spencer JA, Nicholls RJ, et al. Alteration of anal sphincter morphology following vaginal delivery revealed by multiplanar anal endosonography. BJOG 2002;109:942–6 [DOI] [PubMed] [Google Scholar]
- 19.Sultan AH, Loder PB, Bartram CI, Kamm MA, Hudson CN. Vaginal endosonography. New approach to image the undisturbed anal sphincter. Dis Colon Rectum 1994;37:1296–9 [DOI] [PubMed] [Google Scholar]
- 20.Sandridge DA, Thorp JM., Jr Vaginal endosonography in the assessment of the anorectum. Obstet Gynecol 1995;86:1007–9 [DOI] [PubMed] [Google Scholar]
- 21.Alexander AA, Liu JB, Merton DA, Nagle DA. Faecal incontinence: transvaginal US evaluation of anatomic causes. Radiology 1996;199:529–32 [DOI] [PubMed] [Google Scholar]
- 22.Poen AC, Felt-Bersma RJ, Cuesta MA, Meuwissen GM. Vaginal endosonography of the anal sphincter complex is important in the assessment of faecal incontinence and perianal sepsis. Br J Surg 1998;85:359–63 [DOI] [PubMed] [Google Scholar]
- 23.Stewart LK, Wilson SR. Transvaginal sonography of the anal sphincter: reliable, or not? AJR Am J Roentgenol 1999;173:179–85 [DOI] [PubMed] [Google Scholar]
- 24.Ramirez JM, Aguilella V, Martinez M, Gracia JA. The utility of endovaginal sonography in the evaluation of fecal incontinence. Rev Esp Enferm Dig 2005;97:317–22 [DOI] [PubMed] [Google Scholar]
- 25.Valsky DV, Messing B, Petkova R, Savchev S, Rosenak D, Hochner-Celnikier D, et al. Postpartum evaluation of the anal sphincter by transperineal three-dimensional ultrasound in primiparous females after vaginal delivery and following surgical repair of third-degree tears by the overlapping technique. Ultrasound Obstet Gynecol 2007;29:195–204 [DOI] [PubMed] [Google Scholar]
- 26.Hall RJ, Rogers RG, Saiz L, Qualls C. Translabial ultrasound assessment of the anal sphincter complex: normal measurements of the internal and external anal sphincters at the proximal, mid-, and distal levels. Int Urogynecol J Pelvic Floor Dysfunct 2007;18:881–8 [DOI] [PubMed] [Google Scholar]
- 27.Peschers UM, DeLancey JO, Schaer GN, Schuessler B. Exoanal ultrasound of the anal sphincter: normal anatomy and sphincter defects. Br J Obstet Gynaecol 1997;104:999–1003 [DOI] [PubMed] [Google Scholar]
- 28.Lee JH, Pretorius DH, Weinstein M, Guaderrama NM, Nager CW, Mittal RK. Transperineal three-dimensional ultrasound in evaluating anal sphincter muscles. Ultrasound Obstet Gynecol 2007;30:201–9 [DOI] [PubMed] [Google Scholar]
- 29.Huang WC, Yang SH, Yang JM. Three-dimensional transperineal sonographic characteristics of the anal sphincter complex in nulliparous women. Ultrasound Obstet Gynecol 2007;30:210–20 [DOI] [PubMed] [Google Scholar]
- 30.Frudinger A, Bartram CI, Kamm MA. Transvaginal versus anal endosonography for detecting damage to the anal sphincter. AJR Am J Roentgenol 1997;168:1435–8 [DOI] [PubMed] [Google Scholar]
- 31.Beer-Gabel M, Teshler M, Barzilai N, Lurie Y, Malnick S, Bass D, et al. Dynamic transperineal ultrasound in the diagnosis of pelvic floor disorders: pilot study. Dis Colon Rectum 2002;45:239–45, discussion 245–8 [DOI] [PubMed] [Google Scholar]
- 32.Roche B, Deleaval J, Fransioli A, Marti MC. Comparison of transanal and external perineal ultrasonography. Eur Radiol 2001;11:1165–70 [DOI] [PubMed] [Google Scholar]
- 33.Lohse C, Bretones S, Boulvain M, Weil A, Krauer F. Transperineal versus endoanal ultrasound in the detection of anal sphincter defects. Eur J Obstet Gynaecol 2002;103:79–82 [Google Scholar]
- 34.Santoro GA, Wieczorek AP, Dietz HP, Mellgren A, Sultan AH, Shobeiri SA, et al. State of the art: an integrated approach to pelvic floor ultrasonography. Ultrasound Obstet Gynecol 2011;37:381–96 [DOI] [PubMed] [Google Scholar]