Abstract
Objectives
To develop a neonatal MR-compatible incubator for transporting babies between a neonatal intensive care unit and an MRI unit that is within the same hospital but geographically separate.
Methods
The system was strapped to a standard MR-compatible patient trolley, which provides space for resuscitation outside the incubator. A constant-temperature exothermic heat pad was used to maintain temperature together with a logging fluoro-optic temperature monitor and alarm system. The system has been designed to accommodate standard knee-sized coils from the major MR manufacturers. The original incubator was constructed from carbon fibre, but this required modification to prevent radiofrequency shading artefacts due to the conducting properties of the carbon fibre. A high-tensile polyester material was used, which combined light weight with high impact strength. The system could be moved onto the patient bed with the coils and infant in place by one technologist.
Results
Studies in eight neonatal patients produced high quality 1.5 T MR images with low motion artefacts. The incubator should also be compatible with imaging in 3 T MR systems, although further work is required to establish this. Images were acquired using both rapid and high-resolution sequences, including three-dimensional volumes, proton spectra and diffusion weighting.
Conclusion
The incubator provides a safe, quiet environment for neonates during transport and imaging, at low cost.
Neonatal imaging is of growing importance because of the increasing need to make early diagnoses to direct treatment and assist with prognostication [1]. Problems associated with prematurity can often lead to deleterious consequences for the central nervous system (CNS) and other parts of the body, as can some problems in babies born close to term. MR is highly relevant for imaging this population as it is non-invasive and does not use ionising radiation, an important factor in this age group. There have been three main approaches to imaging neonates who require monitoring and preservation of temperature away from the neonatal intensive care unit (NICU):
Ad hoc arrangements using standard MR facilities and non-MR-compatible neonatal transport incubators, which are infrequently used owing to logistical difficulties and concerns about safety.
A dedicated MR system on the NICU where care and support can be easily provided.
A neonatal incubator with full support equipment to transport the baby to a central hospital based MR system.
This study investigates the last approach described above. A number of prototype incubators and a single commercial system have previously been reported. Initial work on imaging neonates used relatively high-field small-bore magnet systems with in-house developed patient-handling systems [2-5]. Emphasis in early neonatal studies was also placed on measuring the physiological stability of preterm neonates for MRI [6,7]. The first attempt to produce a fully engineered neonatal incubator was based around a detachable patient-handling system, and this design has been successful in scanning a large number of neonates over several years [8-10]. However, this design has proven expensive to build and has not been reproduced by the manufacturer commercially. A commercial system was launched a few years ago, providing good control of neonatal physiological parameters [11-14] and proving successful in a number of MR studies, although suffering from some limitations in patient visibility when the incubator is inside the magnet bore.
We report our recent progress in developing a new neonatal transport MR-compatible incubator and monitoring system with a low-cost design integrated from standard components that are already available commercially with the Conformité Européene marking. A preliminary technical evaluation of the incubator has been performed as part of an improvement in clinical service assessment with the written agreement of the Sheffield NHS Hospitals Trust.
Methods and materials
We tested a lightweight, neonatal transport incubator (BabyPod; Advcanced Healthcare Technology Ltd, Hertford, UK) for MR compatibility using a 1.5 T HDx MR (GE Healthcare, Waukesha, WI) and a 3 T Achieva MR (Philips, Best, the Netherlands). This incubator is known to have no metallic components and is routinely used for interhospital transfers and emergency service evacuation procedures. The incubator was originally constructed from a carbon-fibre material, which is extremely strong and protective for the baby. This caused severe shading artefacts on both gradient and spin echo sequences when using the body coil as the transmitter together with received-only knee coils at both field strengths. This was due to shielding of the phantom from the radiofrequency pulses because of conduction in the carbon fibres.
The incubator design was modified to use non-conducting high-tensile polyester (HTP), and the transparent cover, manufactured from polyester polyethyleneterephthalate glycol (PETG), was modified to accept standard knee-sized coils and to improve visibility of the neonate when in the magnet. Subsequent testing on MR scanners at 1.5 and 3.0 T showed no evidence of shading artefact. Quality testing was performed on an MR phantom using a 64-slice X-ray CT system (GE Healthcare). MR images were acquired with a spin echo sequence [repetiton time (TR)/echo time (TE) 640/10 ms, 1 mm in plane, 4 mm slice thickness (SLT), number of excitations (NEX) = 1] from a resolution and slice test object at 3 T located within the carbon-fibre incubator and also the HTP incubator. Images were assessed for uniformity, signal-to-noise ratio (SNR) and geometrical distortion with and without the incubator. Safety testing included assessment of all items located within the incubator for possible magnetic torque and mechanical hazards to the neonate and for possible heating effects due to radiofrequency (RF) absorption.
A combined quadrature uniform transmit and eight-channel phased array receive coil (GE Healthcare) with a minimum inner diameter of 150 mm located inside the incubator was used for this preliminary system evaluation to provide higher SNR than a head coil. The manufacturer of the coil does not make specific reference to its use for imaging neonatal heads, but states “Pay special attention to very young, sedated, or other compromised patients who may not be able to communicate effectively”. With such special attention always being taken through visual and vital signs monitoring, it has been used routinely at our institution for clinically required scans on neonates prior to use with the incubator as there is no available alternative from the manufacturer. Similar small-bore coils have been in use with MR systems from other manufacturers over the past 12 years at our institution to provide the best possible clinical diagnoses with a modality that is optimal for detecting neonatal pathology. As there have been no adverse events related to such use over this extended period, no specific ethical approval was sought for this study, which was designed as an improvement to routine clinical service provision. However, additional testing has been performed as discussed below to ensure safe operation in terms of specific absorption rate (SAR) when used with the incubator.
Experiments were performed with a uniform 120 mm cylindrical saline-containing phantom, which was thermally insulated using the MR system manufacturer's sample holder to assess possible heating effects and located inside the incubator. The fluoroptic temperature probe described above was located at the centre of the phantom inside the fluid to assess possible heating effects. An axial fast-recovery fast spin echo T2 weighted image with 1 mm in plane, 4 mm SLT, NEX = 0.5, TR/TE = 3420/92 ms, echo train length = 16 acquired in 30 s was run continuously for 30 min while temperature was continually monitored. So the experiment was repeated three times, allowing the phantom to cool to ambient scan room temperature (20 °C) in between runs. So far these measurements have only been performed at 1.5 T.
Following the initial image quality and safety checks discussed above, a preliminary technical and radiological evaluation was performed as part of a clinical service assessment. This was undertaken with the full written approval from the Sheffield Hospitals NHS Trust for using the incubator to transport and image a small number of neonates requiring MR for clinical purposes (eight studies performed at the time of writing this report; see Table 1 for details of reasons for clinical investigation).
Table 1. Reasons for clinical investigation.
| Clinical concern requiring MR Scan | Number of cases |
| Complication of prematurity | 3 |
| Assessment of prenatally diagnosed structural brain abnormality | 3 |
| Hypoxic–ischaemic encephalopathy at term | 2 |
Equipment was included to provide a safe environment to transfer neonates within the hospital. Neonatal temperature was maintained using disposable heat pads (TransWarmer Infant Transport Mattress; CooperSurgical, Trumbull, CT) which are widely used during transport of neonates between hospitals. Temperature of the neonate could be monitored using an MR-compatible 15 m fluoroptic probe (PalmSense; Photon Control Inc., Burnaby, Canada) accurate to 0.1 °C and either logged to a computer in the MR control room or monitored locally on the MR trolley in the scan room. Continuous temperature monitoring was not performed owing to initial problems encountered with maintaining good contact between the optical probe and the neonate, mainly due to neonatal foot motion during scanning. Further work is under way to improve the probe positioning and attachment.
The baby could be visually monitored using an MR-compatible wireless (2.4 GHz) colour “spy” camera and receiver system modified by removing magnetic mounting brackets (Maplin, Rotherham, UK) and digitised onto a computer located in the scan control room using a USB interface video capture card (Model USBAV-192; ADS Tech, Guangdong, China). The computer then displayed a live colour video image of the baby inside the incubator. The wireless receiver was located adjacent to the screened room waveguide in the control room. However, we relied on direct visualisation of the baby through the transparent incubator hood by a clinician who remained in the scan room in our preliminary evaluations. The clinician also monitored the baby's vital signs on the monitoring equipment, located on the MR-compatible trolley during the scan. A parent often accompanied the baby in the scan room. None of the babies showed signs of distress during the examinations carried out, but if this had happened they would have been rapidly moved out of the magnet and magnet room on the docking table, with the MR-compatible trolley (with connections to the monitoring equipment remaining in place) also being moved.
Another important consideration in imaging neonates is the high level of acoustic noise encountered with MRI. Each baby was fitted with special neonatal ear defenders that covered the entire ear and provided at least 7 dB of attenuation of the relevant frequency range (MiniMuffs; Natus Medical Inc., Seattle, WA). Standard in-ear protectors can also be used with the MiniMuffs providing an additional 30 dB of protection. In addition, the incubator itself was fitted with acoustic foam pads that reduced external noise by a further 6 dB over the frequency range from 40 to 20,000 Hz. This was measured using a frequency generator and amplified speaker external to the incubator with an audio frequency microphone (SM58; Shure, Niles, IL) located inside the RF coil in the incubator pod and with the lid either on or off. The microphone was positioned within the RF coils and pads in the same location as the neonate. The acoustic attenuation and positioning pads were removable to allow thorough cleaning or sterilisation, which was performed before and after each imaging session.
Babies are usually transported in ambulances at natural humidity, so it was decided to avoid control of this parameter during the short duration of the transport and MR scanning session (typically 1 h from cot back to cot) to avoid issues of sterilisation and infection control associated with humidity control devices. Monitoring of vital signs (pulse rate, SpO2) was provided using an MR-compatible system (Maglife Light; Schiller, Baar, Switzerland).
The neonatal incubator was strapped to a standard MR-compatible trolley (Wardray-Premise, Thames Ditton, UK) with quick-release non-magnetic safety belt fastenings for intrahospital transfers. MR-compatible support gas cylinders and pumps can be transported on the trolley when needed. All equipment used was MR compatible and Conformité Européene marked, fully bolted in place on the trolley and used according to the manufacturer's specifications in terms of operating field and location. The baby was always fed and then placed inside the RF coil with packing cushions to minimise motion artefacts and the monitoring equipment was attached on the NICU, allowing time to settle prior to transport to the MR unit.
The incubator was transported to the MR unit in the presence of a qualified neonatal specialist and a neonatal nurse with suction and resuscitation equipment. A checklist was used to ensure that no magnetic items had been placed on the trolley before entering the MR room. Inside the MR room, the safety fastenings were released and the incubator slid across from the MR-compatible trolley onto the undocked patient trolley adjusted to the correct height. The monitoring equipment can be used in a field up to 40 mT, limiting the approach of the trolley to the magnet. The cables and tubes leading to the monitoring equipment were sufficiently long to allow the connections to be maintained without disturbing the neonate. All cables and tubes entered the incubator through specially created flanges in the transparent lid.
MR images were acquired using a 1.5 T MR system using a range of standard sequences with an MR spin echo (TR/TE 640/10 ms, 1 mm in plane, 4 mm SLT, NEX = 1) and fast imaging sequences including single shot fast spin echo (SSFSE), fast spin echo, T1 weighted, three-dimensional magnetisation-prepared rapid-acquisition gradient echo, gradient echo T2*, diffusion weighted sequences and spectroscopic acquisitions. Other sequences were added as required clinically by the neuroradiologist at the time of examination. All of the studies were assessed by PDG, an experienced paediatric neuroradiologist. No scoring system was used but images were judged as either diagnostic or non-diagnostic quality.
Results
Figure 1 shows the MR- and CT-compatible incubator on the patient table of a 1.5 T MR system (Signa HDx; GE Healthcare) with the RF coil fastened in place. To evaluate image quality prior to neonatal imaging, images were acquired from a resolution and slice test object at 3 T located within the carbon-fibre incubator shown in Figure 2a and also the HTP incubator in Figure 2b. Figure 2c shows an image without the incubator for reference. Similar results were observed at 1.5 T showing the observed artefact in Figure 2a to be due to RF shielding rather than susceptibility effects. Figure 2d shows the CT cross-section of the incubator with the MR phantom in place, showing no streaking or flaring artefacts, suggesting the system could also be used for CT, although we did not perform CT studies on neonates in our initial evaluation [15].
Figure 1.
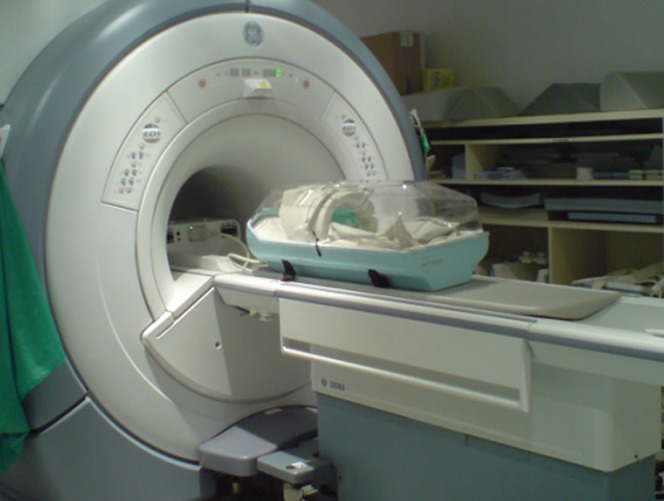
The MR/CT-compatible incubator provides good visibility of the baby during imaging, as well as accommodating knee-sized coils from the major MR manufacturers.
Figure 2.
(a) Spin echo MR image of the resolution and slice location phantom inside the carbon-fibre incubator showing strong radiofrequency screening shading artefact. Similar results were obtained at both 1.5 and 3 T. (b) The phantom in the high-tensile polyester incubator showing no radiofrequency screening shading. (c) Image acquired without the incubator for reference. (d) CT image of the incubator with the resolution and slice location phantom in place. No streaking artefacts were observed owing to the incubator, showing that it is CT compatible.
Images of the thermally insulated saline-containing phantom appeared highly uniform, suggesting no localised regions of increased RF deposition and the mean temperature rise observed after 30 min scanning with a SSFSE sequence was 0.5±0.2 °C (n = 3).
All neonates were stable during the transport and imaging sessions with temperature in the normal range 37±0.5 °C measured before and after the imaging session. SpO2 was monitored continually throughout the transport and examination and was >95% in all cases. Transport time for the baby from out of the cot to the MR unit and back to the cot in the NICU was less than 1h for all studies, including 25 min MRI allocation.
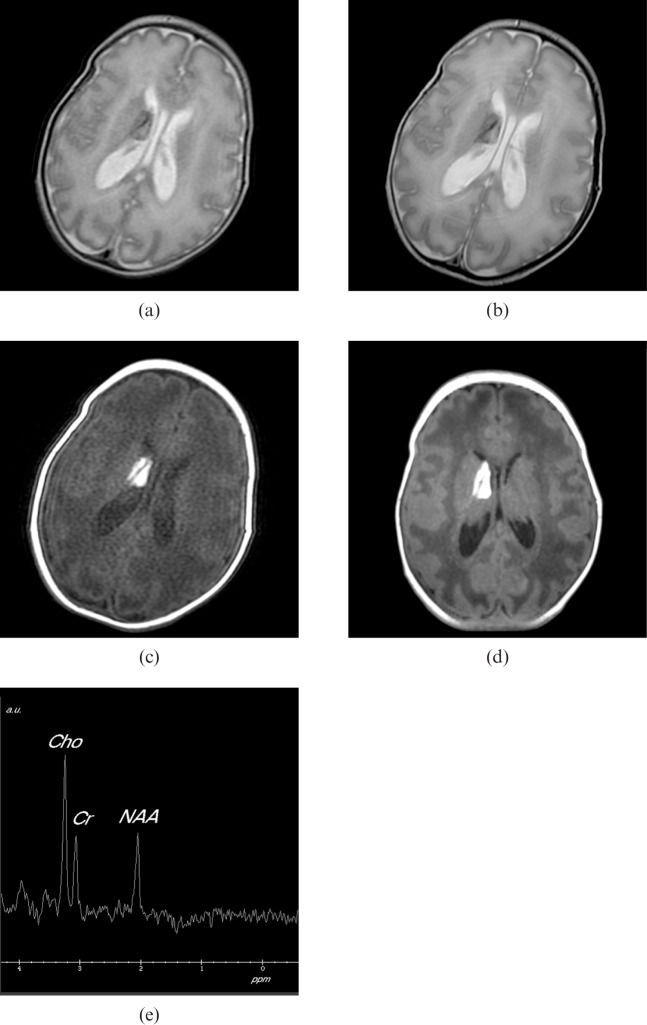
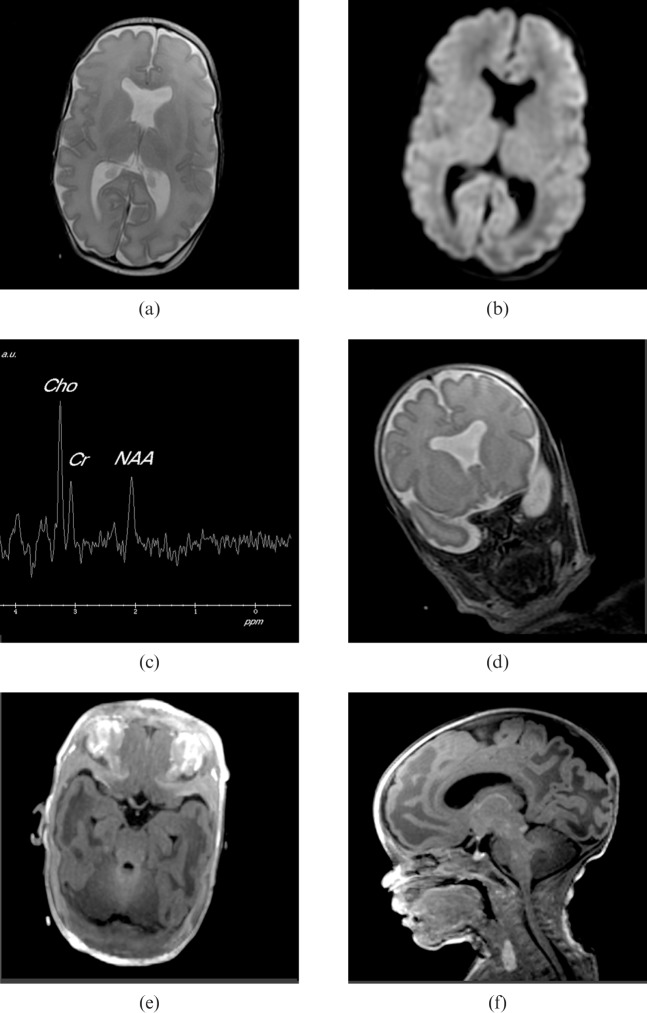
Figures 3 and 4 show two examples of clinical cases examined using the incubator, the first MR images from a male born at 33 weeks′ gestational age by emergency Caesarean section for foetal distress and the second from a female born at 31 weeks′ gestational age by emergency Caesarean section for antepartum haemorrhage, showing good image quality and high-resolution proton spectroscopy.
Figure 3.
MR images from a male born at 33 weeks′ gestational age by emergency Caesarean section for foetal distress. After birth, he was noted to have a bilateral cleft lip and a high arched palate. Cranial ultrasound on day 2 and day 10 showed a small right ependymal haemorrhage with flare in the adjacent periventricular white matter. The MR examination was performed at 2 weeks. (a) Axial fast-recovery fast spin echo T2 weighted image with 1 mm in plane, 4 mm slice thickness (SLT), number of excitations (NEX) = 0.5, repetition time (TR)/echo time (TE) = 3420/92 ms, echo train length = 16 acquired in 30 s. (b) Anatomically equivalent fast spin echo T2 weighted image with TR/TE = 3860/86 ms, with 1 mm in plane resolution, SLT = 4 mm and NEX = 2 acquired in 2 min. Both images clearly show an established subependymal haematoma protruding into the body of the right lateral ventricle. There is some movement artefact on the longer acquisition imaging. The haematoma is also well shown on axial T1 weighted imaging. (c) T1 multiecho multiplanar acquired with TR/TE = 320/11 ms with 1 mm in plane resolution, 4 mm SLT. (d) Axial slice from a three-dimensional acquisition with TR/TE = 10/4 ms, 1 mm in-plane resolution, 1 mm SLT and flip angle = 13°. Note the deep venous structure passing through the haematoma. (e) Single voxel proton spectroscopy from the white matter adjacent to the haematoma was normal for age. The point-resolved spectrum was from a 15×15×15 mm voxel with TR/TE = 1500/144 ms, NEX = 128.
Figure 4.
MR images from a female born at 31 weeks′ gestational age by emergency Caesarean section for antepartum haemorrhage. (a) Axial fast spin-echo (FSE) T2 weighted image with the same parameters as Figure 3b. (b) Anatomically matched diffusion weighted image with 1 mm in plane resolution, 5 mm slice thickness, number of excitations = 1, repetition time/echo time = 10000/75.7 ms, b = 700 s mm–2. These do not show any evidence of hypoxic ischaemic injury. (c) Single voxel proton spectroscopy (shown with the same acquisition parameters as Figure 3e from the posterior hemispheric white matter) was normal. (d) Absence of the cavum septum pellucidum was confirmed, but in addition there was “squaring” of the frontal horns shown on coronal fast-recovery FSE T2 weighted images (shown with the same acquisition parameters as Figure 3b). This is characteristic of septo-optic dysplasia and hypoplasia of the optic nerves (especially the left side). (e) Chiasm was confirmed on axial reconstructions from an axially acquired volume data set (shown with the same acquisition parameters shown for Figure 3d). Opthalmology review confirmed bilateral optic disc hypoplasia, more pronounced on the left than the right. (f) Arrested migration of the posterior pituitary gland was confirmed on sagittal reconstructions of the volume data. This is a recognised associated finding in septo-optic dysplasia.
Discussion and conclusion
A number of options are available for imaging neonates on standard MR systems without the use of an incubator [16]. However, in many cases the use of an incubator provides a number of crucial advantages including improved thermal control and acoustic noise damping. The integrated incubator imaging system developed here has provided a safe, effective, low-cost solution for transporting babies from the NICU to a remote MR system. The image quality was in general judged to be the same or better than in neonates imaged without the incubator in previous studies by the paediatnc neuroradiologist (PDG). The incubator has an MR-compatible temperature maintenance and monitoring system, and is light enough to be moved into the MR magnet by a single operator. Good visibility is provided by the clear design of the incubator lid, allowing the baby to be seen throughout imaging, which is not always possible with other designs. The cost of the incubator and associated equipment is less than £40,000, making this an affordable option for many MR centres.
Avoidance of carbon fibre in the construction of the incubator eliminated MR shading artefacts due to RF screening. The HTP material now used in the design provides a strong mechanical structure, and is fully MR and CT compatible. The incubator is compatible with knee-sized and smaller imaging coils from all the major MR manufacturers, providing operational flexibility and removing the need to purchase an extra neonatal-specific imaging coil, making the basic cost lower. Phantom imaging at both 1.5 and 3 T shows that the HTP incubator can be used at either field strength without artefacts. Specific absorption rate limits enforced by the MR manufacturers provide a wide safety margin for heating effects, and this is not thought to be a problem for imaging neonates at either 1.5 or 3 T, although we only carried out our initial evaluation at 1.5 T. A small temperature rise (0.5±0.2 °C) was noted in the insulated saline phantom heating tests. No temperature rise was noted in any of the neonates following scanning. Of course, the thermally insulated phantom has no way to lose the deposited heat due to RF pulses, unlike a neonate who is fully perfused and loses heat continuously through normal convection and conduction processes. The imaging sequences performed on neonates were intermittent, with relatively long stops in between for localisation, and so much less SAR intensive than that performed on the phantom, which was a worst case scenario. The quadrature transmit, phased array receive RF coil has the advantage that only the part of the neonate inside the coil is exposed to RF absorption, in contrast to the body coil, where the whole body is exposed.
Use of a standard non-magnetic trolley and MR-compatible monitoring equipment allows the entire assembly to be brought into the MR scan room without having to disturb the baby, resulting in reduced motion artefact, in our initial experience. The trolley has sufficient space with the incubator in place so that the lid can be rapidly removed in an emergency, and the baby placed on a flat surface at the rear of the trolley and resuscitated if necessary. Vital signs are monitored through the entire transport and imaging procedure using the battery-powered monitoring device, providing reassurance for the support team. Ensuring safety of the neonate during both transport and imaging is of prime concern in any incubator design, and this involves analysis of human-induced risk factors as well as inherent system hazards. A checklist helps ensure all safety procedures have been completed prior to moving the incubator into the magnet room and magnet.
Neonatal images acquired using the incubator system appear to be of good quality, using the 1.5 T eight-channel phased-array coil (knee-sized) and fast spin echo, T1 weighted diffusion imaging sequences and spectroscopic acquisitions. All eight examinations performed so far provided diagnostic-quality images, with only one baby needing multiple repeat scans to eliminate motion artefact, and this baby was known to be continuously restless prior to imaging. This is, so far, a relatively small number of examinations to establish the technical utility and safety of the transport and imaging system, and further work is required to establish the full clinical impact, which will be reported in future publications.
The array coil was of a closed but split design, which partially restricted visibility of the neonate. A more open array coil design would have been preferred. Alternatively use of surface coils would provide more patient visibility. The absence of motion artefacts in these preliminary studies is thought to be due to the babies being fed immediately prior to imaging, and careful location and gentle restraint within the imaging coil using soft cleanable cushions. The babies had small external self-adhesive ear defenders fitted prior to being located in the array coil, providing 7 dB of hearing protection, which can be added to standard ear defenders (which provide about 30 dB of protection). The padded incubator also provides some acoustic and vibration damping up to an additional 6 dB. In summary, this low-cost, low-weight MR- and CT-compatible incubator design provides a safe and effective method of high-quality imaging of neonates transported away from the NICU.
Acknowledgments
Conflict of interest
M Lait is a director of Advanced Health Technology Ltd, Hertford, UK.
Footnotes
This study was in part sponsored by Westfield Healthcare, Sheffield, UK.
References
- 1.Barkovich AJ. MR imaging of the neonatal brain. Review. Neuroimaging Clin N Am 2006;16:117–35, viii–ix [DOI] [PubMed] [Google Scholar]
- 2.Zuerrer M, Martin E, Boltshauser E. MR imaging of intracranial hemorrhage in neonates and infants at 2.35 Tesla Neuroradiology 1991;33:223–9 [DOI] [PubMed] [Google Scholar]
- 3.Battin M, Maalouf EF, Counsell S, Herlihy A, Hall A, Azzopardi D, et al. Physiological stability of preterm infants during magnetic resonance imaging. Early Hum Dev 1998;52:101–10 [DOI] [PubMed] [Google Scholar]
- 4.Thornton JS, Amess PN, Penrice J, Chong WK, Wyatt JS, Ordidge R. Cerebral tissue water spin-spin relaxation times in human neonates at 2.4 tesla: methodology and the effects of maturation. Magn Reson Imaging 1999;17:1289–95 [DOI] [PubMed] [Google Scholar]
- 5.Steinlin M, Dirr R, Martin E, Boesch C, Largo RH, Fanconi S, et al. MRI following severe perinatal asphyxia: preliminary experience. Pediatr Neurol 1991;7:164–70 [DOI] [PubMed] [Google Scholar]
- 6.Counsell SJ, Maalouf EF, Fletcher AM, Duggan P, Battin M, Lewis HJ, et al. MR imaging assessment of myelination in the very preterm brain. AJNR Am J Neuroradiol 2002;23:872–81 [PMC free article] [PubMed] [Google Scholar]
- 7.Groenendaal F, Leusink C, Nijenhuis M, Janssen MJ. Neonatal life support during magnetic resonance imaging. J Med Eng Technol 2002;26:71–4 [DOI] [PubMed] [Google Scholar]
- 8.Dumoulin CL, Rohling KW, Piel JE, Rossi CJ, Giaquinto RO, Watkins DR, et al. An MRI compatible neonate incubator. Concepts Magn Reson B 2002;15:117–28 [Google Scholar]
- 9.Bartha AI, Yap KR, Miller SP, Jeremy RJ, Nishimoto M, Vigneron DB, et al. The normal neonatal brain MR imaging, diffusion tensor imaging, and 3D MR spectroscopy in healthy term neonates. AJNR Am J Neuroradiol 2007:28:1015–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim DH, Barkovich AJ, Vigneron DB. Short echo time MR spectroscopic imaging for neonatal pediatric imaging. AJNR Am J Neuroradiol 2006;27:1370–2 [PMC free article] [PubMed] [Google Scholar]
- 11.Erberich SG, Friedlich P, Seri I, Nelson MD, Jr, Blüml S. Functional MRI in neonates using a neonatal head coil and MR compatible incubator. Neuroimage 2003;20:683–92 [DOI] [PubMed] [Google Scholar]
- 12.Blüml S, Friedlich P, Erberich S, Wood JC, Seri I, Nelson MD., Jr MR imaging of newborns by using an MR-compatible incubator with integrated radiofrequency coils: initial experience. Radiology 2004;231:594–601 [DOI] [PubMed] [Google Scholar]
- 13.Whitby EH, Griffiths PD, Lonneker-Lammers T, Srinivasan R, Connolly DJ, Capener D, et al. Ultrafast magnetic resonance imaging of the neonate in a magnetic resonance-compatible incubator with a built-in coil. Pediatrics 2004;113:150–2 [DOI] [PubMed] [Google Scholar]
- 14.Bohnen TM. Incubators for magnetic resonance imaging use. Griffiths PD, Paley MNJ, Whitby EH, Imaging the central nervous system of the fetus and neonate. New York, NY: Taylor and Francis Group, 2006. 233–44 [Google Scholar]
- 15.Chau V, Poskitt KJ, Sargent MA, Lupton BA, Hill A, Roland E, et al. Comparison of computer tomography and magnetic resonance imaging scans on the third day of life in term newborns with neonatal encephalopathy comparison of computer tomography and magnetic resonance imaging scans. Pediatrics 2009;123:319–26 [DOI] [PubMed] [Google Scholar]
- 16.Mathur AM, Neil JJ, McKinstry RC, Inder TE. Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol 2008;38:260–4 [DOI] [PubMed] [Google Scholar]