Abstract
Objective
It is not established whether myalgic encephalomyelitis/chronic fatigue syndrome (CFS) is associated with structural brain changes. The aim of this study was to investigate this by conducting the largest voxel-based morphometry study to date in CFS.
Methods
High-resolution structural 3 T cerebral MRI scanning was carried out in 26 patients with CFS and 26 age- and gender-matched healthy volunteers. Voxel-wise generalised linear modelling was applied to the processed MR data using permutation-based non-parametric testing, forming clusters at t>2.3 and testing clusters for significance at p<0.05, corrected for multiple comparisons across space.
Results
Significant voxels (p<0.05, corrected for multiple comparisons) depicting reduced grey matter volume in the CFS group were noted in the occipital lobes (right and left occipital poles; left lateral occipital cortex, superior division; and left supracalcrine cortex), the right angular gyrus and the posterior division of the left parahippocampal gyrus. Significant voxels (p<0.05, corrected for multiple comparisons) depicting reduced white matter volume in the CFS group were also noted in the left occipital lobe.
Conclusion
These data support the hypothesis that significant neuroanatomical changes occur in CFS, and are consistent with the complaint of impaired memory that is common in this illness; they also suggest that subtle abnormalities in visual processing, and discrepancies between intended actions and consequent movements, may occur in CFS.
Myalgic encephalomyelitis, or chronic fatigue syndrome (CFS), as defined by the revised diagnostic criteria of the Centers for Disease Control and Prevention, is mainly characterised by persistent or relapsing fatigue lasting for at least 6 consecutive months [1]. As the aetiology of the disorder is currently unknown, it is important to establish whether it is associated with cerebral abnormalities; however, MRI has provided conflicting results when used to search for brain abnormalities in sufferers [2].
A recent, large British MRI study by Perrin et al [2] of 18 CFS patients and 9 healthy volunteers, in which the images were examined for abnormalities in brain atrophy, deep white matter hyperintensities, and cerebral blood and cerebrospinal fluid flow, reported no significant differences in brain structure between the 2 groups at either baseline or 1-year follow-up, with the authors concluding that “These results throw open the debate into whether MRI scanning can reveal diagnostic signs of CFS and clinically questions the diagnoses of CFS made on the basis of previous research conclusions.”
A small number of previous cerebral MRI studies have been conducted in CFS. A 1993 study involving the comparison by two radiologists of the scans of CFS patients and of controls whom had undergone imaging because of histories of head trauma or headache reported that the former had significantly more abnormal scans than controls (27% vs 2%) [3]; abnormalities included foci of increased white matter T2 signal in 17% of the CFS patients and ventricular or sulcal enlargement in 10%. On the other hand, a 1997 study of white matter abnormalities found no significant difference between CFS patients and controls [4]. A 1999 study involving the comparison of MR scans by two to three radiologists found, overall, no significant differences between CFS patients and healthy controls, although those CFS patients without a psychiatric diagnosis since illness onset had more brain abnormalities on T2 weighted images (mostly small, punctate, subcortical white matter hyperintensities, predominantly in the frontal lobes) than patients with such a diagnosis [5].
Brain MRI analysis using voxel-based morphometry offers advantages over the methodologies used in the above studies. It is an objective method that is not operator dependent and that does not require a priori information about the location of possible differences between groups. The technique involves spatially normalising all the MR images to the same stereotactic space (by registering each of the images to the same template image, by minimising the residual sum of squared differences between them), segmenting the grey matter from the normalised images, correcting for volume changes arising from spatial normalisation and, finally, carrying out a statistical analysis to localise differences between groups; the output from the method is a statistical parametric map that shows regions where grey matter concentration differs significantly between groups [6,7].
Thus far, just one voxel-based morphometry study of CFS has been published. In this 2004 Japanese study of 16 CFS patients, reduced grey matter volume was reported in the bilateral prefrontal cortex [8]. This represents the first report of focal grey matter atrophy in the prefrontal cortex of CFS patients. There have been no attempts, until now, to replicate this finding.
Here, we report the largest voxel-based morphometry study of the brain in CFS.
Methods and materials
Subjects
26 patients and 26 normal controls underwent cerebral structural MRI. All the patients met the revised diagnostic criteria for CFS of the Centers for Disease Control and Prevention [1], with a mean duration of symptoms of 10.9 [standard error (SE) 1.7] years; none of the healthy controls met the CFS criteria, nor did they suffer from undue fatigue or from any history of neurological or psychiatric disorder. The study was carried out according to the Declaration of Helsinki. Each subject gave written informed consent. The study had research ethics committee approval.
Imaging
High-resolution three-dimensional T1 weighted turbo field echo anatomical images of the brain were acquired on all subjects using the same 3 T Philips Achieva system (Philips Healthcare, Best, the Netherlands) as a series of 150 sagittal slices [1.15 mm slice thickness, 208×208 matrix, repetition time (TR)=0.7 ms, echo time (TE) 4.6 ms (in-phase), flip angle=8°, maximum water-fat shift, turbo field echo shot interval=1779.5 ms, minimum time interval (TI) delay=562.3 ms] with a Cartesian acquisition mode, linear profile order and using cerebral grey matter as the reference tissue; specific absorption rate <0.5 W kg−1.
Voxel-based morphometry protocol
The structural data were analysed with FSL-VBM (The Oxford Centre for Functional MRI of the Brain, Oxford, UK), a voxel-based morphometry-style analysis [6,9] carried out with The Oxford Centre for Functional MRI of the Brain software library tools [10]. First, the values in the T1 images were scaled to lie between 0 and 10 000, and these rescaled structural images were brain-extracted using the brain extraction tool [11]. Next, tissue-type segmentation was carried out using The Oxford Centre for Functional MRI of the Brain automated segmentation tool [12]. The resulting grey matter partial volume images were then aligned to MNI152 standard space using affine registration [13]. The resulting images were averaged to create a study-specific template, to which the native grey matter images were then non-linearly reregistered. The registered partial volume images were then modulated (to correct for local expansion or contraction owing to the non-linear component of the transformation) by dividing by the Jacobian of the warp field. The modulated segmentated images were then smoothed with an isotropic gaussian kernel with a sigma of 3 mm.
Statistical analyses
Statistical analyses were carried out using SPSS v. 16 statistical program (SPSS Inc., Chicago, IL). In the voxel-based morphometry analysis, randomised testing with 5000 permutations was used for statistical inference. Voxel-wise generalised linear modelling was applied using permutation-based non-parametric testing, forming clusters at t>2.3 and testing clusters for significance at p<0.05, corrected for multiple comparisons across space. Significant clusters were then overlaid on the MNI152 template.
Results
Subjects
The mean (±SE) age of the patients (42.9±2.2 years) did not differ significantly from that of the healthy controls (38.2±2.2 years, p>0.05). The male-to-female ratio of the patients (7:19) also did not differ significantly from that of the controls (13:13, p>0.05).
Voxel-wise analyses
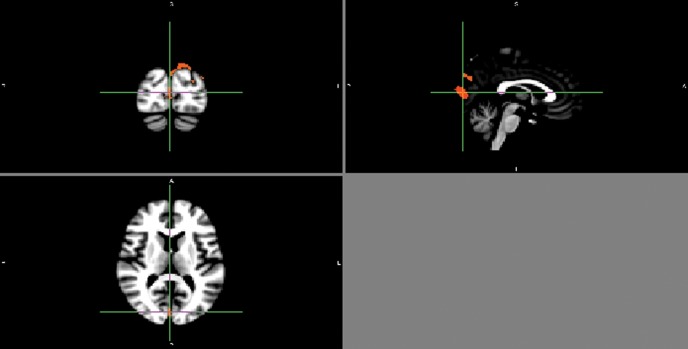
Significant voxels (p<0.05, corrected for multiple comparisons) depicting reduced grey matter volume in the CFS group compared with the control group were noted in the occipital lobes (right and left occipital poles; left lateral occipital cortex, superior division; and left supracalcrine cortex); the right angular gyrus; and the left parahippocampal gyrus, posterior division. Significant voxels (p<0.05, corrected for multiple comparisons) depicting reduced white matter volume in the CFS group were also noted in the left occipital lobe. All these anatomical locations were confirmed by an operator-independent electronic atlas, namely the Harvard-Oxford Cortical Structural Atlas. Figure 1 shows p-value maps in which some of these significant clusters, corrected for multiple comparisons, have been overlaid on the MNI152 template.
Figure 1.
A p-value map in which significant clusters, corrected for multiple comparisons, have been overlaid on the MNI152 template. The upper left panel shows the coronal plane, the upper right panel the sagittal plane and the lower panel the transverse plane.
Discussion
This largest voxel-based morphometry study of CFS has shown evidence of reduced grey and white matter volume in the occipital lobes, as well as reduced grey matter in the right angular gyrus and the left parahippocampal gyrus. Thus, in contrast to the recent report in this journal by Perrin et al [2] in Manchester, our study confirms that, using voxel-based morphometry, there are indeed significant neuroanatomical changes in CFS.
We have not replicated the findings of the first voxel-based morphometry study [8]; this may be (at least partly) a result of the fact that we studied 63% more CFS patients in our voxel-based morphometry analysis.
The occipital lobe is well established as being a part of the brain involved in visual processing. The angular gyrus is a circumscribed area strategically positioned between the parietal and temporal lobes, and close to the occipital lobe; its functions have been unclear until recently [14]. The right angular gyrus has now been shown to have a critical role in perceptual sequence learning [15]. It also computes action awareness representations; in particular, it is associated with both awareness of discrepancy between intended action and movement consequences, and awareness of action authorship [16]. Farrer et al [16] have proposed that the right angular gyrus is involved in higher-order aspects of motor control that allow one consciously to access different aspects of one's own actions; specifically, it processes discrepancies between intended action and movement consequences in such a way that these will be consciously detected by the subject: this joint processing is at the core of experiences used to interpret one's actions. On the basis of our results in the occipital lobes and right angular gyrus, we would suggest that subtle abnormalities in visual processing, and discrepancies between intended actions and consequent movements, should be investigated in CFS patients.
The parahippocampal gyrus is important in such mnemonic functions as encoding and retrieval. The fact that we found reduced grey matter in the posterior part of the left parahippocampal gyrus is of particular interest in light of a recent MRI study by Burgmans et al [17] showing that the posterior parahippocampal gyrus is preferentially affected in age-related memory decline. Impaired memory is indeed recognised as a clinical feature of CFS.
The potential implications of these findings in respect of the aetiology and pathophysiology of myalgic encephalomyelitis/CFS is of interest. This disease is of unknown aetiology, but recently there has been renewed speculation that it may be related to persistent viral infection. Such infections are likely to impair the ability of the body to biosynthesise n-3 and n-6 long-chain polyunsaturated fatty acids by inhibiting the Δ-6 desaturation of the precursor essential fatty acids α-linolenic acid and linoleic acid, which would in turn impair the proper functioning of cell membranes, including cell signalling, and have an adverse effect on the biosynthesis of eicosanoids from the long-chain polyunsaturated fatty acids dihomo-γ-linolenic acid, arachidonic acid and eicosapentaenoic acid [18]. The resulting reduced availability of appropriate long-chain polyunsaturated fatty acids at the Sn2 position of phospholipid molecules might be expected to be associated with a reduced rate of anabolism of membrane phospholipids, and therefore a relative increase in the level of free choline, which would otherwise form the polar head groups of some of these phospholipid molecules. Interestingly, an increase in the choline resonance has been reported in systematic controlled proton MR neurospectroscopy studies in this patient group [19,20]. Moreover, the first of these studies specifically reported an increased level in the occipital lobe, which is consistent with our present finding of reduced grey and white matter volume in this part of the brain.
The related issue also arises as to whether the present study would have produced similar results if the mean duration of symptoms in the patients had been much less than 10.9 years. Clearly, a longitudinal study would be an appropriate way of addressing this issue. In the absence of such data, however, it may still be possible to shed some light on the likely answer by examining the published results of a non-systematic proton neurospectroscopy study of choline resonances in myalgic encephalomyelitis/CFS of short duration. Tomoda et al [21] examined three cases (11-, 12- and 13-year-old children) with illness of relatively recent origin; all three children showed marked elevations in the choline resonances. These results would tend to suggest that the brain changes are of relatively early onset and that, accordingly, our study might have produced similar results if the mean duration of symptoms had been much less than 10.9 years.
In summary, the present study supports the hypothesis that CFS patients have structural abnormalities in the brain [22].
Acknowledgments
We also wish to acknowledge the involvement of several myalgic encephalomyelitis/CFS charities, including ME Research UK (formerly known as MERGE) and ME Solutions. We are very grateful to the patients, their families and the healthy volunteers who took part in this study.
Footnotes
We should like to thank the MRC for funding this study.
References
- 1.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Intern Med 1994;121:953–9 [DOI] [PubMed] [Google Scholar]
- 2.Perrin R, Embleton K, Pentreath VW, Jackson A. Longitudinal MRI shows no cerebral abnormality in chronic fatigue syndrome. Br J Radiol 2010;83:419–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Natelson BH, Cohen JM, Brassloff I, Lee HJ. A controlled study of brain magnetic resonance imaging in patients with the chronic fatigue syndrome. J Neurol Sci 1993;120:213–17 [DOI] [PubMed] [Google Scholar]
- 4.Greco A, Tannock C, Brostoff J, Costa DC. Brain MR in chronic fatigue syndrome. AJNR Am J Neuroradiol 1997;18:1265–9 [PMC free article] [PubMed] [Google Scholar]
- 5.Lange G, DeLuca J, Maldjian JA, Lee H, Tiersky LA, Natelson BH. Brain MRI abnormalities exist in a subset of patients with chronic fatigue syndrome. J Neurol Sci 1999;171:3–7 [DOI] [PubMed] [Google Scholar]
- 6.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage 2000;11:805–21 [DOI] [PubMed] [Google Scholar]
- 7.May A, Ashburner J, Buchel C, McGonigle DJ, Friston KJ, Frackowiak RS, et al. Correlation between structural and functional changes in brain in an idiopathic headache syndrome. Nat Med 1999;5:836–8 [DOI] [PubMed] [Google Scholar]
- 8.Okada T, Tanaka M, Kuratsune H, Watanabe Y, Sadato N. Mechanisms underlying fatigue: a voxel-based morphometric study of chronic fatigue syndrome. BMC Neurol 2004;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001;14:21–36 [DOI] [PubMed] [Google Scholar]
- 10.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23:S208–19 [DOI] [PubMed] [Google Scholar]
- 11.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002;17:143–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 2001;20:45–57 [DOI] [PubMed] [Google Scholar]
- 13.Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging 1999;18:712–21 [DOI] [PubMed] [Google Scholar]
- 14.Kombos T, Picht T, Suess O. Electrical excitability of the angular gyrus. J Clin Neurophysiol 2008;25:340–5 [DOI] [PubMed] [Google Scholar]
- 15.Rosenthal CR, Roche-Kelly EE, Husain M, Kennard C. Response-dependent contributions of human primary motor cortex and angular gyrus to manual and perceptual sequence learning. J Neurosci 2009;29:15115–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrer C, Frey SH, Van Horn JD, Tunik E, Turk D, Inati S, et al. The angular gyrus computes action awareness representations. Cereb Cortex 2008;18:254–61 [DOI] [PubMed] [Google Scholar]
- 17.Burgmans S, van Boxtel MP, van denBerg KE, Gronenschild EH, Jacobs HI, Jolles J, et al. The posterior parahippocampal gyrus is preferentially affected in age-related memory decline. Neurobiol Aging 2009;32:1572–8 [DOI] [PubMed] [Google Scholar]
- 18.Puri BK. Long-chain polyunsaturated fatty acids and the pathophysiology of myalgic encephalomyelitis (chronic fatigue syndrome). J Clin Pathol 2007;60:122–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puri BK, Counsell SJ, Zaman R, Main J, Collins AG, Hajnal JV, et al. Relative increase in choline in the occipital cortex in chronic fatigue syndrome. Acta Psychiatr Scand 2002;106:224–6 [DOI] [PubMed] [Google Scholar]
- 20.Chaudhuri A, Condon BR, Gow JW, Brennan D, Hadley DM. Proton magnetic resonance spectroscopy of basal ganglia in chronic fatigue syndrome. Neuroreport 2003;14:225–8 [DOI] [PubMed] [Google Scholar]
- 21.Tomoda A, Miike T, Yamada E, Honda H, Moroi T, Ogawa M, et al. Chronic fatigue syndrome in childhood. Brain Dev 2000;22:60–4 [DOI] [PubMed] [Google Scholar]
- 22.Chen R, Liang FX, Moriya J, Yamakawa J, Sumino H, Kanda T, et al. Chronic fatigue syndrome and the central nervous system. J Int Med Res 2008;36:867–74 [DOI] [PubMed] [Google Scholar]