Abstract
Objectives
To report on complications from transrectal ultrasound-guided insertion of fiducial markers for prostate image-guided radiotherapy.
Methods
234 patients who underwent transrectal fiducial marker insertion for prostate cancer image-guided radiotherapy were assessed retrospectively by questionnaire with regard to the duration and severity of eight symptoms experienced following the procedure. Pain during the implantation procedure was assessed according to the Wong–Baker faces pain scale.
Results
Of 234 patients, 32% had at least one new symptom after the procedure. The commonest new symptom following the procedure was urinary frequency affecting 16% of patients who had not been troubled by frequency beforehand. Haematuria, rectal bleeding, dysuria and haematospermia affected 9–13% of patients, mostly at Grade 1 or 2. Pain, obstruction, and fever and shivers affected 3–4% of patients. Grade 3 rectal bleeding, haematuria, fever and shivers, and urinary frequency affected 0.5–1.5% of patients. Only one patient had a Grade 4 complication (i.e. fever and shivers). Overall, 9% of patients had symptoms lasting more than 2 weeks. The commonest symptoms that lasted more than 2 weeks were frequency, dysuria, obstructive symptoms and rectal bleeding. Mean pain score during the procedure was 1.1 (range 0–5).
Conclusion
Transrectal ultrasound-guided fiducial marker insertion for image-guided radiotherapy is well tolerated in the majority of prostate cancer patients. Most symptoms were Grade 1 or 2 in severity. Symptoms in the majority of patients last under 2 weeks. The most serious complication was sepsis in our study.
Background
Image-guided radiotherapy (IGRT) in prostate cancer refers to the use of daily pre-treatment imaging and adjustment of the treatment couch to correct for prostate motion [1]. The prostate gland can be displaced by up to 2 cm on day-to-day imaging compared with the planning CT scan, which can result in geographical miss of the target and unintentional irradiation of surrounding critical structures without IGRT [2]. Fiducial marker implantation in the prostate gland improves visibility of the target and is central to the IGRT process [3]. Bony anatomy has been shown to be a poor surrogate as the prostate is independently mobile of bony anatomy [4,5]. Even with cone-beam CT where soft tissue is visible, the accuracy of image registration is increased from 70% to 95% in the anteroposterior direction with the utility of fiducial markers [6]. Fiducial marker IGRT has been the standard practice for all radically treated prostate cancer patients in our centre since 2007 [7].
The fiducial markers are usually inserted transrectally under ultrasound guidance [8,9]. In our centre, the procedure is carried out in the radiology department as an outpatient, and in other centres it may be done by the urologist or, less commonly, by the radiation oncologist. Being a recently introduced procedure, at present, incidence rates for complications from fiducial marker implantation are poorly documented in the scientific literature on the whole [10,11]. In addition, as the procedure is done as a day case, patients are discharged home immediately after the procedure and are not seen routinely by the treating radiation oncologist until the start of radiotherapy, by which time any symptoms from the procedure may have subsided. Therefore only the most severe complications would come to the attention of the treating radiation oncologist, and, unless specifically sought, less severe complications are likely to go underreported.
The aim of our study was to evaluate the side effects from the insertion of fiducial markers for radiotherapy treatment for patients with prostate cancer. Our study was conducted at this time because our clinicians sensed that the initial reports of little or no toxicity were not what we found in our practice [8,9]. We also evaluated the severity of pain experienced during fiducial marker insertion.
Method
This study had approval from our institutional human ethical review board. All patients who underwent gold seed fiducial marker implantation for prostate IGRT between October 2006 and June 2009 at our centre were identified through a local database. Patients were excluded if fiducials were implanted during high dose rate brachytherapy treatment, if the clinical notes indicated that they were illiterate, if they were known to have dementia, or if they required an English language translator for their clinic appointments.
Gold seed implantation procedure
Prior to fiducial marker implantation, all patients were given an information sheet outlining the procedure, the risks of the procedure and the rationale for undergoing fiducial marker implantation. All patients were prescribed prophylactic antibiotics with ciprofloxacin for 4 days to start the day before the procedure, and also two microlax enemas to use the night before and morning of the procedure, as prostate biopsy performed without an enema is a risk factor for the development of prostatitis [12]. Patients were instructed to stop anticoagulant and antiplatelet medications 7–10 days before the implant, if appropriate [13]. The procedure was conducted by a specialist radiologist who checked that the patient had had his bowel prep, stopped antiplatelet and anticoagulant therapy if appropriate and taken their prophylactic antibiotics before proceeding with the procedure. During the procedure, a transrectal ultrasound probe was used to localise the prostate. A local nerve block was used by injecting a local anaesthetic at the angle between the seminal vesicle and prostate on either side immediately prior to insertion of the fiducials. The fiducial markers were made of 24 carat gold and measured 1 mm in diameter by 5 mm in length. Markers were implanted in the prostate base, apex and contralateral mid-zone, and placed away from the urethra. Orthogonal pelvic radiographs were routinely carried out to confirm the position of the fiducial markers. Patients were instructed to contact the treating radiation oncologist if there were complications.
Questionnaire outline
Questionnaires were sent by post in October 2009. Questionnaires returned after December 2009 were excluded from the analysis. The questionnaire was 10 pages long, and contained 28 main questions and 18 subquestions relating to duration and severity of symptoms. Patients were asked to grade the severity of pain during the insertion of gold seeds on a Wong–Baker faces pain scale (a visual scale ranging from smiling to crying, where 0 implied no pain and 5 was severe pain) [14].
The questions about symptoms enquired about pain in the week after the procedure, fever or shivers, dysuria, frequency of urination more than usual, rectal bleeding, haematuria, haematospermia and obstructive symptoms. The questionnaire was structured such that the symptom question required a “yes”, “no”, “no more than usual” (NMTU) or “don't remember” (DR) answer for each symptom. If the patient answered yes to a symptom, they were further questioned about duration in terms of number of days. Duration was grouped as days: 0–3, 4–6, 7–14 and >14. For yes answers, severity was assessed according to the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0 [15]. The grades of severity for each adverse event are generally: Grade 1, mild adverse event; Grade 2, moderate adverse event; Grade 3, severe adverse event; Grade 4, life-threatening or disabling adverse event; Grade 5, death related to adverse event.
We also collected data regarding patients' age, T stage, body mass index, hypertension, diabetes, smoking history, overseas travel in the month before implantation, diarrhoea in the month before implantation, development of infection after transrectal ultrasound-guided prostate biopsy and whether patients noticed the passage of gold seeds after the procedure. The returned questionnaires were collected and data inserted into an Access database (Microsoft Corporation, Redmond, WA).
Statistical analysis
T stage, Gleason score, and age for patients who completed and returned the questionnaire and those who did not return the questionnaire are reported as absolute figures and percentages. Percentages of patients who experienced each symptom, didn't experience, didn't remember or didn't think it was worse than usual are described, derived using Excel (Microsoft Corporation). For patients who had symptoms, duration was presented according to day grouping, as a proportion of those that were affected only. The grade of symptoms was reported as a percentage of all patients answering yes and no (no included NMTU but excluded DR). For descriptive purposes, patients were said to have “prostatitis” if they suffered from prostate pain, fever or shivers, dysuria or frequency after the procedure [16].
Results
There were 416 patients in total who had fiducial marker image-guided radiotherapy during the study period. 77 patients were excluded from our study: 36 patients because of requiring an English language interpreter for clinic appointments, 31 patients had fiducials inserted at high dose rate brachytherapy, 8 patients were deceased and 2 patients had a history of dementia. Out of 339 remaining patients, 234 patients (69%) returned the completed questionnaire. The median time from fiducial insertion to being sent the questionnaire was 21 months (range, 5–37 months). Of all answers, 7% answered DR, 76% answered no, 7% answered yes and 9% answered NMTU.
Comparisons of T stage, Gleason score and age between responders and non-responders are shown in Table 1, illustrating that the groups were equally distributed. Most patients were stage T2 (54%), followed by T1 (25%), T3 (21%) and T4 (0.4%). The median age at the time of fiducial marker implant among responders was 71 years (range, 49–84 years). The median body mass index was 26.5 kg m−2 (range, 18.4–42.8 kg m−2). Of 234 patients, 5 patients reported a history of foreign travel in the preceding month (3 to Europe, 2 to the USA). 7 patients (3%) reported diarrhoea 1 month before the procedure. 59% had hypertension and 16% had diabetes. 23 patients (10%) were current smokers, 112 patients (48%) were ex-smokers, 98 patients (42%) were never smokers. 6 patients (3%) reported a prostate infection after the prostate biopsy procedure.
Table 1. Visual comparison of T stage, Gleason score and median age at time of completion of questionnaire between patients who completed and returned the questionnaires (responders) and those that did not return the questionnaires (non-responders).
| TNM stage | Responders (n = 234) | Non-responders (n = 105) |
| T1 | 59 (25%) | 23 (22%) |
| T2 | 126 (54%) | 48 (46%) |
| T3 | 48 (21%) | 29 (28%) |
| T4 | 1 (0.4%) | 0 (0%) |
| TX | 0 (0%) | 5 (5%) |
| Gleason <7 | 58 (25%) | 27 (26%) |
| Gleason 7 | 126 (54%) | 46 (44%) |
| Gleason 8 | 25 (11%) | 20 (19%) |
| Gleason 9 | 23 (10%) | 12 (11%) |
| Gleason 10 | 2 (1%) | 0 (0%) |
| Median age at time of completion of questionnaire (years) | 73 (range = 50–86) | 71 (range = 52–86) |
Wong–Baker faces pain scores during the implantation procedure are given in Figure 1. Of 234 patients, 229 (98%) completed a pain score. 38% reported no pain and 35% reported a pain score of only 1. 15% reported pain between 3 and 5 in severity.
Figure 1.
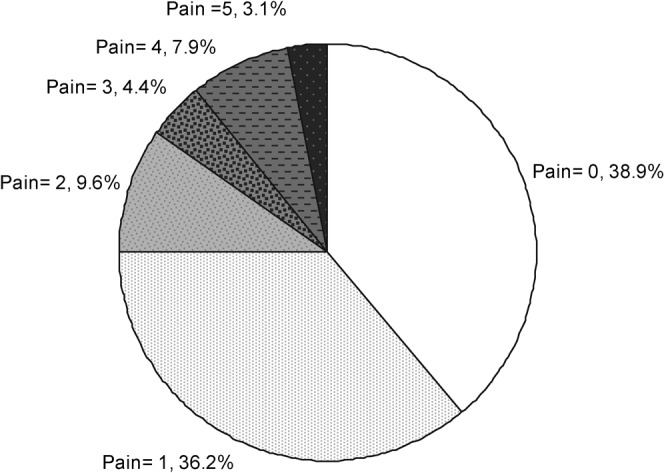
Pain score during implantation of fiducial markers (Wong–Baker faces pain scale).
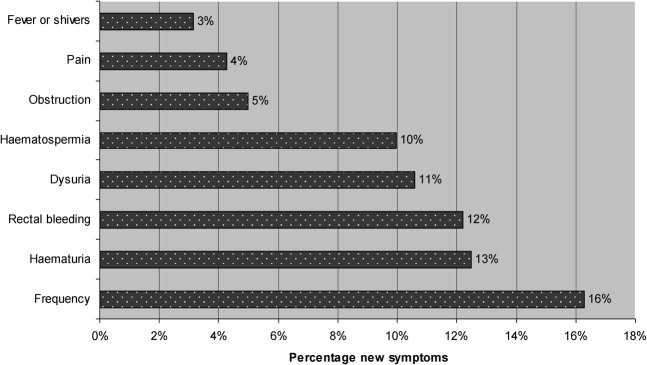
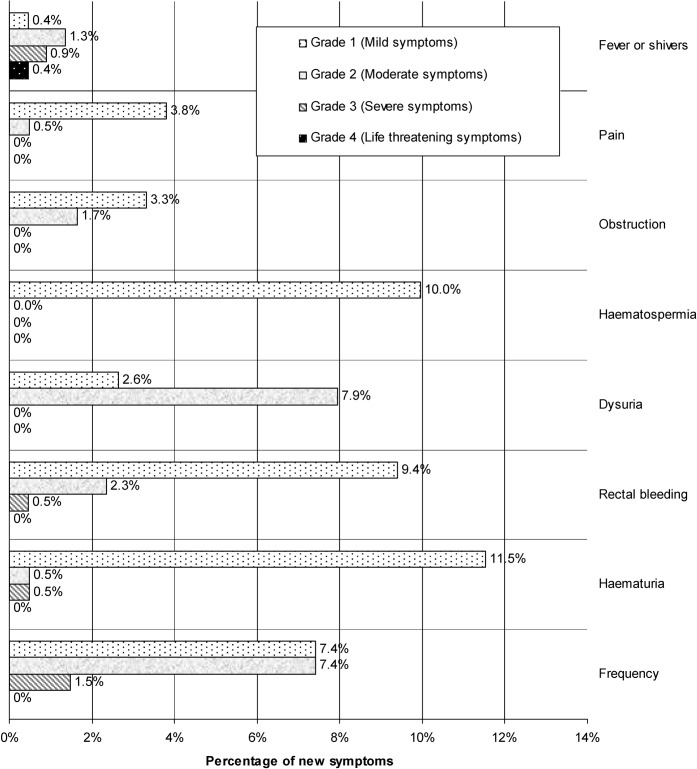
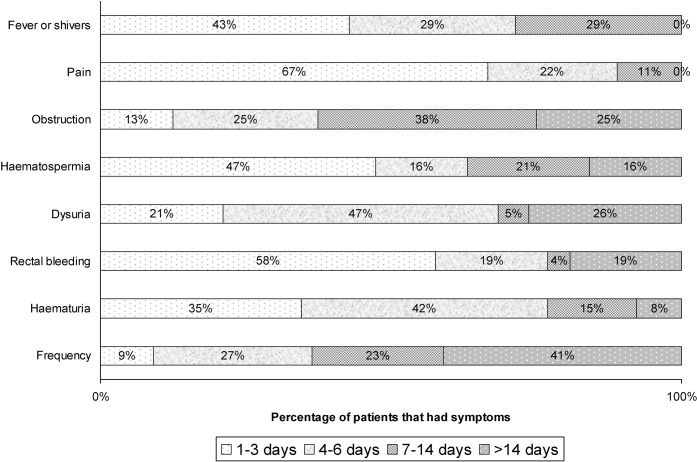
The frequency of new symptoms experienced in the week following the implantation of fiducial markers is summarised in Figure 2. 32% of patients had at least one symptom, and overall 17% experienced at least one “prostatitis” symptom (pain, fever or shivers, dysuria or frequency). Figure 3 shows the distribution of grade of symptoms among those who were affected, excluding patients who responded as NMTU or DR. Figure 4 shows the duration of the symptom experienced as a percentage of all patients who developed that new symptom, excluding those who didn't remember or felt that their symptom was no worse than usual.
Figure 2.
Frequency of new symptoms following insertion of fiducial markers (excluding “no more than usual” and “don't remember”).
Figure 3.
Severity of complications by Common Terminology Criteria for Adverse Events (version 3.0) for patients with new symptoms following fiducial implantation, excluding patients that responded as “no more than usual” or “don't remember”.
Figure 4.
Duration of symptoms as a percentage excluding patients that did not have symptoms, did not remember or had no more than usual symptoms.
9 out of 211 patients reported experiencing new pain in the week following the procedure (i.e. 4% of patients as shown in Figure 2). Of the nine patients reporting pain, eight reported that the pain did not require analgesics (Grade 1) and one reported that the pain required mild analgesic medication (Grade 2), as shown in Figure 3. Six out of nine patients reported that pain settled within 3 days and all reported that pain settled within 2 weeks, as shown in Figure 4.
Dysuria, frequency and obstructive symptoms required medical treatment in at least one-third (most probably an α-1-receptor antagonist). Although dysuria and frequency are common in prostate cancer patients, new dysuria or frequency after fiducial marker implantation may be the result of infection or oedema from trauma following the procedure. 26 (11%) and 71 (30%) patients reported that they had a baseline dysuria and frequency, respectively, and that it was no worse than usual. 20 (9%) patients developed new dysuria after the implantation procedure; of these, 5 patients had Grade 1 and 15 patients had Grade 2 dysuria. 23 (10%) patients developed new frequency after the implantation procedure, and of these 10 patients had Grade 1, 10 had Grade 2, 2 had Grade 3 frequency and 1 did not grade frequency. 41 patients (18%) reported baseline urinary obstruction symptoms that did not get any worse than usual after the implant procedure, and 9 (4%) reported new obstruction. Six of these patients did not require specific treatment and three received specific treatment. No Grade 3 or 4 obstruction was reported. Duration of obstructive symptoms was more than 14 days in two patients.
For haemorrhagic symptoms (rectal bleeding, haematospermia, haematuria), most patients did not require specific treatment (Grade 1). Haematospermia affected 20 patients (10%). All patients reported this as mild, although two patients reported haematospermia lasting for more than 2 weeks. New haematuria immediately post-implantation could have either been a traumatic or an infective symptom. Two patients had baseline haematuria that was no worse than usual. Of 208 patients who responded with yes or no, 26 had haematuria after the procedure, lasting less than 7 days in the majority (20/26) of cases. However, two patients reported having haematuria lasting for more than 14 days. Rectal bleeding affected 26 (11%) patients (20 Grade 1, 5 Grade 2 and 1 Grade 3). The patient who had a Grade 3 rectal bleed (requiring blood transfusion) had been taking regular aspirin, which was stopped prior to his implant, and he only developed rectal bleeding after recommencing aspirin. Of the patients who had rectal bleeding, 58% lasted less than 3 days, and 19% lasted more than 2 weeks. Seed expulsion suggests probable implantation in the bladder or, less likely, urethra. Seed expulsion was reported by 3 patients out of 190, excluding those who did not remember. One of these patients also had a Grade 2 frequency after the procedure lasting more than 14 days and another had a Grade 1 rectal bleed, while the third did not report any other symptoms.
Overall, the most serious complication was infection, with one patient experiencing a Grade 4 septicaemia that was life-threatening. In this patient, Escherichia coli was isolated from urine culture, which was ciprofloxacin and gentamicin resistant. Two other patients experienced a Grade 3 infection (requiring admission to hospital). In total, 3% (7 out of 223 patients) experienced fever and shivers suggestive of infection. Of these, one was Grade 1, three were Grade 2, two were Grade 3 and one was Grade 4.
Overall, 39 out of 234 patients (17%) experienced at least one symptom for 0–3 days, 28 out of 234 (12%) experienced at least one symptom for 4–6 days, 15 patients (6%) had at least one symptom that lasted 7–14 days, and 20 patients (9%) had at least one symptom that lasted longer than 2 weeks after the procedure.
Discussion
In summary, our study shows that the commonest complications following transrectal fiducial marker implantation were frequency, haematuria, dysuria, rectal bleeding and haematospermia, affecting 9–11% of patients (Figure 2). Urinary obstruction occurred in 4% of patients, and although only 3% reported fever and shivers, three patients in total required admission for sepsis and one patient reported a Grade 4 toxicity.
There is limited information in the medical literature reporting complication rates of fiducial marker insertion. The main potential for bias is in the underreporting of symptoms by patients to the referring department, especially for less serious complications. Even though patients in our study were told to contact us if they developed any complications after fiducial marker implantation, many did not report these symptoms before they were sent the questionnaire. In another study of 705 patients who had fiducial marker implantation and were told to contact the urology and radiation oncology departments in the event of severe or prolonged adverse effects, only one patient reported a urinary tract infection requiring antibiotics [8]. No severe rectal bleeding or haematuria was recorded in that series. In another series of 98 patients who had prostate fiducial markers implanted for IGRT, no complications at all were reported [9].
Conversely, in a Dutch mainly retrospective series of 209 prostate cancer patients who had fiducial marker placements, 6.2% had a moderate complication [10]. In that study, haematuria more than 3 days, haematospermia in those who were potent and rectal bleeding occurred in 3.8%, 18.5% and 9.1% of patients, respectively. Fever occurred in 1.9% of patients. Complications were more frequent in younger patients, more advanced T stage and shorter duration of hormonal treatment. In another retrospective questionnaire-based study on 135 patients who underwent transrectal implantation of fiducial markers, Igdem et al [11] specifically enquired about haematuria, rectal bleeding and fever, and found that 15%, 4% and 2% experienced these symptoms, respectively. The grade of toxicity was not reported in that study, but the authors noted that no toxicity requiring intervention was necessary.
Pain was a significant issue for 15% of the patients in our study who scored 3–5 on the Wong–Baker faces pain scale, and overall the mean pain score was 1.1. Igdem et al [11] also used the Wong–Baker faces pain scale, although their patients did not have a periprostatic nerve block. The mean pain score in their study was 1.7 [11]. Without nerve block, Langenhuijsen et al [10] in the Dutch study reported a mean pain score of 3.2 on a 10 point scale and 15% had a score of 6–9 on their scale. The benefit of periprostatic nerve block has not previously been confirmed in fiducial marker implantation studies. There is conflicting evidence for the role of periprostatic nerve block in prostate biopsy studies, some suggesting benefit and others no benefit [17,18]. In one randomised, double-blind, placebo-controlled trial, the group who had a periprostatic nerve block for prostate biopsy reported a mean pain score of 0.76 on a 10-point scale compared with 3.62 in the group that received a placebo [17]. However, another study with the same design reported no benefit [18]. A meta-analysis of 20 studies involving 1685 patients who underwent prostate biopsy showed a significant reduction in pain score in the group that received anaesthetic compared with no anaesthesia or placebo [19]. Although it is not possible to compare across studies, the lower mean pain score in our study may suggest a possible benefit of periprostatic nerve block in fiducial marker implantation.
The main limitation of our study is that the information was not collected immediately after the fiducial marker implantation procedure and therefore it is difficult to estimate the effect of recall bias. To reduce recall bias, the option of DR was allowed in our questionnaire, and only 7% of responders selected this option out of all responses. Fiducial marker IGRT became the standard of care in our centre in 2007, and we prospectively collected a database of all patients treated. Symptoms following implantation of fiducial markers are not reliably documented in our clinical notes and therefore a questionnaire was thought to be the best way to collect this information. Fiducial marker implantation is likely to be a significant event in the patients' life, and therefore they are more likely to remember it. Evidence for this is partly suggested by the Dutch study, whereby 88% completed the questionnaire retrospectively 22 months after the procedure, and 12% completed the questionnaire prospectively immediately after the procedure [10]. That study found that there was no difference in the rate of reported complication between the two groups. Igdem et al [11] compared the mean pain score of patients completing the questionnaire under 6 months vs longer than 6 months after the procedure, and also found no difference between the two groups. For comparison, in a prospective study looking at 1051 men who underwent a transrectal ultrasound-guided prostate biopsy, the rate of post-procedure fever was 2.9%, dysuria, haematuria and haematospermia affected 7.2%, 15.9% and 9.8%, respectively, and urosepsis affected 0.1% of all patients, overall representing findings very similar to our study [20].
This study raises some important issues. It is unknown whether compliance with medication (antibiotic prophylaxis) and discontinuation of anticoagulants may have affected complication rates. Compliance with antibiotics could be addressed by using intravenous gentamicin rather than oral ciprofloxacin as the prophylactic treatment. The patient in our study who experienced a Grade 4 sepsis had E. coli resistant to both ciprofloxacin and gentamycin. There is evidence in the literature of emerging strains of E. coli resistant to fluoroquinolones [21,22]. Alternatively, transperineal implantation of gold seeds may reduce the introduction of rectal pathogens into the prostate gland [23]. 15% of patients did score pain experienced during the procedure ≥3. In the Dutch study, patients were specifically asked in the questionnaire if they thought the marker implantation was more painful, equally painful or less painful compared with the prostate biopsy procedure, and about 90% noted that the pain was comparable or less severe [10]. Therefore we may be able to identify patients who might benefit from stronger analgesics or anaesthetic during fiducial marker implantation on the basis of severe pain reported during the prostate biopsy procedure. The use of topical nitrates may be an option to reduce discomfort for some patients [24].
Conclusion
Fiducial marker insertion for image-guided radiotherapy was well tolerated in the majority (85%) of prostate cancer patients in terms of pain. However, 32% of patients experience at least one symptom. The most frequent symptoms were frequency, haematuria, dysuria, rectal bleeding and haematospermia. 9% had symptoms lasting more than 2 weeks.
Footnotes
Peter MacCallum Cancer Centre and investigators receive a Varian Medical Systems research grant.
References
- 1.Kupelian PA, Langen KM, Willoughby TR, Zeidan OA, Meeks SL. Image-guided radiotherapy for localized prostate cancer: treating a moving target. Semin Radiat Oncol 2008;18:58–66 [DOI] [PubMed] [Google Scholar]
- 2.Langen KM, Jones DT. Organ motion and its management. Int J Radiat Oncol Biol Phys 2001;50:265–78 [DOI] [PubMed] [Google Scholar]
- 3.Beltran C, Herman MG, Davis BJ. Planning target margin calculations for prostate radiotherapy based on intrafraction and interfraction motion using four localization methods. Int J Radiat Oncol Biol Phys 2008;70:289–95 [DOI] [PubMed] [Google Scholar]
- 4.Nederveen AJ, Dehnad H, van derHeide UA, van Moorselaar RJA, Hofman P, Lagendijk JJW. Comparison of megavoltage position verification for prostate irradiation based on bony anatomy and implanted fiducials. Radiother Oncol 2003;68:81–8 [DOI] [PubMed] [Google Scholar]
- 5.Schallenkamp JM, Herman MG, Kruse JJ, Pisansky TM. Prostate position relative to pelvic bony anatomy based on intraprostatic gold markers and electronic portal imaging. Int J Radiat Oncol Biol Phys 2005;63:800–11 [DOI] [PubMed] [Google Scholar]
- 6.Moseley DJ, White EA, Wiltshire KL, Rosewall T, Sharpe MB, Siewerdsen JH, et al. Comparison of localization performance with implanted fiducial markers and cone-beam computed tomography for on-line image-guided radiotherapy of the prostate. Int J Radiat Oncol Biol Phys 2007;67:942–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson A, Fox C, Foroudi F, Styles C, Tai KH, Owen R, et al. Planning and implementing an implanted fiducial programme for prostate cancer radiation therapy. J Med Imaging Radiat Oncol 2008;52:419–24 [DOI] [PubMed] [Google Scholar]
- 8.Shinohara K, Roach M., 3rd Technique for implantation of fiducial markers in the prostate. Urology 2008;71:196–200 [DOI] [PubMed] [Google Scholar]
- 9.Linden RA, Weiner PR, Gomella LG, Dicker AP, Suh DB, Trabulsi EJ, et al. Technique of outpatient placement of intraprostatic fiducial markers before external beam radiotherapy. Urology 2009;73:881–6 [DOI] [PubMed] [Google Scholar]
- 10.Langenhuijsen JF, van Lin EN, Kiemeney LA, van derVight LP, McColl GM, Visser AG, et al. Ultrasound-guided transrectal implantation of gold markers for prostate localization during external beam radiotherapy: complication rate and risk factors. Int J Radiat Oncol Biol Phys 2007;69:671–6 [DOI] [PubMed] [Google Scholar]
- 11.Igdem S, Akpinar H, Alco G, Agacayak F, Turkan S, Okkan S. Implantation of fiducial markers for image guidance in prostate radiotherapy: patient-reported toxicity. Br J Radiol 2009;82:941–5 [DOI] [PubMed] [Google Scholar]
- 12.Kim SJ, Kim SI, Ahn HS, Choi JB, Kim YS. Risk factors for acute prostatitis after transrectal biopsy of the prostate. Korean J Urol 2010;51:426–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Hakim A, Moussa S. CUA guidelines on prostate biopsy methodology. Can Urol Assoc J 2010;4:89–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong DL, Baker CM. Smiling faces as anchor for pain intensity scales. Pain 2001;89:295–300 [DOI] [PubMed] [Google Scholar]
- 15.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176–81 [DOI] [PubMed] [Google Scholar]
- 16.Benway BM, Moon TD. Bacterial prostatitis. Urol Clin North Am 2008;35:23–32; v [DOI] [PubMed] [Google Scholar]
- 17.Berger AP, Frauscher F, Halpern EJ, Spranger R, Steiner H, Bartsch G, et al. Periprostatic administration of local anesthesia during transrectal ultrasound-guided biopsy of the prostate: a randomized, double-blind, placebo-controlled study. Urology 2003;61:585–8 [DOI] [PubMed] [Google Scholar]
- 18.Ingber MS, Ibrahim I, Turzewski C, Hollander JB, Diokno AC. Does periprostatic block reduce pain during transrectal prostate biopsy? A randomized, placebo-controlled, double-blinded study. Int Urol Nephrol 2010;42:23–7 [DOI] [PubMed] [Google Scholar]
- 19.Tiong HY, Liew LC, Samuel M, Consigliere D, Esuvaranathan K. A meta-analysis of local anesthesia for transrectal ultrasound-guided biopsy of the prostate. Prostate Cancer Prostatic Dis 2007;10:127–36 [DOI] [PubMed] [Google Scholar]
- 20.Djavan B, Waldert M, Zlotta A, Dobronski P, Seitz C, Remzi M, et al. Safety and morbidity of first and repeat transrectal ultrasound guided prostate needle biopsies: results of a prospective European prostate cancer detection study. J Urol 2001;166:856–60 [PubMed] [Google Scholar]
- 21.Livermore DM, James D, Reacher M, Graham C, Nichols T, Stephens P, et al. Trends in fluoroquinolone (ciprofloxacin) resistance in enterobacteriaceae from bacteremias, England and Wales, 1990–1999. Emerg Infect Dis 2002;8:473–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lingaratnam S, Thursky KA, Slavin MA. Fluoroquinolone prophylaxis: a word of caution. Leuk Lymphoma 2010;52:5–6 [DOI] [PubMed] [Google Scholar]
- 23.Henry AM, Wilkinson C, Wylie JP, Logue JP, Price P, Khoo VS. Trans-perineal implantation of radio-opaque treatment verification markers into the prostate: an assessment of procedure related morbidity, patient acceptability and accuracy. Radiother Oncol 2004;73:57–9 [DOI] [PubMed] [Google Scholar]
- 24.McCabe JE, Hanchanale VS, Philip J, Javle PM. A randomized controlled trial of topical glyceryl trinitrate before transrectal ultrasonography-guided biopsy of the prostate. BJU Int 2007;100:536–8 (discussion: 8–9) [DOI] [PubMed] [Google Scholar]