Abstract
Objective
The purpose of this study is to compare the dose-volumetric results of RapidArc® (RA Varian Medical Systems, Palo Alto, CA) with those of intensity-modulated radiation therapy (IMRT) for hepatocellular carcinoma.
Methods
20 patients previously treated for hepatocellular carcinoma were the subjects of this planning study. 10 patients were treated for portal vein tumour thrombosis (Group A), and 10 patients for primary liver tumour (Group B). Prescription dose to the planning target volume was 54 Gy in 30 fractions, and the planning goal was to deliver more than 95% of prescribed dose to at least 95% of planning target volume.
Results
In Group A, mean doses to liver were increased with RA vs IMRT (22.9 Gy vs 22.2 Gy, p=0.0275). However, V30 Gy of liver was lower in RA vs IMRT (31.1% vs 32.1%, p=0.0283). In Group B, in contrast, neither mean doses nor V30 Gy of liver significantly differed between the two plans. V35 Gy of duodenum and V20 Gy of kidney were decreased with RA in Groups A and B, respectively (p=0.0058 and 0.0124, respectively). Both maximal doses to spinal cord and monitor unit were significantly lower in the RA plan, regardless of the group.
Conclusion
The dose-volumetric results of RA vs IMRT were different according to the different target location within the liver. In general, RA tended to be more effective in the sparing of non-liver organs at risk such as duodenum, kidney, and/or spinal cord. Moreover, RA was more efficient in the treatment delivery than IMRT in terms of total monitor unit used.
With the advent of three-dimensional conformal radiotherapy and more understanding of partial liver tolerance, radiotherapy has been increasingly incorporated into the treatment of hepatocellular carcinoma (HCC). Reported results of novel techniques to deliver higher dose radiation, such as stereotactic body radiotherapy and proton beam therapy, are not only feasible but also promising [1,2]. In contrast, HCC has rarely been an indication of intensity-modulated radiation therapy (IMRT) due to the organ motion during respiration, although several dosimetric studies have been reported on the benefit of IMRT in HCC [3,4]. However, with image-guidance technology, liver tumours can be treated with IMRT safely and efficiently. Recently, a study on IMRT using Hi-Art tomotherapy (TomoTherapy Inc., Madison, WI) for patients with unresectable HCC reported lower toxicity with promising local control [5].
RapidArc® (RA; Varian Medical Systems, Palo Alto, CA), which delivers intensity-modulated beams during gantry rotation, is shown to reduce doses to organs at risk (OARs) compared with static field IMRT in a number of tumours at various anatomical sites [6-8]. However, little is known about the benefit of RA in the treatment of HCC to date. Herein, a planning study comparing RA vs IMRT for patients with HCC was performed.
Methods and materials
Patient population
After institutional review board approval, 20 patients who underwent radiotherapy for HCC between December 2004 and February 2009 were entered into this planning study. There were 19 males and 1 female, and their median age was 59 (range, 38–74) years. Among them, 10 patients were treated for portal vein tumour thrombosis (Group A), and 10 patients were treated for primary liver tumour (Group B). Of 10 patients in Group B, 4 patients had left lobe tumours and 6 patients had right lobe tumours.
Simulation
All patients underwent three-phase CT, including free breathing, full inhalation and full exhalation. Clinical target volume included only portal vein tumour thrombosis in Group A, and only intrahepatic tumour in Group B. Internal target volume was delineated based on the three phases of CT images. Planning target volume (PTV) was defined as internal target volume plus 8 mm in all directions. Organs at risk were also delineated, including whole liver, non-target liver (whole liver minus PTV), stomach, duodenum, kidney, spinal cord, and so on.
Planning
Treatment planning was performed using the Eclipse system with 15 MV photons in a Varian 21IX machine with a 120 leaf millennium multileaf collimator (Varian Medical Systems, Palo Alto, CA). For IMRT, the sliding window technique was used. The Anisotropic Analytical Algorithm (AAA, version 8.6) dose calculation algorithm was used for both IMRT and RA. The dose calculation grid was set to 2.5 mm. A total of 54 Gy/30 fx was prescribed to the PTV with the planning goal of delivering more than 95% of the prescribed dose to at least 95% of the PTV. To achieve these objectives, a constraint for D100%, where Dn% is the dose received by the n% volume of the target volume, was set to receive ≥102% of prescription and a constraint for maximum dose (Dmax) was set to receive ≤104% of the prescription in the optimisation process for both plans. Optimisation constraints and their weightings are summarised in Table 1. These constraints and weightings were set initially and then were modified by either relaxing or tightening or adding during the optimisation process based on the real-time updated dose–volume histograms (DVHs) of structures. We used a 2-cm-wide ring structure, pseudo target, which is 7 mm apart from the PTV.
Table 1. Dose–volume constraints.
| Volume | Dose–volume constraints | Relative weighting |
| PTV | D100%≥102% | 120 |
| D98%≥102.5% | ||
| D2%≤103.5% | ||
| Dmax≤104% | ||
| CTV | D100%≥102.5% | 130 |
| Dmax≤104% | ||
| Whole liver | V30 Gy≤40% | 80 |
| Kidneys | V20 Gy≤30% | 60 |
| Duodenum | V35 Gy≤30% | 50 |
| Stomach | V35 Gy≤30% | 50 |
| Spinal cord | Dmax≤45 Gy | 50 |
CTV, clinical target volume; PTV, planning target volume.
For both IMRT and RA, the isocentre of beams was set at the centre of the PTV initially and then determined by rounding off to the nearest integer. For IMRT plans, five coplanar gantry angles of beams were manually selected based on morphological relationships of the PTVs and OARs. An RA plan was generated using 2.5 arcs rotating from 179.9° to 180.1° anticlockwise, from 180.1° to 179.9° clockwise, and 179.9° to 0° anticlockwise. All plans used 15 MV photons and a fixed dose rate (or maximum dose rate for RA) of 600 MU min−1. Planning was done by a single physicist (JMP) and clinical aspects were reviewed by a single oncologist (KK).
Dose-volumetric analysis
Dose-volumetric analysis was performed using DVHs of the treatment plans of individual patients. Conformity index (CI) was calculated as (volume within the 95% isodose)/(volume of the PTV). For liver, both mean dose and V30 Gy of the whole liver and non-target liver were calculated. For non-liver OARs, V35 Gy of stomach and duodenum, V20 Gy of kidney, and maximal dose to spinal cord were calculated. Average DVHs for each OAR were also built from the individual DVHs. A paired t-test was used to calculate the statistical difference of dose-volumetric results between RA and IMRT. Total monitor unit and treatment time were also compared to access the efficiency of treatment delivery.
Results
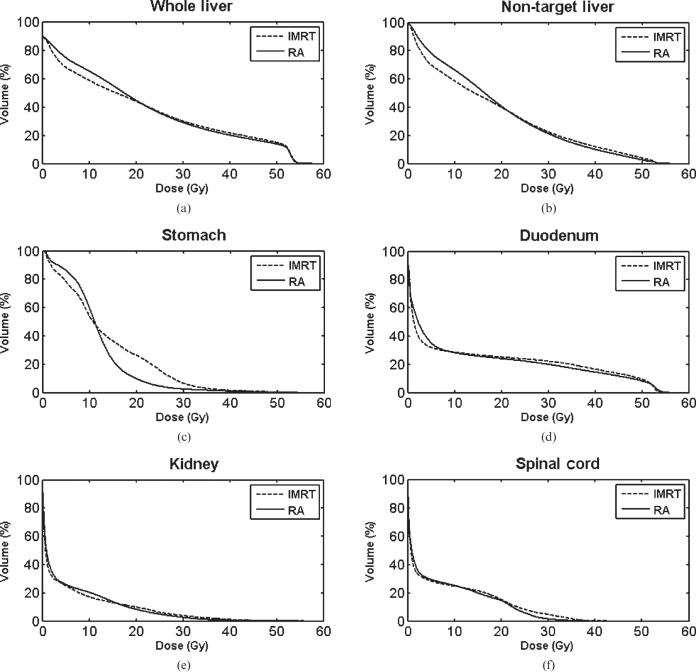
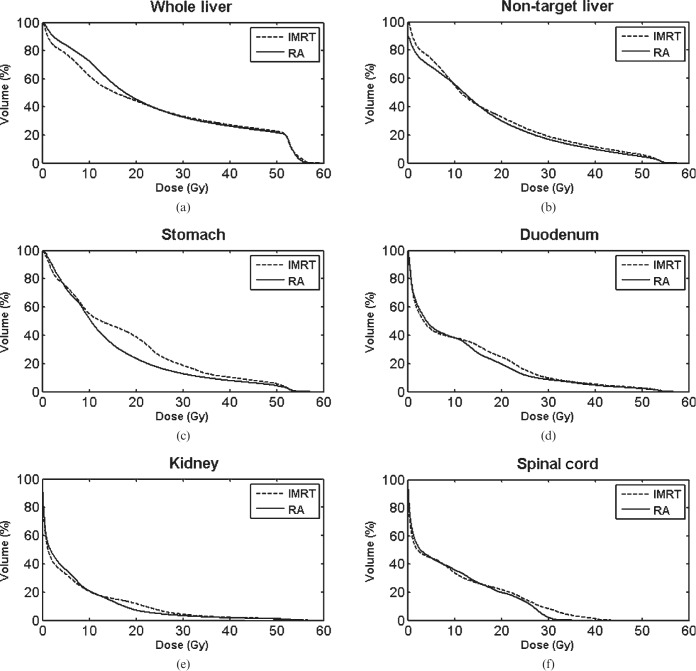
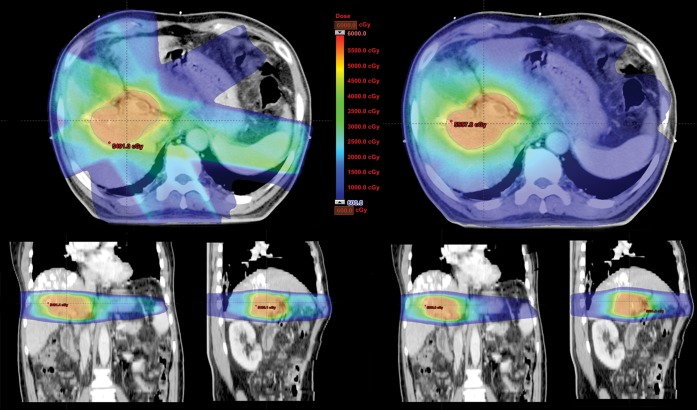
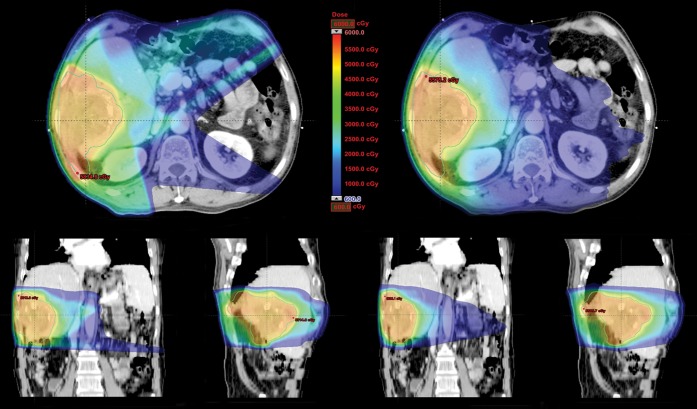
The dose-volumetric results of RA compared with IMRT in Groups A and B are shown in Tables 2 and 3, respectively. In group A, mean doses to the whole liver and non-target liver were increased with RA vs IMRT (22.9 vs 22.2 Gy and 18.7 vs 18.0 Gy, p=0.0275 and 0.0307, respectively). However, V30 Gy of the whole liver and non-target liver was lower in RA vs IMRT (31.1% vs 32.1% and 21.4% vs 22.6%, p=0.0283 and 0.0351, respectively). In Group B, in contrast, neither mean doses nor V30 Gy of the whole liver and non-target liver significantly differed between the two plans. As for non-liver OARs, V35 Gy of duodenum was decreased with RA vs IMRT in group A (17.9% vs 20.9%, p=0.0058), while V20 Gy of kidneys was decreased in group B (7.3% vs 11.9%, p=0.0124). When divided into right and left kidneys, the statistical significance of difference in V20 Gy of each kidney in Group B was marginal, but a similar trend of lowered value with RA was observed in each kidney. V35 Gy of stomach was decreased with RA in both groups, but statistical significance was not reached. Maximal dose to the spinal cord and total monitor unit were significantly lower in RA, regardless of the group. In terms of treatment time, RA was more efficient than IMRT; statistical significance was reached in Group B, but marginal in Group A. The average DVHs of RA vs IMRT for (a) whole liver, (b) non-target liver, (c) stomach, (d) duodenum, (e) kidney and (f) spinal cord are shown in Figures 1 and 2. Also, the dose distributions of RA vs IMRT for a selected patient in the Groups A and B are compared in Figures 3 and 4, respectively.
Table 2. Dose-volumetric comparison of intensity-modulated radiation therapy (IMRT) and RapidArc® [RA; (Varian Medical Systems, Palo Alto, CA)] in patients with portal vein tumour thrombosis (Group A).
| Variable | Mean±standard deviation |
p–value | |
| IMRT | RA | ||
| Conformity index | 1.1±0.03 | 1.0±0.008 | <0.0001 |
| Mean whole liver dose (Gy) | 22.2±6.6 | 22.9±6.0 | 0.0275 |
| Mean non-target liver dose (Gy) | 18.0±5.9 | 18.7±5.3 | 0.0307 |
| Max spinal cord dose (Gy) | 32.1±6.0 | 26.7±6.9 | 0.0217 |
| V30 Gy of whole liver (%) | 32.1±12.1 | 31.1±12.2 | 0.0283 |
| V30 Gy of non-target liver (%) | 22.6±10.2 | 21.4±10.1 | 0.0351 |
| V35 Gy of duodenum (%) | 20.9±10.0 | 17.9±9.9 | 0.0058 |
| V35 Gy of stomach (%) | 2.9±2.6 | 1.5±2.0 | 0.0891 |
| V20 Gy of kidney (%) | 9.9±7.6 | 8.3±7.1 | 0.2388 |
| Right kidney | 18.6±13.9 | 16.5±13.8 | 0.3794 |
| Left kidney | 1.8±4.0 | 0.7±1.4 | 0.2785 |
| Monitor unit | 778±122 | 463±45 | <0.0001 |
| Treatment time (s) | 225.6±62.0 | 188.8±0.8 | 0.0929 |
p–values in italics are statistically significant.
Table 3. Dose-volumetric comparison of intensity-modulated radiation therapy (IMRT) and RapidArc® [RA; (Varian Medical Systems, Palo Alto, CA)] in patients with primary liver tumour (Group B).
| Variable | Mean±standard deviation |
p–value | |
| IMRT | RA | ||
| Conformity index | 1.1±0.05 | 1.0±0.05 | <0.0001 |
| Mean whole liver dose (Gy) | 23.1±7.4 | 23.7±7.6 | 0.0526 |
| Mean non-target liver dose (Gy) | 16.7±6.7 | 17.4±7.0 | 0.0568 |
| Max spinal cord dose (Gy) | 33.9±7.2 | 27.7±7.2 | 0.0008 |
| V30 Gy of whole liver (%) | 33.3±14.0 | 32.9±14.2 | 0.4597 |
| V30 Gyza of non-target liver (%) | 19.0±11.8 | 18.5±12.3 | 0.4268 |
| V35 Gy of duodenum (%) | 7.1±9.9 | 6.6±8.9 | 0.5518 |
| V35 Gy of stomach (%) | 12.9±15.9 | 9.9±11.8 | 0.0762 |
| V20 Gy of kidney (%) | 11.9±13.7 | 7.3±9.5 | 0.0124 |
| Right kidney (%) | 18.7±25.0 | 12.7±17.3 | 0.0836 |
| Left kidney (%) | 5.0±9.4 | 1.9±4.8 | 0.0848 |
| Monitor unit | 791±138 | 457±77 | <0.0001 |
| Treatment time (s) | 198.6±12.8 | 188.9±0.5 | 0.0359 |
p–values in italics are statistically significant.
Figure 1.
Average dose–volume histograms of RapidArc® (RA; Varian Medical Systems, Palo Alto, CA) vs intensity-modulated radiation therapy (IMRT) for (a) the whole liver, (b) non-target liver, (c) stomach, (d) duodenum, (e) kidney and (f) spinal cord (Group A).
Figure 2.
Average dose–volume histograms of RapidArc® (RA; Varian Medical Systems, Palo Alto, CA) vs intensity-modulated radiation therapy (IMRT) for (a) the whole liver, (b) non-target liver, (c) stomach, (d) duodenum, (e) kidney and (f) spinal cord (Group B).
Figure 3.
Dose distributions of RapidArc® (Varian Medical Systems, Palo Alto, CA) vs intensity-modulated radiation therapy for one patient in Group A.
Figure 4.
Dose distributions of RapidArc® (Varian Medical Systems, Palo Alto, CA) vs intensity-modulated radiation therapy for one patient in Group B.
Discussion
As mentioned earlier, there are several dosimetric studies evaluating the benefit of IMRT for HCC. Cheng et al [3] compared IMRT vs three-dimensional conformal radiotherapy for HCC, and reported that IMRT resulted in an increased mean liver dose, while reducing (or at least achieving similar) non-liver OAR sparing, such as spinal cord, kidneys or stomach. Eccles et al [4] compared IMRT vs three-dimensional conformal radiotherapy in the hypofractionation setting, and tried a dose escalation using IMRT. Although dose escalation was feasible only in 35% of patients, a significant increase in the minimal dose to 93% of the PTV was observed with IMRT for all patients without increase in the doses to non-liver OARs such as stomach, duodenum and spinal cord. They also evaluated the implication of the PTV location on the dose-volumetric results, and observed that cases with a PTV overlapping or directly adjacent to non-liver OARs had a greater advantage with IMRT than “non-overlapping” cases in which the liver itself was the OAR.
In this study, we compared two different IMRT techniques: RA vs static field IMRT. In general, RA tended to be more effective in the sparing of non-liver OARs such as duodenum, kidney and spinal cord. However, the dose-volumetric results of RA vs IMRT were different according to the target location within the liver. Our patients were divided into two groups by the PTV location. Group A consisted of 10 patients with portal vein tumour thrombosis, and their PTVs were located in the central portion of the liver. In contrast, Group B consisted of 10 patients with primary liver tumour, and their PTVs were located in the peripheral portion of the liver, either in the right lobe adjacent to the right kidney (n=4) or left lobe adjacent to the stomach (n=6). As a result, V35 Gy of duodenum, which is located adjacent to the central portion of the liver, was decreased with RA in group A, while V20 Gy of kidneys, which was located adjacent to peripheral portion of the liver (especially, right kidney), was decreased with RA in group B. From these observations, it can be summarised that adjacent OARs were more effectively spared with RA.
However, the increased mean liver dose in group A with RA should not be overlooked. As for the implication of mean liver dose in the development of radiation-induced liver disease, however, there are several conflicting reports. Some investigators suggested mean liver dose was associated with the hepatic toxicity [9,10], while others did not [11,12]. Cheng et al [3] reported increased mean liver dose but reduced normal tissue complication probability of liver with IMRT. Although the delivery of beams during gantry rotation did increase mean liver dose the difference was balanced by the decreasing V30 Gy of the liver, and this dose–volume effect on the development of radiation-induced liver disease has not been evaluated (and needs to be elucidated in the clinical setting).
Despite the statistical significance, however, the absolute difference in dose-volumetric results (including non-liver OAR sparing, increased mean liver dose and decreased V30 Gy of liver) between IMRT and RA plans is quite small. In contrast, there was only a trend towards decreased V35 Gy of stomach with RA in both groups, but the mean DVHs suggest that there may be much reduction of irradiated stomach volume in the lower dose range of about 20 Gy. Recently, Kim et al [13] reported that V35 Gy was the most significant factor predicting gastroduodenal complications in HCC patients treated with radiotherapy, but V5 Gy, V10 Gy, V15 Gy, V20 Gy, V25 Gy and V30 Gy were also associated with the toxicity. Moreover, the dose-volumetric improvement in the lower dose range may be meaningful in the stereotactic body radiotherapy (SBRT) setting. Murphy et al [14] analysed the dosimetric determinants of duodenal toxicity in single-fraction SBRT for pancreatic cancer, and reported that V10 Gy, V15 Gy, V20 Gy and V25 Gy were significantly correlated with duodenal toxicity. Further investigation is needed to confirm the dose–volume relationship of gastric and/or duodenal toxicity, and for evaluating the clinical implication of the improved dose-volumetric results with RA shown in our study.
Lastly, treatment efficiency is an important factor to be considered in the treatment. In our study, regardless of the group, total monitor unit was much smaller in RA plans. Treatment time was also reduced with RA, but the magnitude of reduction was less than that of the previous reports from other treatment sites [6,15,16]. This might be due to the employed techniques of RA and IMRT in our study. 2.5 arcs were employed to maximise the benefit of RA, whereas the number of fields of IMRT was limited to five to save the liver as much as possible. Despite this, however, the treatment time of RA was still shorter than that of IMRT. Plan efficiency can also be improved with RA, because the dose distribution of static field IMRT is more dependent on the beam angle, and the selection of the optimal beam angle relies on the experience of the planning dosimetrist or physicist.
There are concerns that the organ motion during respiration can lead to the under-dosed area within the target. Some may argue that the hollow viscus (such as duodenum and stomach) can move unpredictably, even regardless of respiration. However, we delineated the internal target volume based on three-phase CT including free breathing, full inhalation and full exhalation. Moreover, this study is a comparative planning study using the same data set, and therefore the comparison of the treatment plans between RA and IMRT was not affected by the organ motion. There are several reports evaluating the interplay effect between multileaf collimator motion and organ motion in the treatment using RA. Some investigators noted that the interplay effect of RA was minimal compared with IMRT [17], whereas others did not [18]. This issue is beyond the scope of discussion in our study, but further studies are needed to minimise the error in the delivered dose during treatment.
Conclusion
The dose-volumetric results of RA vs IMRT were different by the different target location within the liver. In general, RA tended to be more effective in sparing non-liver OARs such as duodenum, kidney and spinal cord. Moreover, RA was more efficient in the treatment delivery compared with IMRT in terms of total monitor unit used.
References
- 1.Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, Knox J, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2008;26:657–64 [DOI] [PubMed] [Google Scholar]
- 2.Kawashima M, Furuse J, Nishio T, Konishi M, Ishii H, Kinoshita T, et al. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol 2005;23:1839–46 [DOI] [PubMed] [Google Scholar]
- 3.Cheng JC, Wu JK, Huang CM, Liu HS, Huang DY, Tsai SY, et al. Dosimetric analysis and comparison of three-dimensional conformal radiotherapy and intensity-modulated radiation therapy for patients with hepatocellular carcinoma and radiation-induced liver disease. Int J Radiat Oncol Biol Phys 2003;56:229–34 [DOI] [PubMed] [Google Scholar]
- 4.Eccles CL, Bissonnette JP, Craig T, Taremi M, Wu X, Dawson LA. Treatment planning study to determine potential benefit of intensity-modulated radiotherapy versus conformal radiotherapy for unresectable hepatic malignancies. Int J Radiat Oncol Biol Phys 2008;72:582–8 [DOI] [PubMed] [Google Scholar]
- 5.McIntosh A, Hagspiel KD, Al-Osaimi AM, Northup P, Caldwell S, Berg C, et al. Accelerated treatment using intensity-modulated radiation therapy plus concurrent capecitabine for unresectable hepatocellular carcinoma. Cancer 2009;115:5117–25 [DOI] [PubMed] [Google Scholar]
- 6.Cozzi L, Dinshaw KA, Shrivastava SK, Mahantshetty U, Engineer R, Deshpande DD, et al. A treatment planning study comparing volumetric arc modulation with RapidArc and fixed field IMRT for cervix uteri radiotherapy. Radiother Oncol 2008;89:180–91 [DOI] [PubMed] [Google Scholar]
- 7.Verbakel WF, Cuijpers JP, Hoffmans D, Bieker M, Slotman BJ, Senan S. Volumetric intensity-modulated arc therapy vs conventional IMRT in head-and-neck cancer: a comparative planning and dosimetric study. Int J Radiat Oncol Biol Phys 2009;74:252–9 [DOI] [PubMed] [Google Scholar]
- 8.Palma D, Vollans E, James K, Nakano S, Moiseenko V, Shaffer R, et al. Volumetric modulated arc therapy for delivery of prostate radiotherapy: comparison with intensity-modulated radiotherapy and three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys 2008;72:996–1001 [DOI] [PubMed] [Google Scholar]
- 9.Lawrence TS, Ten Haken RK, Kessler ML, Robertson JM, Lyman JT, Lavigne ML, et al. The use of 3-D dose volume analysis to predict radiation hepatitis. Int J Radiat Oncol Biol Phys 1992;23:781–8 [DOI] [PubMed] [Google Scholar]
- 10.Xu ZY, Liang SX, Zhu J, Zhu XD, Zhao JD, Lu HJ, et al. Prediction of radiation-induced liver disease by Lyman normal-tissue complication probability model in three-dimensional conformal radiation therapy for primary liver carcinoma. Int J Radiat Oncol Biol Phys 2006;65:189–95 [DOI] [PubMed] [Google Scholar]
- 11.Kim TH, Kim DY, Park JW, Kim SH, Choi JI, Kim HB, et al. Dose-volumetric parameters predicting radiation-induced hepatic toxicity in unresectable hepatocellular carcinoma patients treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys 2007;67:225–31 [DOI] [PubMed] [Google Scholar]
- 12.Cheng JC, Wu JK, Lee PC, Liu HS, Jian JJ, Lin YM, et al. Biologic susceptibility of hepatocellular carcinoma patients treated with radiotherapy to radiation-induced liver disease. Int J Radiat Oncol Biol Phys 2004;60:1502–9 [DOI] [PubMed] [Google Scholar]
- 13.Kim H, Lim DH, Paik SW, Yoo BC, Koh KG, Lee JH, et al. Predictive factors of gastroduodenal toxicity in cirrhotic patients after three-dimensional conformal radiotherapy for hepatocellular carcinoma. Radiother Oncol 2009;93:302–6 [DOI] [PubMed] [Google Scholar]
- 14.Murphy JD, Christman-Skieller C, Kim J, Dieterich S, Chang DT, Koong AC. A dosimetric model of duodenal toxicity after stereotactic body radiotherapy for pancreatic cancer. Int J Radiat Oncol Biol Phys 2010;78:1420–6 [DOI] [PubMed] [Google Scholar]
- 15.Popescu CC, Olivotto IA, Beckham WA, Ansbacher W, Zavgorodni S, Shaffer R, et al. Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes. Int J Radiat Oncol Biol Phys 2010;76:287–95 [DOI] [PubMed] [Google Scholar]
- 16.Zhang P, Happersett L, Hunt M, Jackson A, Zelefsky M, Mageras G. Volumetric modulated arc therapy: planning and evaluation for prostate cancer cases. Int J Radiat Oncol Biol Phys 2010;76:1456–62 [DOI] [PubMed] [Google Scholar]
- 17.Ong C, Verbakel WF, Cuijpers JP, Slotman BJ, Senan S. Dosimetric impact of interplay effect on Rapidarc lung stereotactic treatment delivery. Int J Radiat Oncol Biol Phys 2011;79:305–11 [DOI] [PubMed] [Google Scholar]
- 18.Court L, Wagar M, Berbeco R, Reisner A, Winey B, Schofield D, et al. Evaluation of the interplay effect when using RapidArc to treat targets moving in the craniocaudal or right-left direction. Med Phys 2010;37:4–11 [DOI] [PubMed] [Google Scholar]