Abstract
Objectives
Radiofrequency ablation of the pulmonary veins is an accepted treatment for atrial fibrillation. An accurate knowledge of pulmonary venous anatomy and dimensions is desirable prior to such a procedure. The objective of this study was to use 64-detector row cardiac CT to investigate the changes in pulmonary venous dimensions during the cardiac cycle.
Methods
Data from 44 consecutive patients with no significant cardiovascular pathology who underwent electrocardiogram (ECG)-gated 64-detector row coronary angiography were retrospectively analysed. Average diameter and cross-sectional area were measured at 5 mm intervals from each pulmonary vein ostium, in ventricular end-diastole and ventricular end-systole, using curved multiplanar reformats.
Results
4 (9.1%) patients had pulmonary vein anomalies and were excluded. In the remaining 40 patients, pulmonary vein diameter and area at the ostium were significantly larger in end-systole in all four veins, with the largest differences in the superior pulmonary veins. Dimensional changes for diameter (millimetres) and area (square millimetres) were as follows: left superior pulmonary vein, 2.5 (p<0.001), 65.48 (p<0.001); right superior pulmonary vein, 1.63 (p<0.001), 56.27 (p<0.001); left inferior pulmonary vein, 1.1 (p<0.001), 30.41 (p<0.001); and right inferior pulmonary vein, 0.68 (p = 0.005), 30.14 (p = 0.005). Less marked changes were seen at measurement sites further from the atrium. Interobserver correlation was high (all but one measurement >0.9).
Conclusion
Pulmonary vein dimensions change significantly between end-systole and end-diastole, and the ostia of the superior pulmonary veins are potentially the most vulnerable to dimensional inaccuracies. ECG-gated cardiac CT may provide a more precise method of pulmonary venous dimensional measurement than non-gated techniques. Knowledge of change in pulmonary vein diameter offers interesting potential research into the effect of pulmonary vein function.
With the technological advances in multidetector row CT (MDCT) and electrocardiographic gating capabilities, cardiac morphology and coronary arterial anatomy may be reconstructed in detail.
The pulmonary veins (PVs) are an important source of ectopic atrial activity and have been implicated in chronic and paroxysmal atrial fibrillation; radiofrequency ablation of the PVs has been shown to be successful in treating this condition [1]. An accurate prior knowledge of the pulmonary venous anatomy is desirable, which may ultimately shorten the duration of this often prolonged procedure. An understanding of normal pulmonary venous anatomy and measurements is therefore important.
CT has become an increasingly important tool in the pre-procedural assessment of these patients, providing a detailed anatomical depiction of the left atrium (LA) and PVs [2], and allows three-dimensional (3D) models to be reconstructed using dedicated software algorithms (Figure 1). The presence of anatomical variations and congenital pulmonary venous anomalies may be delineated (Figures 2 and 3), and unsuspected persistent atrial septal defects and patent foramen ovale may be demonstrated, which are of relevance in the setting of a planned trans-septal puncture.
Figure 1.
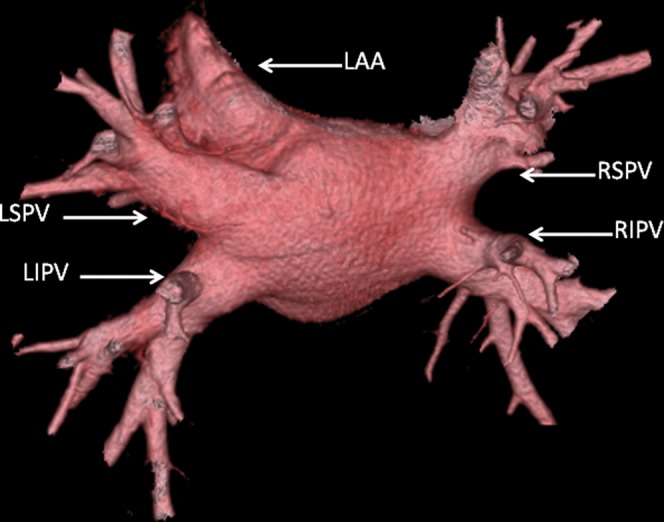
Three-dimensional volume rendered image (CardEP; GE Healthcare Technologies, Waukesha, WI) showing normal pulmonary venous anatomy with a superior (RSPV, right superior pulmonary vein; LSPV, left superior pulmonary vein) and inferior (RIPV, LIPV) vein on each side of the left atrium. The left atrial appendage (LAA) is also shown.
Figure 2.
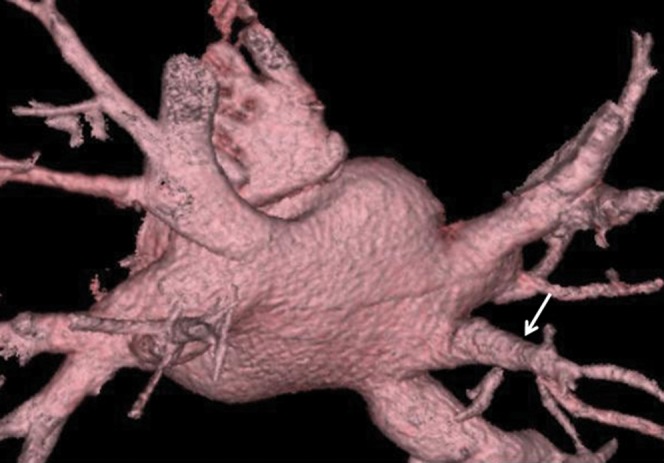
Three-dimensional volume rendered image of the most common pulmonary venous anomaly with a unilateral middle pulmonary vein (arrow).
Figure 3.
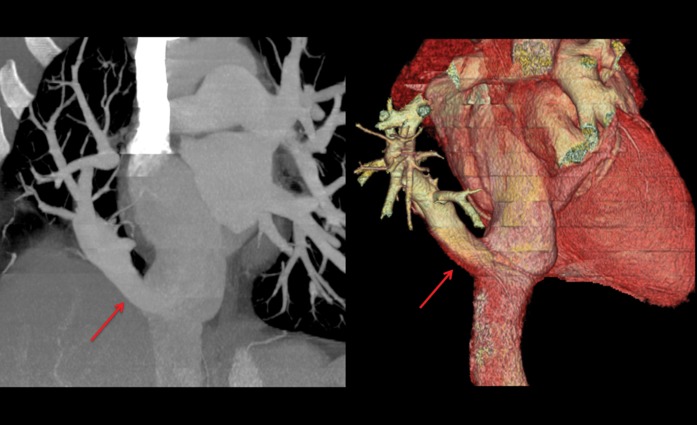
Coronal thick maximum intensity projection (left) and three-dimensional volume rendered (right) images showing partial anomalous pulmonary venous drainage with a Scimitar vein (red arrows) draining all venous blood from the right lung into the inferior vena cava.
There have been a number of studies regarding the assessment of PV diameters [3-5]. The majority of these have used non-gated CT. During standard coronary CT, we observed that pulmonary venous dimensions appeared to change with relation to the phase of reconstruction interrogated during the cardiac cycle. This finding has been demonstrated on cine MRI, with one study finding a reduction in the mean PV diameter of 32.5% from atrial diastole to atrial systole [6]. We therefore postulated that electrocardiogram (ECG)-gated cardiac CT might provide more precise pulmonary venous dimensional information than non-ECG-gated studies such as those acquired during a standard CT pulmonary angiogram [4]. However, to date, only one study has evaluated the variation in PV diameter between end-systole (ES) and end-diastole (ED) using ECG-gated CT and found a significant difference in PV ostial diameter between the cardiac phases [7]. To our knowledge, no studies have examined these variations using 64-slice MDCT.
The objective of this study was to use 64-detector row cardiac CT to demonstrate whether central pulmonary venous dimensions alter significantly during the cardiac cycle between ES and ED.
Methods and materials
This study involved retrospective data collection and analysis of consecutive patients who underwent 64-detector row coronary angiography from January 2005 to November 2005 as part of their clinical care, for the exclusion of coronary artery disease, and in whom no significant cardiovascular pathology was found (i.e. normal coronary arteries and left ventricular function). Exclusion criteria were significant thoracic or congenital cardiac abnormality, significant coronary artery disease, valvular heart disease and cardiac failure. Patients who had anatomical variations and anomalous pulmonary venous drainage were documented but excluded from the overall analysis.
Electrocardiogram-gated CT coronary angiogram
All patients underwent a retrospectively ECG-gated CT coronary angiogram using 64-detector row technology (GE Healthcare Technologies, Waukesha, WI) with a gantry rotation time of 350 ms and 64×0.625 mm slice collimation. The patient was positioned feet-first with arms above their head following 18-gauge cannulation in a right antecubital fossa vein. An anteroposterior (AP) and lateral “scout” or localiser was first performed, centred at the sternal notch with a ∼50 cm field of view. A non-contrast enhanced calcium score was performed with: 120 kV, 200 mA, 2.5 mm slice collimation.
Intravenous (iv) beta-blocker (metoprolol tartrate; Betaloc, AstraZeneca UK, London, UK) was administered to patients in normal sinus rhythm with rates >70 beats per minute. Scan coverage to just include the entire heart was determined from the localising scout incorporating 2–3 cm inferiorly to allow for anatomical excursion during the breath-hold.
A 90 ml bolus of iodinated iv contrast (ioversol; Optiray 300, Tyco Health Care UK, Gosport, UK) was injected at 5 ml s–1. A bolus-tracking method (SmartPrep, GE Healthcare; 120 kV, 50 mA, 5 mm thickness) was used in order to time the scan trigger while imaging at the level of the ascending aorta/pulmonary artery bifurcation with a region of interest in the ascending aorta. This was followed by a 50 ml saline bolus “chaser”, also injected at 5 ml s–1 via a dual pump injector (Tyco Health Care UK Ltd).
Post-processing
10 sets of phase reconstruction data, ranging from 5% to 95% in 10% intervals of the R wave to R wave interval on the ECG, were performed on each study and sent to a GE workstation for analysis. Standard “C1” reconstruction kernel was used with “soft-tissue” window levels.
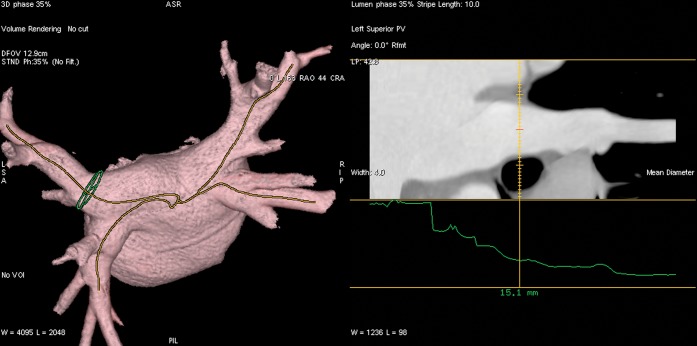
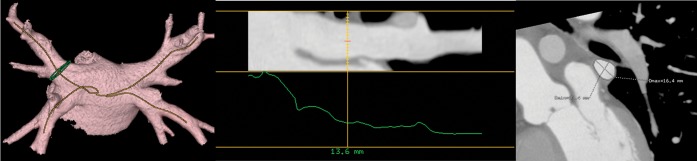
CardIQ semi-automated left ventricular ejection fraction software (GE Healthcare Technologies) was initially used to determine the ES and ED phases. For the purpose of this study, ED and ES refer to the appropriate ventricular phase of the cardiac cycle and should be taken as such when referred to throughout the paper. These selected phases were then loaded into the cardiac electrophysiology (Card EP) software (GE Healthcare Technologies). By using PV and LA model analysis algorithm, 3D anatomical representations of the LA and PVs were reconstructed. Curved vessel multiplanar reconstruction was used to define a plane through a vessel using the centre of the lumen as the axis and depicting the vessel (in this case the PVs) in a straight line. This permits the average diameter and cross-sectional area to be measured at any user-defined interval and position along the vein. In this study, these measurements were taken at 5 mm intervals from each PV ostium, in the ED and ES phases, in the curved oblique and long axis planes (Figures 4 and 5); inner lumen measurements were performed.
Figure 4.
Ventricular end-systole. Left atrial reconstruction and centre line curved multiplanar reconstruction, which stretch the pulmonary veins out into a straight line to allow more accurate measurement of diameter and circumference at intervals from the pulmonary venous ostia.
Figure 5.
Ventricular end-diastole. Left atrial reconstruction and centre line curved multiplanar reconstruction, which stretch the pulmonary veins out into a straight line to allow more accurate measurement of diameters and circumference at intervals from the pulmonary venous ostia.
Two radiologists experienced in cardiac CT performed these measurements. A semi-automated circumference measurement (which tracks tissue contrast attenuation levels), with manual adjustment, was used to obtain a cross-sectional area at each site. Cross-sectional area was also calculated using the formula Csa = π(r2) (π(d2/4), where Csa is the cross-sectional area, r is radius and d is the average diameter, from average diameter at each of three levels along the pulmonary veins: level 1 was defined as the point of inflection between the pulmonary vein and atrial wall, and levels 2 and 3 were defined at 5 mm intervals along the pulmonary vein from level 1. Measurements were obtained in both ES and ED. Echocardiographic PV Doppler flow assessment was not performed.
Statistical analysis
Patient demographic data and measurements are expressed as mean ±1 standard deviation. The Wilcoxon signed-rank test was used to compare systolic and diastolic diameter and area. Correlation was made between the estimated and the calculated cross-sectional areas, and compared using non-parametric tests.
Results
CT scans of 44 subjects (26 male and 18 female) with a mean age of 55±10.1 years were included in the study. 3/44 (6.8%) patients were excluded owing to the presence of unilateral middle pulmonary veins and 1/44 (2.3%) was excluded owing to the presence of anomalous pulmonary venous drainage (APVD; see above). A total of 40/44 (90.9%) patients had 4 pulmonary veins (1 superior and inferior PV on each side of the LA). The automated software algorithm reliably volume rendered a 3D model of the LAs and PVs, and contrast tracked the lumen of the PVs. Figures 4 and 5 show ED and ES phases, respectively—3D volume rendered, PV vessel tracking and PV in cross-section.
All veins were found to be elliptical in cross-section with supero-inferior axis greater than the anteroposterior axis. PV dimensions are summarised in Tables 1 and 2.
Table 1. Mean pulmonary vein diameter (mm) and cross-sectional area (mm2) at each measurement site in end-systole (ES) and end-diastole (ED).
| Pulmonary vein dimensions |
Level 1 |
Level 2 |
Level 3 |
|||
| ES | ED | ES | ED | ES | ED | |
| LSPV | ||||||
| Diameter | 19.7±4.03 | 17.2 ±4.8 | 16.15±2.8 | 15.06±2.8 | 14.2±2 | 13.8±2.81 |
| Area | 317.27±129.21 | 251.79±128.41 | 210.91±68.41 | 199.91±97.23 | 151.46±60.87 | 157.37±60.61 |
| RSPV | ||||||
| Diameter | 20.7 ±4.15 | 19.07±3.4 | 16.17±3.15 | 15.4±2.8 | 13.7±3.02 | 13.998±2.7 |
| Area | 350.73±130.84 | 294.46±102.53 | 213.04±80.59 | 192.73±65.0 | 155.02±64.49 | 159.64±60.36 |
| LIPV | ||||||
| Diameter | 17.3 ±3.9 | 16.2 ±3.7 | 15.8±2.00 | 14.61±2.1 | 13.7 ±2.9 | 14.05±2.2 |
| Area | 248.21±117.02 | 217.80±104.68 | 199.41±49.87 | 170.88±49.38 | 155.96±66.6 | 158.64±48.76 |
| RIPV | ||||||
| Diameter | 17.295±3.67 | 16.61±3.70 | 14.07±3.7 | 14.07±3.16 | 12.05 ±3.4 | 12.09±3.28 |
| Area | 245.11±97.48 | 227.50±93.82 | 166.11±83.9 | 163.05±66.57 | 123.06±66.85 | 123.13±65.25 |
LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
Table 2. Dimensional change of the mean measurements in pulmonary vein diameter and area at each measurement site with p-values.
| Pulmonary vein |
Level 1 |
Level 2 |
Level 3 |
|||
| Diameter (mm) | Csa (mm2) | Diameter (mm) | Csa (mm2) | Diameter (mm) | Csa (mm2) | |
| LSPV | 2.5 | 65.48 | 1.09 | 11 | 0.4 | −5.9 |
| p<0.001 | p<0.001 | p<0.001 | p = 0.028 | p = 0.003 | p = 0.004 | |
| RSPV | 1.63 | 56.27 | 0.77 | 20.3 | −0.2 | −4.6 |
| p<0.001 | p<0.001 | p = 0.018 | p = 0.016 | p = 0.91 | p = 0.92 | |
| LIPV | 1.1 | 30.41 | 1.19 | 28.53 | −0.3 | −2.68 |
| p<0.001 | p<0.001 | p<0.001 | p<0.001 | p = 0.91 | p = 0.97 | |
| RIPV | 0.68 | 30.14 | 0 | 3.06 | −0.04 | −0.07 |
| p = 0.005 | p = 0.005 | p = 0.37 | p = 0.32 | p = 0.98 | p = 0.97 | |
Csa, cross-sectional area; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
At the ostium, the right superior PV was found to be the largest and the right inferior PV (RIPV) the smallest. Generally, the PV are larger in ES, with the greatest dimensional change occurring at the ostia in the superior pv than that of the inferior PV (p<0.001), with the left superior PV (LSPV) exhibiting the greatest change.
Interobserver correlation between the two observers were almost all very high, with just one (LIPVS2) <0.90.
Discussion
With the use of ECG-gated 64-detector row CT, this study has demonstrated that there were significant pulmonary venous dimensional differences between ES and ED, and these differences would appear to become less significant further from the LA. Dimensions of the superior pair of PVs vary more than the inferior pair.
The pulmonary ostia have been implicated in the aetiology of atrial fibrillation owing to the extension of sleeves of myocardium [1,8]. Electrical isolation of this ectopic atrial tissue in the pulmonary venous ostia by radiofrequency ablation, using guided parametric mapping and a multipolar loop catheter such as the Lasso (Biosense Webster, Diamond Bar, CA) [9], is a current treatment option for such patients.
The LA and pulmonary venous anatomy is therefore important for the cardiac electrophysiologist to enable procedural planning and selection of the correct catheter [2].
Kim et al [4] determined PV dimensions in patients who underwent CT pulmonary angiography (CTPA) for suspected pulmonary embolism without the use of ECG gating. Cronin et al [5] investigated pulmonary venous anatomy and attempted to define normal values for PV ostial diameters and the distance to the first bifurcation in 200 patients referred for assessment prior to radiofrequency ablation for atrial fibrillation using non-gated thoracic CT. In their study, 82% of patients had 4 PVs, a similar proportion to our study. Our studies also concurred with the finding of the right upper PV having the largest diameter, although they found the RIPV to be larger than the left upper vein, which is discrepant with our analysis. However, the results of the present study might suggest that the measurements in these previous studies are potentially inaccurate. This is also reflected in the diverse set of measurements quoted in the literature.
Choi et al [7] assessed the change in PV diameter throughout the cardiac cycle in 19 patients in sinus rhythm without cardiac disease using retrospective ECG-gated studies performed on a 16-detector row CT. Our results mirror the finding that PV ostia significantly enlarge at ES compared with ED. Our results also confirm their findings that the upper PVs were both larger than the lower veins and their ostial areas showed a greater variation during the cardiac cycle. Their study was limited in that they only measured ostial area and did not assess the more proximal vein. They also relied on one axial section for the measurement of RIPV diameter, whereas we utilised curved mulitplanar reformats and 3D reconstruction, which we hope would improve the precision of all measurements. Ito and Dajani [2] used retrospectively gated MDCT to evaluate 30 patients prior to PV ablation for atrial fibrillation. However, they assessed the change in LA volume rather than PV measurements.
PV measurements have also been investigated using MRI. A study by Lickfett et al [6] utilised cine MRI, and found a reduction in the mean PV diameter of 32.5% from atrial diastole to atrial systole. A study comparing CT and MRI for the assessment of PV anatomy and dimensions (maximum and minimum diameter and cross-sectional area) revealed good correlation between the imaging modalities [10].
It is likely that a key explanation in the pulmonary ostial dimensional changes is related to the extent and degree of the myocardial sleeves and their contraction within the pulmonary ostia. Pathological studies have revealed that the myocardial sleeves of the PVs vary in length. Interestingly, a number of studies have shown that the superior veins have longer sleeves than the inferior veins, with the LSPV having the longest sleeve and the RIPV having the shortest [11,12]. Ho et al [11] showed that myocardial sleeve thickness is maximal at the ostium of the left superior PV. These findings mirror the variation in dimensional changes between the PVs demonstrated in our study (i.e. the LSPV showing the largest dimensional change; the RIPV showing the smallest change). In addition, pathological studies have shown that myocardial sleeve thickness reduces with increasing distance from the PV ostium [11], which, again, may be reflected in the finding in our study of a reduction in dimensional change of the vein at the measurement sites more remote from each vein ostium. These correlations add weight to the theory that the myocardial sleeve plays a pivotal role in PV contraction. Given these findings, it could be postulated that quantification of PV dimensional change on CT prior to ablation could potentially guide the operator as to the depth of tissue at each site, and reduce the likelihood of over- or underablation, recognised procedural risks that can result in PV stenosis or failure of the procedure, respectively.
Several studies have found that myocardial sleeves are longer [13,14] and thicker [14] in patients with atrial fibrillation than in patients without. This would suggest that increased dimensional changes may be seen in the PV in patients with atrial fibrillation, which may be a topic for future research.
Comparison with pre- and post-procedure CT could also possibly predict the likelihood of success of the procedure and may indicate possible sites of incomplete isolation in the setting of recurrent atrial fibrillation. Such comparison would clearly also be useful in the assessment of complications such as PV stenosis. It is also useful to have some understanding of the underlying haemodynamic physiology in order to augment the explanation for these dimensional differences.
Typical pulmonary venous blood flow, assessed using pulsed-wave Doppler, is biphasic, with a peak in the systolic and the diastolic phase of the cardiac cycle [15]. PV orifice area changes considerably through the cardiac cycle. Insights from pulmonary venous pulsed Doppler provide a potential explanation for this [16]. In subjects with normal cardiac function there is a large ventricular systolic (S) wave as blood moves from the PVs into the LA. This is driven by left ventricular long-axis shortening – this lengthens the atria, increasing the pressure gradient between veins and LA, and in effect “sucks” blood into the atria. As left ventricular long-axis function deteriorates, this pulmonary venous S wave is reduced. In ventricular diastole, the lengthening (now low pressure) ventricle, in conjunction with an open mitral valve, causes another large, this time diastolic (D), wave of pulmonary venous flow into the atrium. By mid- to end ventricular diastole, pulmonary venous pressure is equalising with ventricular diastolic pressure, so antegrade pulmonary venous flow begins to cease.
The effect of atrial systole on PV function is interesting and deserves special attention. In pulmonary venous Doppler, a small retrograde wave representing flow of blood is seen from the LA into the veins. This is called the atrial (A) wave and matches the A wave seen with transmitral Doppler, where atrial contraction completes ventricular filling. We also speculate that the reason there is a sleeve of atrial myocardium in the pulmonary venous ostia is so that in atrial systole the pulmonary venous ostia contract. This will reduce ostial area and may have the effect of reducing reflux of blood into the veins from atrial systole at the expense of ventricular filling. If this occurs it would make physiological sense.
This phenomenon may be of greater importance on exercise where reflux of blood into the veins would reduce the ability of the LA to fill the left ventricle – thus reducing left ventricular end diastolic volume, stroke volume and cardiac output. This should be the subject of future research.
It is also reasonable to postulate that, in our population of patients with structurally and functionally normal hearts, a dominant PV systolic wave was occurring. We might infer that the change in blood velocity and flow in the PV during ventricular systole (53–89 cm s–1) contributes to the increase in end-systolic pulmonary venous dimensions observed, and the decrease in dimensions during diastole is also reflective of the smaller peak during diastole, with lower pulmonary venous velocities and flow (20–56 cm s–1) [17].
It is uncertain whether accurate measurement of pulmonary venous ostial diameter is helpful to the electrophysiologist, and, if done, it is also uncertain at which phase of the cardiac cycle this should be performed. Nonetheless, knowledge of the extent of change in PV diameter offers interesting potential research into the effect of PV function.
Study limitations
It is acknowledged that the overall study numbers are small (although larger than in previous studies).
The patients in our cohort were considered normal (i.e. free from cardiovascular disease) on the basis of CT evidence of normal coronary arteries and ventricular and valvular function. While CT is clearly not the reference standard for confirming normal cardiac function, and thus could be considered a limitation of our study, we suggest that normal CT findings coupled with no evidence of cardiac disease on clinical examination would make significant cardiac pathology unlikely.
Patients with normal variant and anomalous venous drainage were excluded. While it would be of interest to study this group, the number of patients was too low to enable a meaningful analysis.
As discussed above, this study was conducted using patients in normal sinus rhythm without significant cardiac disease, and the findings therefore may not be extrapolated to those in atrial fibrillation. However, it is important to note that many of the patients undergoing ablation procedures do so for paroxysmal atrial fibrillation and thus are often in sinus rhythm at the time of scanning. Further studies in patients with persistent atrial fibrillation may be of value, however.
PV movement during the cardiac cycle could contribute to interphase inaccuracies at each site of measurement.
This study was conducted using a retrospective scan technique, which the authors acknowledge is not the current optimal technique for performing very low radiation dose cardiac CT. However, in our more recent experience with 128-detector row scanner technology, retrospective ECG-gated scanning can be performed at an acceptably low radiation dose. Utilising a more aggressive dose modulation technique, with concurrent close attention to the optimisation of scanning parameters such as body mass index, adjusted kilovoltage and delivered milliampere seconds, along with attention to an optimal scan field of view, doses as low as 0.5 mSv can be achieved in adult patients without using prospective/“step and shoot” scanning (unpublished single centre audit data), thus enabling functional and anatomical assessments to be performed with diagnostic image quality outside the more optimal usual phases of reconstruction in mid- to late diastole.
Conclusion
The authors suggest that submillimetre ECG-gated cardiac CT as a method of pulmonary venous dimensional measurement is more precise than previously reported techniques. This study has shown that PV dimensions change significantly in ventricular ES when compared with ED, with the most marked changes closest to the vein ostia. It would seem that the superior PVs (in particular the LSPV) are most vulnerable to dimensional measurement inaccuracies if a non-ECG-gated technique is employed.
It is as yet uncertain whether accurate measurement of pulmonary venous ostial diameter is helpful to the electrophysiologist. It is also uncertain at which phase of the cardiac cycle this should be performed. However, knowledge of change in PV diameter offers interesting potential research into the effect of PV function.
Further studies using ECG-gated cardiac CT would be useful to evaluate the PVs in patients with either controlled atrial fibrillation or paroxysmal atrial fibrillation (imaged during sinus rhythm) before and after radiofrequency ablation for comparison with a normal population.
Acknowledgment
The work was undertaken at Derriford Hospital, Plymouth, UK.
Footnotes
This work was supported by The Royal College of Radiologists Research Fellowship Award, funding Dr Nathan Manghat to study The Clinical Applications of Cardiac Computed Tomography.
References
- 1.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Eng J Med 1998;339:659–66 [DOI] [PubMed] [Google Scholar]
- 2.Ito H, Dajani KA. Evaluation of the pulmonary veins and left atrial volume using multidetector computed tomography in patients undergoing catheter ablation for atrial fibrillation. Curr Cardiol Rev 2009;5:17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato R, Lickfett L, Meininger G, Dickfeld T, Wu R, Juang G, et al. Pulmonary vein anatomy in patients undergoing catheter ablation of atrial fibrillation: lessons learned by use of magnetic resonance imaging. Circulation 2003;107:2004–10 [DOI] [PubMed] [Google Scholar]
- 4.Kim YH, Marom EM, Herndon JE, 2nd, McAdams HP. Pulmonary vein diameter, cross-sectional area, and shape: CT analysis. Radiology 2005;235:43–9; discussion 49–50 [DOI] [PubMed] [Google Scholar]
- 5.Cronin P, Kelly AM, Desjardins B, Patel S, Gross BH, Kazerooni EA, et al. Normative analysis of pulmonary vein drainage patterns on multidetector CT with measurements of pulmonary vein ostial diameter and distance to bifurcation. Acad Radiol 2007;14:178–88 [DOI] [PubMed] [Google Scholar]
- 6.Lickfett L, Dickfeld T, Kato R, Tandri H, Vasamreddy CR, Berger R, et al. Changes of pulmonary vein orifice size and location throughout the cardiac cycle: Dynamic analysis using magnetic resonance cine imaging. J Cardiovasc Electrophysiol 2005;16:582–8 [DOI] [PubMed] [Google Scholar]
- 7.Choi SI, Seo JB, Choi SH, Lee SH, Do KH, Ko SM, et al. Variation of the size of pulmonary venous ostia during the cardiac cycle: optimal reconstruction window at ECG-gated multi-detector row CT. Eur Radiol 2005;15:1441–5 [DOI] [PubMed] [Google Scholar]
- 8.Haywood G. Can we ablate permanent atrial fibrillation? Heart 2006;92:152–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pappone C, Oreto G, Rosanio S, Vicedomini G, Tocchi M, Gugliotta F, et al. Atrial electroanatomic remodelling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation 2001;104:2539–44 [DOI] [PubMed] [Google Scholar]
- 10.Hamdan A, Charalampos K, Roettgen R, Wellnhofer E, Gebker R, Paetsch I, et al. Magnetic resonance imaging versus Computed tomography for characterization of pulmonary vein morphology before radiofrequency catheter ablation of atrial fibrillation. Am J Cardiol 2009;104:1540–6 [DOI] [PubMed] [Google Scholar]
- 11.Ho S, Cabrera J, Tran V, Farre J, Anderson R, Sanchez-Quintana D. Architecture of the pulmonary veins: relevance to radiofrequency ablation. Heart 2001;86:265–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito T, Waki K, Becker A. Left atrial myocardial extension onto pulmonary veins in humans: anatomic observations relevant for atrial arrhythmias. J Cardiovasc Electrophysiol 2000;11:888–94 [DOI] [PubMed] [Google Scholar]
- 13.Hassink R, Aretz H, Ruskin J, Keane D. Morphology of atrial myocardium in human pulmonary veins: a post-mortem analysis in patients with and without atrial fibrillation. J Am Coll Cardiol 2003;42:1108–14 [DOI] [PubMed] [Google Scholar]
- 14.Kholova I, Kautzner J. Anatomic characteristics of extensions of atrial myocardium into the pulmonary veins in subjects with and without atrial fibrillation. Pacing Clin Electrophysiol 2003;26:1348–55 [DOI] [PubMed] [Google Scholar]
- 15.Appelton C, Fristenberg M, Garcia M, Thomas JD. The echo-Doppler evaluation of the left ventricular diastolic function: a current perspective. Cardiol Clin 2000;18:513–42 [DOI] [PubMed] [Google Scholar]
- 16.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009;10:165–93 [DOI] [PubMed] [Google Scholar]
- 17.Cohen G, Pietrolungo J, Thomas J. A practical guide to assessment of ventricular diastolic function using doppler echocardiography. J Am Col Cardiol 1996;27:1754. [DOI] [PubMed] [Google Scholar]