Abstract
Objectives
Cocaine is a commonly used illicit drug that leads to the most emergency department (ED) visits. Chest pain is the most common presentation, reported in 40% of patients. Our aim was to evaluate the incidence of previous myocardial infarction among young cocaine users (18–40 years) with cocaine-associated chest pain by the assessment of myocardial fibrosis by cardiovascular MRI. Second, we also intended to evaluate the coronary tree by CT angiography (CTA).
Methods
24 cocaine users (22 males) who frequently complained about cocaine-associated chest pain underwent CTA and cardiovascular MRI. Mean age of patients was 29.7 years and most of them (79%) had frequently used inhalatory cocaine.
Results
The calcium score turned out to be positive in only one patient (Agatston=54). Among the coronary segments evaluated, only one patient had calcified plaques at the anterior descending coronary artery (proximal and medium segments). Assessment of regional ventricular function by the evaluation of 17 segments was normal in all patients. None of the patients showed myocardial delayed enhancement, indicative of myocardial fibrosis. CTA therefore confirmed the low cardiovascular risk of these patients, since most of them (96%) had no atherosclerosis detected by this examination. Only one patient (4%) had coronary atherosclerosis detected, without significant coronary stenosis.
Conclusion
Cardiovascular MR did not detect the presence of delayed enhancement indicative of myocardial fibrosis among young cocaine users with low cardiovascular risk who had complained of cocaine-associated chest pain.
Cocaine is a commonly used illicit drug, used worldwide by more than 15 million people in 2009 [1], and the drug that leads to the most emergency department (ED) visits [2]. Chest pain is the most common presentation, reported in 40% of patients [3,4]. Cocaine-related cardiovascular complications include acute myocardial infarction (MI), arrythmias, sudden death, myocarditis, cardiomyopathy, hypertensive crises, aortic dissection and endocarditis [5,6]. The majority of patients with cocaine-associated MI events appear to be young, non-white smokers with a history of drug use in the preceding 24 h [6,7]. These characteristics are similar in patients with cocaine-associated chest pain [8], making it difficult to predict those at risk for MI, given the relatively low incidence of cocaine-associated MI [8-11].
In recent years, MRI and CT angiography (CTA) have emerged as powerful non-invasive tools for myocardial assessment and evaluation of the coronary tree [12-14], which might be very important in this special scenario. The aim of this study was to evaluate the incidence of previous MI among young cocaine users with a history of chest pain through the diagnosis of myocardial fibrosis (MF) by MRI. A secondary aim was the assessment of the coronary tree by CTA.
Methods
We performed a prospective pilot study including outpatients from the Interdisciplinary Study Group in Alcohol and Drugs (GREA) of the Institute of Psychiatry at the University of São Paulo Medical School. During the period from August 2008 to January 2009, from 100 outpatients who were cocaine users, we selected 52 patients with drug-associated chest pain (several episodes with more than 20 min duration), aged between 18 and 40 years and no risk factors for coronary heart disease. 28 individuals from the sample of 52 subjects did not participate due to a number of reasons, such as lack of commitment, refusal to participate in the study and contact misinformation. Therefore, we studied 24 patients with complaints of cocaine-associated chest pain (during or shortly after its use), and without emergency department admission. None of the patients had exclusion criteria for MRI or CTA studies [12-14]. The study was approved by the Institutional Ethics Committee, and all patients gave written informed consent.
MRI data acquisition and analyses
Cardiac MRI images were acquired by a 1.5 T clinical scanner (Signa CV/i; GE Medical Systems, Waukesha, WI). After localisation of the heart, 8–12 contiguous short-axis slices encompassing the entire left ventricle (LV) and 4 long-axis slices were prescribed. Cine images were acquired with a steady-state free precession pulse sequence. Delayed-enhancement images were acquired 5–15 min after a bolus injection of 0.2 mmol kg–1 (Magnevistan; Schering AG, Berlin, Germany), with an inversion recovery fast gradient-echo pulse sequence [12,13]. Inversion time was adjusted visually to null the signal of normal myocardium. All MRI analyses were performed in a commercially available workstation (ReportCARD; GE Medical Systems, Waukesha, WI). Cine images were used for assessment of LV volumes, mass and function. Quantification of MF by MRI was based on the assessment of the short-axis delayed-enhancement images. The criteria for MF regions were defined as areas of pixels with higher signal intensity (SI) at least two standard deviations above a mean SI of a remote area.
Acquisition and analysis of data from multidetector CT angiography
CT studies were performed in a 64-slice scanner (Aquilion 64; Toshiba, Ottawara, Japan). Calcium scoring was performed with the use of prospective electrocardiographic (ECG) gating with 400 ms gantry rotation, 100–120 kV tube voltage and 300 mA tube current. For multidetector CTA, retrospective ECG gating was used, with heart rate-adjusted gantry rotations of 350–500 ms to enable adaptive multisegmented reconstruction [14]. Pitch and tube currents of 240–400 mA were determined by patients' weight to ensure the lowest individual radiation dose (6–10 mSv). Sublingual nitrates were given before multidetector CTA, along with intravenous contrast (Iopamiron 370; Schering AG, Berlin, Germany) at an injection rate of 5 ml s–1 (mean volume 70–85 ml) using a power injector (Stellant; Medrad, Indianola, PA), followed by a flush of 40 ml of saline at a rate of 5 ml s–1. Beta-blockers (intravenous metoprolol in 5–15 mg dose) were given, if the heart rate was above 70 beats per minute.
Multisegmented reconstruction was performed with 0.5 mm slice thickness, 0.3 mm overlap, multiple phases and ECG editing [14]. Two independent and experienced observers reviewed MRI and CTA images, and eventual discrepancies were solved by consensus. Descriptive statistics were provided as mean±standard deviation for continuous values, and as absolute and relative frequency for categorical values using a commercially available software (SPSS v. 17.0, IBM, Armonk, NY; Excel 2007, Microsoft Corporation, Redmond, WA).
Results
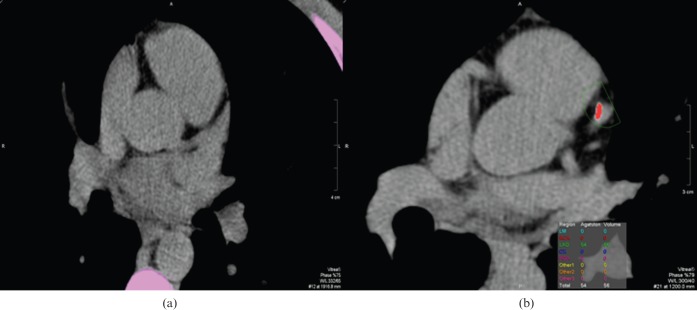
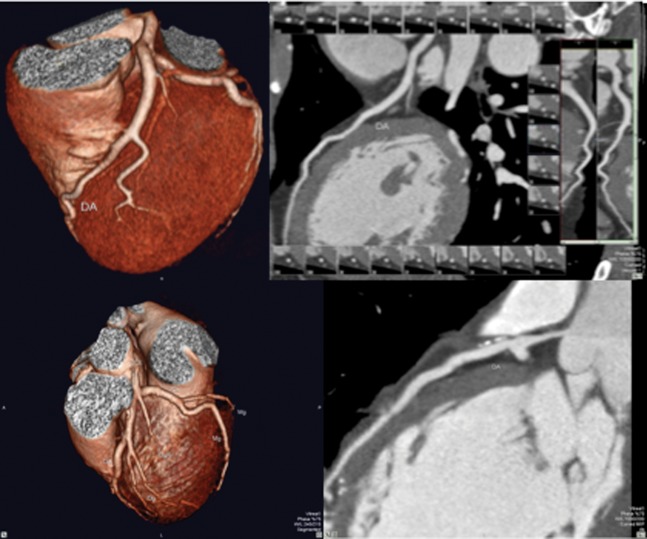
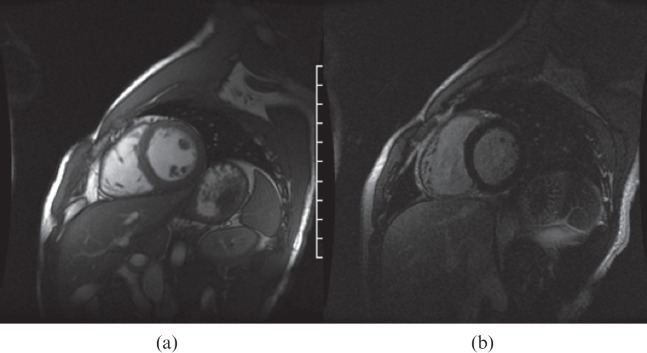
24 young patients (mean age 29.7 years; 22 males) were enrolled in the protocol and underwent MRI and CTA studies. Clinical data are listed in Table 1. All patients had complaints of chest pain and no risk factors for coronary heart disease other than cigarette smoking. Only 21% of the patients (5 of 24) did not report a smoking history. 71% of the patients (17 of 24) reported marijuana abuse, most of them on a frequent basis (more than 10 times a month). 96% of the patients (23 of 24) used cocaine in inhalatory form, and 79% of them (19 of 24) reported a frequency of more than 20 times a month. The non-inhalatory user reported only crack abuse. 71% of the patients reported crack use (17 of 24). It is notable that 42% of the patients (10 of 24) reported use of cocaine in both inhalatory and crack form on a frequent basis. The calcium score was zero in all but one patient (Agatston=54; volume, 56; Figure 1). None of them showed significant coronary stenosis evaluated by CTA scans. Among the coronary segments evaluated, only one patient showed calcified plaques at the anterior descending coronary artery (proximal and medium segments; Figure 2). The overall analysis of the left ventricular function showed ejection fraction (EF), end diastolic volume (EDV), end systolic volume (ESV) and ventricular mass considered normal in all patients. Mean EF, EDV and ESV were 60.7%, 147.7 ml and 59.1 ml, respectively. The cardiac index was normal in all patients. None of the patients presented myocardial hypertrophy. Assessment regional ventricular function by the evaluation of 17 segments was normal in all patients. None of the patients showed myocardial delayed enhancement, indicative of MF (Figure 3).
Table 1. Clinical data.
| Patient details | Patients (n=24) |
| Male | 22 (92%) |
| Female | 2 (8%) |
| White race | 22 (92%) |
| Black race | 2 (8%) |
| Tabagism | 19 (79%) |
| Body mass index <25 | 17 (71%) |
| Body mass index >25 | 7 (29%) |
| Alcoholism | 7 (29%) |
| Sedentarism | 11 (46%) |
| Active | 13 (54%) |
Figure 1.
Calcium score showing no (a) calcified plaques and (b) a calcified plaque at the left descending artery.
Figure 2.
Coronary anatomy showing normal three- and two-dimensional images (top), and three- and two-dimensional images showing proximal calcified plaques (bottom).
Figure 3.
MR images showing (a) normal cine and (b) delayed enhanced short axis planes.
Discussion
To the best of our knowledge, this study is the first to assess previous MI in young cocaine users without risk factors for coronary artery disease who complained about chest pain, using CTA and cardiovascular MRI as diagnostic tools. These patients frequently complained about cocaine-associated chest pain (several episodes related to the use of the drug for more than 20 min), without emergency department admissions. Despite this, we have not found myocardial delayed-enhancement indicative of MF (from previous acute MI) in any of them. The CTA results reinforced the low risk profile of these individuals, since there was only one patient with small calcified plaques at the anterior descending coronary artery.
Cocaine use is concentrated among a select population of young individuals, more males than females [15]. These young patients have low thrombolysis in MI risk score for CAD; therefore most of them do not require hospitalisation because of their complaints of chest pain. In the present study, most of the patients were male (91.6%), with mean age of 29.7 years, which matches the known profile of cocaine users. This age range is at a low risk for CAD according to the Framingham criteria [16]. The only risk factor for CAD was cigarette smoking, found in 79% of our patients (>20 cigarettes a day). It is also interesting to notice that several patients reported frequent use of marijuana (71%) and alcohol (96%), drugs that usually precede and lead to cocaine use. All individuals reported cocaine ingestion in inhalatory or crack forms, and 42% of them reported frequent ingestion of both forms. The low risk profile of our sample was corroborated by the CTA results, since just one patient had a positive calcium score (Agatston=54) and small calcified plaques in the anterior left descending artery.
Cocaine use is highly prevalent in young patients who present acute chest pain to the ED [6] and it is known to cause myocardial ischaemia in a multifactor fashion, including increased oxygen demand, decreased oxygen supply and the induction of a prothrombotic state [17-20].
Although pathophysiological effects of cocaine are well documented, its effect on the formation of atherosclerosis is controversial. Kolodgie et al [21] found that 30-year-old cocaine users already had significant coronary atherosclerosis. Small autopsy studies suggest that atherosclerotic plaque burden is high in cocaine users [22], and there is some evidence that cocaine causes endothelial damage, therefore accelerating atherosclerosis [23].
Lai et al [24] reported a borderline significant association between cocaine use and extent of coronary calcification in 197 intravenous drug users. Pletcher et al [25] did not detect an association between cocaine exposure and the extent of coronary calcification in the Coronary Risk Development in Young Adults (CARDIA) study, which enrolled 3038 participants. Bamberg et al [26] confirmed these observations, and demonstrated no association between history of cocaine use and coronary calcification for the presence and extent of calcified and non-calcified plaques. They also found that in patients presenting with acute chest pain history of cocaine use is associated with a six fold increase in risk for acute coronary syndrome [26].
The overall literature with imaging diagnostic tools for this matter has little available data [24-27]. In these studies, the mean age of the patients was higher, with other risk factors for CAD involved, in contrast to the population of this study, which were very young (mean age 29.7 years). In this population, since the CTA results excluded the presence of atheromatosis in most of the patients, it is more likely that the chest pain could be associated with a vasospasm mechanism caused by the drug. Its importance is, therefore, to highlight the low risk profile of these patients and avoid survey of CAD of atherosclerotic origin focusing on diagnostic imaging confirmation of the absence of vascular disease in young patients, low risk and cocaine users presenting with chest pain at the ED.
MRI did not detect MF due to any previous acute MI in these patients. Therefore, we might conclude that even though they reported prolonged chest pain, it did not cause irreversible myocardial damage. The literature reports a 6% incidence of acute MI among cocaine users who have chest pain and seek medical emergency services [21]. In this study, most patients (90%) did not visit an emergency department due to chest pain.
In conclusion, cardiovascular MRI did not detect the presence of delayed enhancement indicative of MF among young cocaine users with low cardiovascular risk with complaints of chest pain during or after cocaine abuse. CTA confirmed low conventional cardiovascular risk for these patients, since most of them (96%) had no atherosclerosis detected by this examination.
Limitations
A limitation of this study was the number of patients enrolled. Access to these individuals is very difficult because of the illegal nature of their activity. Owing to the recruitment of patients, the incidence of MI may have been underestimated. This method was intended to diagnose MI in young cocaine users, with no risk factors for coronary disease and who did not recognise chest pain as a possible clinical presentation of MI. The reliability of the reported type and duration of chest pain cannot be ensured because the patients did not visit the emergency department, although prolonged chest pain can be considered suggestive of cardiac origin. Finally, a reliable evaluation should be done in this population because the literature reports there is a risk (albeit small, at 6%) of myocardial infarction. Further and multicentre studies are needed to evaluate the incidence and impact of cocaine use on this young subset of patients [28].
References
- 1.UNODC World drug report 2010. Publication no. E.10.XI.13 New York, NY: United Nations, 2010 [Google Scholar]
- 2.Colliver JD, Kopstein AN. Trends in cocaine abuse reflected in emergency room episodes reported to DAWN Drug Abuse Warning Network. Public Health Rep 1991;106:59–68 [PMC free article] [PubMed] [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration, Office of Applied Studies Drug abuse warning network, 2005: national estimates of drug-related emergency department visits. DAWN series D-29. DHHS publication no. (SMA) 07-4256 Rockville, MD: Substance Abuse and Mental Health Services Administration, 2007 [Google Scholar]
- 4.Brody SL, Slovis CM, Wrenn KD. Cocaine-related medical problems: consecutive series of 233 patients. Am J Med 1990;88:325–31 [DOI] [PubMed] [Google Scholar]
- 5.Isner JM, Chokshi SK. Cardiovascular complications of cocaine. Curr Probl Cardiol 1991;16:89–123 [DOI] [PubMed] [Google Scholar]
- 6.Lange RA, Hillis LD. Cardiovascular complications of cocaine use. N Engl J Med 2001;115:99–111 [DOI] [PubMed] [Google Scholar]
- 7.Hollander JE, Hoffman RS, Burstein JL, Shih RD, Thode HC., Jr Cocaine-associated myocardial infarction. Mortality and complications. Cocaine-Associated Myocardial Infarction Study Group. Arch Intern Med 1995;155:1081–6 [PubMed] [Google Scholar]
- 8.Hollander JE, Hoffman RS, Gennis P, Fairweather P, DiSano MJ, Schumb DA, et al. Prospective multicenter evaluation of cocaine-associated chest pain. Cocaine Associated Chest Pain (COCHPA) Study Group. Acad Emerg Med 1994;1:330–9 [DOI] [PubMed] [Google Scholar]
- 9.Weber JE, Chudnofsky CR, Boczar M, Boyer EW, Wilkerson MD, Hollander JE. Cocaine-associated chest pain: how common is myocardial infarction? Acad Emerg Med 2000;7:873–7 [DOI] [PubMed] [Google Scholar]
- 10.Feldman JA, Fish SS, Beshansky JR, Griffith JL, Woolard RH, Selker HP. Acute cardiac ischemia in patients with cocaine-associated complaints: results of a multicenter trial. Ann Emerg Med 2000;36:469–76 [DOI] [PubMed] [Google Scholar]
- 11.Kontos MC, Schmidt KL, Nicholson CS, Ornato JP, Jesse RL, Tatum JL. Myocardial perfusion imaging with technetium-99m setamibi in patients with cocaine-associated chest pain. Ann Emerg Med 1999;33:639–45 [PubMed] [Google Scholar]
- 12.Sensky PR, Jivan A, Hudson NM, Keal RP, Morgan B, Tranter JL, et al. Coronary artery disease: combined stress MR imaging protocol-one-stop evaluation of myocardial perfusion and function. Radiology 2000;215:608–14 [DOI] [PubMed] [Google Scholar]
- 13.Schneider G, Fries P, Ahlhelm F, Kindermann I, Kramann B, Böhm M. Contrast-enhanced cardiac MR imaging. Eur Radiol. 2003;13:N11–18 [DOI] [PubMed] [Google Scholar]
- 14.Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008;359:2324–36 [DOI] [PubMed] [Google Scholar]
- 15.McCord J, Jneid H, Hollander JE, de Lemos JA, Cercek B, Hsue P, et al. Management of cocaine-associated chest pain and myocardial infarction: a scientific statement from the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology. Circulation 2008;117:1897–907 [DOI] [PubMed] [Google Scholar]
- 16.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–47 [DOI] [PubMed] [Google Scholar]
- 17.Flores ED, Lange RA, Cigarroa RG, Hillis LD. Effect of cocaine on coronary artery dimensions in atherosclerotic coronary artery disease: enhanced vasoconstriction at sites of significant stenoses. J Am Coll Cardiol 1990;16:74–9 [DOI] [PubMed] [Google Scholar]
- 18.Kuhn FE, Gillis RA, Virmani R, Visner MS, Schaer GL. Cocaine produces coronary vasoconstriction independent of an intact endothelium. Chest 1992;102:581–5 [DOI] [PubMed] [Google Scholar]
- 19.Mittleman MA, Mintzer D, Maclure M, Tofler GH, Sherwood JB, Muller JE. Triggering of myocardial infarction by cocaine. Circulation 1999;99:2737–41 [DOI] [PubMed] [Google Scholar]
- 20.Siegel AJ, Sholar MB, Mendelson JH, Lukas SE, Kaufman MJ, Renshaw PF, et al. Cocaine-induced erythrocitosis and increase in von Willebrand factor: evidence for drug-related blood doping and prothrombotic effects. Arch Intern Med 1999;159:1925–9 [DOI] [PubMed] [Google Scholar]
- 21.Kolodgie FD, Virmani R, Cornhill JF, Herderick EE, Smialek J. Increase in atherosclerosis and adventitial mast cells in cocaine abusers: an alternative mechanism of cocaine-associated vasospasm and thrombosis. J Am Coll Cardiol 1991;17:1553–60 [DOI] [PubMed] [Google Scholar]
- 22.Dressler FA, Malekzadeh S, Roberts WC. Quantitative analysis of amounts of coronary arterial narrowing in cocaine addicts. Am J Cardiol 1990;65:303–8 [DOI] [PubMed] [Google Scholar]
- 23.Benzaquen BS, Cohen V, Eisenberg MJ. Effects of cocaine on coronary arteries. Am Heart J 2001;142:402–10 [DOI] [PubMed] [Google Scholar]
- 24.Lai S, Lai H, Meng Q, Tong W, Vlahov D, Celentano D, et al. Effect of cocaine use on coronary calcium in Black adults in Baltimore, Maryland. Am J Cardiol 2002;90:326–8 [DOI] [PubMed] [Google Scholar]
- 25.Pletcher MJ, Kiefe CI, Sidney S, Carr JJ, Lewis CE, Hulley SB. Cocaine and coronary calcification in young adults: the coronary artery risk development in young adults (CARDIA) study. Am Heart J 2005;150:921–6 [DOI] [PubMed] [Google Scholar]
- 26.Bamberg F, Schlett C, Truong QA, Rogers IS, Koenig W, Nagurney JT, et al. Presence and extent of coronary artery disease by cardiac computed tomography and risk for acute coronary syndrome in cocaine users among patients with chest pain. Am J Cardiol 2009;103:620–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh K, Chang AM, Perrone J, McCusker C, Shofer F, Collin M, et al. Coronary computerized tomography angiography for rapid discharge of low-risk patients with cocaine-associated chest pain. J Med Toxicol 2009;5:111–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchholz S, Grieve SM, Maher M, Figtree GA. Cardiovascular flashlight. Cocaine-induced myocardial injury seen as multiple mid-wall foci of late enhancement by contrast-enhanced cardiac magnetic resonance imaging. Eur Heart J 2010;31:1422. [DOI] [PubMed] [Google Scholar]