Abstract
Objectives
Musculoskeletal structures often appear brighter on imaging in the elderly, which makes it difficult to accurately delineate a peripheral nerve during ultrasound-guided regional anaesthetic procedures. The echo intensity of skeletal muscles is significantly increased in the elderly. However, there are no data comparing the echo intensity of peripheral nerves in the young and the elderly, which this study was designed to evaluate.
Methods
13 healthy, young volunteers (aged <30 years) and 11 elderly patients (aged >60 years) who were scheduled to undergo orthopaedic lower limb surgery were recruited. The settings of the ultrasound system were standardised and a high-frequency linear array transducer was used for the scan. A transverse scan of the median nerve (MN) and the flexor muscles (FMs) at the left mid-forearm was performed and three video loops of the ultrasound scan were recorded for each subject. Still images were captured from the video loops and normalised. Computer-assisted greyscale analysis was then performed on these images to determine the echo intensity of the MN and the FMs of the forearm.
Results
The echo intensity of the MN and FMs of the mid-forearm was significantly increased in the elderly (p<0.005). There was also a reduction in contrast between the MN and the adjoining FM in the elderly (p=0.04).
Conclusion
Under the conditions of this study, the MN and the FMs in the forearm appeared significantly brighter than those in the young, and there was a loss of contrast between these structures in sonograms of the elderly.
Recently, there has been an increase in interest in the use of ultrasound to guide peripheral nerve blocks [1-3]. We have observed during such procedures that musculoskeletal structures often appear significantly brighter and that there is loss of contrast between the nerve and its adjoining muscles in the elderly, which often makes it difficult to accurately delineate a peripheral nerve using ultrasound in this age group. There are published data showing that the echo intensity (EI) of skeletal muscles is significantly increased in the elderly [4]. However, there are no data comparing the EI of a peripheral nerve in the young and the elderly, which this study was designed to evaluate.
Methods and materials
The study was approved by the research ethics committee (The Joint CUHK-NTEC Clinical Research Ethics Committee, Hong Kong, People's Republic of China), and written informed consent was obtained from all subjects. 13 healthy young volunteers (<30 years old, group Y) and 11 elderly patients (>60 years old, group E) who were due to undergo orthopaedic lower limb surgery at the Department of Anaesthesia and Intensive Care, the Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong, China, were recruited for the study. Patients were excluded if they had diabetes mellitus, documented peripheral or autonomic neuropathy or musculoskeletal disorders, or if they had performed physical exercise prior to the ultrasound examination. The median nerve (MN) and the adjoining flexor muscles (flexor digitorum superficialis, flexor pollicis longus and flexor digitorum profundus; FMs) in the mid-forearm were selected for the ultrasound scan because they are relatively superficial structures and readily accessible for ultrasound imaging. The ultrasound scans were performed by a single investigator (MK), who was experienced in ultrasound imaging, using the same ultrasound system (MicroMaxx v.3.1, ARM 30.80.301.014 with no autogain or compound imaging facility; Sonosite Inc., Bothell, WA) and a high-frequency linear array transducer (HFL38, 13–6 MHz, 38 mm footprint). Since the EI of musculoskeletal structures is affected by the amount of gain used during the ultrasound examination, the gain setting was standardised for every subject. This was done by setting the delta key in the ultrasound system to “reset to factory defaults”, on the advice of the manufacturer, to ensure that the starting gain was the same for every patient whenever an examination was commenced after booting the ultrasound machine and it was not changed during the scan. The “small parts” preset, “general” image optimisation setting and “dynamic range” (preset=0) were chosen before every scan and the MN was focused so that it was in the centre of the image. The “depth-setting” was also standardised to 2.7 cm, which was considered adequate for imaging the MN and the FMs in the mid-forearm. These standardised scan settings were used in every subject studied. The ultrasound scans were performed on the left mid-forearm with the subject lying comfortably in the supine position. The left arm was abducted and externally rotated, with the palm of the hand facing the ceiling, and resting on a padded arm rest. The left arm was chosen for the ultrasound scan because it is readily accessible to the anaesthetist during anaesthesia. Moreover, there are no published data showing that there are differences in EI of the FMs in the dominant and non-dominant arms. A liberal amount of ultrasound gel was applied for acoustic coupling between the skin and the ultrasound transducer and it was positioned midway between the flexor crease of the elbow joint and the distal flexor crease of the wrist joint. Transverse scans of the MN and the FMs of the forearm were thereby obtained (Figures 1 and 2). Care was taken not to exert undue pressure over the area scanned because pressure can affect the greyscale intensity of the pixels in the image. Moreover, to minimise anisotropy (angular dependence), the transducer was also aligned such that it was perpendicular to the forearm bones and the MN. This was done by gently tilting the transducer during the scan until maximum echogenicity of the bones and the MN was achieved. Once an optimal image of the MN and FM was obtained, three video loops (AVI format, 6 s each) were recorded onto the compact flash card of the ultrasound machine in each subject for analysis at a later date. Video loops as opposed to still images were recorded during the scan to ensure than an optimal still image was captured at a later stage for the computer-assisted greyscale analysis.
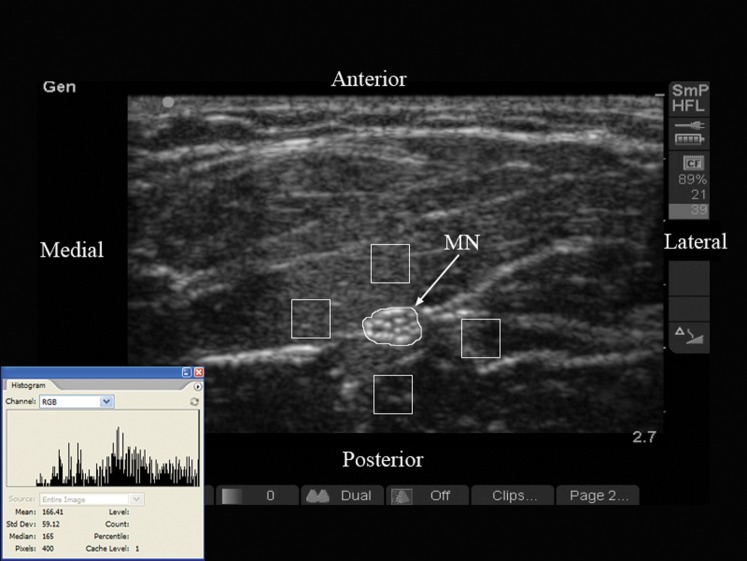
Figure 1.
Typical transverse sonogram of the forearm in a young subject (male, 26 years old). Note the polygonal region of interest (ROI) that has been placed around the outlines of the median nerve (MN), and the four ROI boxes with dimensions of 40×40 pixels that have been placed anterior, lateral, posterior and medial to the MN on the flexor muscles of the forearm. The inset image shows the histogram of the echo intensity of the ROI over the MN. Numbers on the right margin of the image indicate the amount of storage space (89%) remaining in the compact flash card and the number of still (21) and video loop (39) images stored in the card.
Figure 2.
Typical transverse sonogram of the forearm in an elderly subject (female, 80 years old). Note the polygonal region of interest (ROI) that has been placed around the outlines of the median nerve (MN) and the four ROI boxes with dimensions 40×40 pixels that have been placed anterior, lateral, posterior and medial to the MN on the flexor muscles of the forearm. Inset showing the histogram of the echo intensity of ROI over the MN. Numbers on the right margin of the image indicate the amount of storage space (95%) remaining in the compact flash card and the number of still (6) and video loops (13) stored in the card.
The digital video images were processed by a single investigator (XL). SiteLink Image Manager 3.4.1 (Sonosite Inc.) software was used to transfer the video loops from the ultrasound system to a laptop computer. Representative optimal still images (three per subject, TIFF, 720×480 pixels and 8 bit grey levels) were then captured from the video loops using Adobe Premier Pro 2.0 (Adobe Systems Inc., San Jose, CA) and randomly assigned a file number (1–72) to blind the investigator, who later performed the computer-assisted greyscale analysis on these images, from patient details. The still images were then “normalised” (Adobe Photoshop CS2; Adobe Systems Inc.) to ensure that the brightness and contrast of the whole image was at the same level before any greyscale median (GSM) measurement. Normalisation is a simple digital image-processing technique that attempts to evenly distribute the intensities in an image by stretching the range of intensity values that it contains over a desired range (0–255). It is used as a pre-processing step to aid computer-assisted greyscale analysis [4,5] and is often done to eliminate image variations that are due to conditions of image acquisition and not related to the object identity. However, although the intensity of individual pixels in the image may change during normalisation, the brightness of the pixels relative to one another remains the same. In this study, normalisation was performed by initially defining the GSM of two reference points (background, black; text, pure white) in the two-dimensional (TIFF) image. Algebraic (linear) scaling was then performed using the “curves function” in Adobe Photoshop CS2 such that the GSM of the pure black equalled 0 and that of the pure white equalled 255 in the resultant image. This way, the greyscale values of all the pixels in the images were adjusted according to the input and output values of the two reference points. Computer-assisted greyscale analysis (Adobe Photoshop CS2) was then performed on these images, to measure the EI of the MN and the FMs, by a computer technician who was blinded to patient details and otherwise not involved in the study. A polygonal region of interest (ROI) was drawn around the outlines of the MN using the freehand lasso tool in Adobe Photoshop CS2 and four ROI boxes with dimension 40×40 pixels were placed anterior, lateral, posterior and medial to the MN on the FMs (Figures 1 and 2). Four small ROI boxes, as opposed to a single large ROI box, were used to measure the EI of the FMs because the MN is located between the FMs in the forearm, which made it difficult to include the FMs without the MN. We also hoped that this would minimise selection bias and intra-observer variability. While placing the ROI boxes on the muscles, care was taken to include only muscle in the ROI box and exclude any fascia or bone. The greyscale intensity or EI (mean (standard deviation (SD)) and median (range)) values of the pixels inside the ROI were determined using the standard histogram function in Adobe Photoshop CS2 [4,5]. An EI value of 0 represents “pure black” and a value of 255 represents pure white. The EI measurements were also repeated to test for intra-observer and interobserver reproducibility. 10 images (from 5 young subjects) were randomly selected and they were tested twice by the first observer (XL) on two different days, and the process was repeated by a second observer (TL, computer technician).
Prospective power analysis based on pilot data showed that 9 subjects per group would have an 80% power to detect a mean difference of 60 in the EI of the MN, the primary outcome variable of this study, assuming that the common SD was 40 using a 2 group t-test with a 0.05 2-sided significance level. The data were analysed using SPSS for Windows (version 14; IBM Corporation, Armonk, NY). The Kolmogorov–Smirnov test was used to test the normality of the data recorded. If the data were normally distributed, they are presented as a mean [SD; 95% confidence interval (CI)], otherwise they are presented as a median (range). The mean EIs of the MN from the three images from each subject were averaged into one group mean EI for statistical analysis (Table 1). The mean EI at each ROI box in the FMs was also determined for the two study groups and one-way repeated measures analysis of variance (ANOVA) was used to compare the differences in EI at these four sites (Table 2). The mean EIs of the FMs from the four ROI boxes were then averaged into one mean EI for the group (Table 1). The contrast between the MN and the FMs was also determined by subtracting the EI of the FM from that of the MN (Table 1). An independent sample t-test was used to test the differences in the EI of the MN and the FMs, and the contrast between the MN and the FMs between the two study groups. ANOVA was also performed to test the effect of the study groups on the EI of the MN and the FMs, and the contrast between the nerve and muscles. The χ2 test was used to compare the number of male and female subjects in the two study groups. Interobserver and intra-observer variability was tested using the correlation coefficient. The paired samples t-test was used to compare the differences in the results obtained by the two observers, and that on both days that the EI measurements were made. This also allowed us to determine the reliability and consistency of the EI measurement. A p-value of <0.05 was considered statistically significant.
Table 1. Echo intensity (EI) of the median nerve and flexor muscles of the forearm, and the contrast between the nerve and muscle in the young and elderly subjects.
| Young (n=13) | Elderly (n=13) | p-value | |
| EI of median nerve | 126 (11.6; 119–133) | 153.3 (13.1; 144.5–162.2) | <0.0005 |
| EI of flexor muscles | 50.7 (5.3; 47.4–53.9) | 90.2 (15.6; 79.7–100.6) | <0.0005 |
| Contrast between the nerve and muscle | 75.3 (13.4; 67.2–83.4) | 63.2 (15.2; 52.9–73.4) | 0.04 |
Data are presented as mean (standard deviation; 95% confidence interval). p<0.05 was considered statistically significant.
Table 2. Echo intensity of the flexor muscles of the forearm at the four ROI boxes (anterior, lateral, posterior and medial) around the median nerve.
| Group | Anterior | Lateral | Posterior | Medial | p-value |
| Young (n=13) | 52.4 (10.1; 45.6–59.2) | 53.3 (9.5; 45.9–58.7) | 40.7 (9.8; 34.1–47.3) | 53.9 (7.5; 48.9–58.9) | 0.024a |
| Elderly (n=11) | 86.2 (24.2; 69.9–102.4) | 95.9 (16.9; 84.5–107.2) | 85.7 (12; 77.7–93.8) | 89.6 (15.7; 79.1–100.2) | 0.09 |
ROI, region of interests.
Data are presented as mean (standard deviation; 95% confidence interval). p<0.05 was considered statistically significant.
aAnterior vs posterior, p=0.03; lateral vs posterior, p=0.005; medial vs posterior, p=0.001; with adjustments for multiple comparisons.
Results
The mean (SD; range) age of the young group was 25.8 (3.44; 19–30) years, and that of the elderly group was 81.7 (7.48; 67–89) years. The number of male and female subjects in each study group (Group Y, seven males and six females; Group E, two males and nine females) was comparable (p=0.08). The mean (SD; range) weight, height and body mass index (BMI) in group Y was 61.5 (8.92; 48–76) kg, 166 (6.19; 156–175) cm and 21.5 (2.53; 19.4–27.3) kg m–2, respectively. Accurate measures of body habitus of only three subjects were obtained in Group E because most had fractures in their lower limbs and could not be moved out of bed. The mean weight, height and BMI of the three elderly subjects were 60.7 (52–67) kg, 165.7 (157–172) cm and 22 (21.1–22.6) kg m–2, respectively. The mean EI of the MN in Group E was significantly higher than that in Group Y (p<0.0005) (Table 1). The mean EI of the FMs at the four sites (anterior, lateral, posterior and medial to the MN) are presented in Table 2. There was a significant difference in the mean EI of the FMs at the four sites in Group Y (p=0.024) but not in Group E (p=0.09). The overall mean EI of the FMs in Group E was also significantly higher than that in Group Y (p<0.0005). The mean contrast between the MN and the FMs was significantly higher in Group Y than in Group E (p=0.04) (Table 1). Univariate ANOVA showed that the study group had a significant effect on the EI of the MN (p=0.001) and the FMs (p<0.001), and also on the contrast between the MN and the FMs (p=0.001). There were no significant differences between the EI measurements made by the two observers (p=0.89), nor were there any differences between measurements that were made on different days (p=0.55), and the interobserver and intra-observer correlation coefficients were 0.80 and 0.92, respectively. This proves the validity of the method used for measuring the EI in this study.
Discussion
In this study, we used computer-assisted greyscale analysis to measure the EI of the MN and the FMs in the forearm in the young and the elderly. We have demonstrated that the EIs of the MN and the FMs in the forearm are significantly increased, and there is a reduction in the contrast between the MN and the adjoining FMs in the elderly.
We have also demonstrated that computer-assisted greyscale analysis is a valid and reproducible method of measuring the EI of the FMs of the forearm and the MN. This is in agreement with previous reports, which have demonstrated that computer-assisted greyscale analysis is a sensitive, quantitative [4,6], reproducible [6] and valid [6] method of analysing and characterising ultrasound images of skeletal muscles [6], and has been used to determine normal muscle parameters [4,7] and to diagnose [4,7] and follow-up neuromuscular disease [4]. It is more sensitive and accurate, and also achieves a higher interobserver agreement than visual evaluation of skeletal muscle sonograms [5]. We therefore believe that the same should apply when computer-assisted greyscale analysis is used to measure the EI of the MN and FMs in the forearm. Moreover, the ultrasound images from both study groups were processed in the same way and we had determined that the dimension of a pixel in the still images was 0.07×0.07 mm. The average velocity of sound when it travels through soft tissues is 1540 m s–1 and the frequency of ultrasound used in this study was between 13 and 6 MHz. Hence, the resolution of the ultrasound system used was 0.12–0.26 mm (V=f×λ). Therefore, one can conclude that no data were lost during the digitisation of the image, which is in accordance with previously published data [6].
Our results must be interpreted after considering the following facts. We were unable to obtain accurate data on body habitus (weight, height and BMI) in all our elderly subjects, for practical reasons outlined above. It is known that skeletal muscle size and subcutaneous fat thickness varies with age, sex, weight, BMI [4] and whether the muscles are trained or not [8]. Variations in muscle size and subcutaneous fat thickness, by altering the acoustic impedance of the tissues, may affect the EI of a musculoskeletal structure. The BMIs of the young volunteers, and those of the three subjects from Group E, are comparable to those of elderly Hong Kong Chinese patients, calculated from data previously published (BMI 20–24) from our institution [9]. Therefore, we believe that our findings are unlikely to have been influenced by a lack of accurate data on body habitus in the elderly subjects. Moreover, there is fatty replacement of muscles with age and this is more prominent in women. In this study, although there were more female subjects in the elderly group, this was not statistically significant. One may argue that this is due to chance and may influence our results. However, it would require MRI to measure intramuscular fat content, which was not done in this study. Future studies should investigate the possible effects of sex, body weight, height and BMI on the EI of peripheral nerves. Moreover, we also did not measure the distance from the skin to the MN in this study, which may decrease in the elderly compared with that in the young owing to loss of muscle mass. The reduction in depth to the MN may result in less attenuation of the incident ultrasound energy and thereby an apparent increase in the EI of the MN. One may also question the validity of using computer-assisted greyscale analysis rather than the raw digital imaging and communications in medicine (DICOM) data to measure the EI in this study. We did not have a DICOM facility in our ultrasound system and therefore used computer-assisted greyscale analysis to analyse the mean greyscale values of the pixels in the ultrasound images. Our results show that no data were lost in the digitisation of the images. Therefore, the method (computer-assisted greyscale analysis) used to measure the mean greyscale values of the pixels in the ultrasound images is valid. In addition, anisotropy or angular dependence can also affect the echogenicity of an imaged structure [10]. In this study, we tried to minimise the effects of anisotropy by aligning the transducer at right angles to the forearm bones so that it produced maximum echogenicity of the bones and the median nerve, a method that has previously been described [4,5,7,11], and used the average of three EI measurements of the MN and FMs.
The EI of the FMs in the forearm was significantly increased in the elderly subjects. This is in agreement with results previously published by Maurits et al [4], who demonstrated a strong correlation between the EI of muscles (EI of the biceps increases by 1.8% per year and that of the quadriceps increases by 1.9% per year) and age, and suggested that the increased EI of muscles with increasing age was due to age-related changes in the muscle [4]. In the elderly, there is a reduction in skeletal muscle mass [12,13], replacement of the contractile elements in the muscle by fat and connective tissue [4] and an increase in extracellular water content in the muscle [14]. There is also an increase in body fat [13]. Normally, subcutaneous fat, water and skeletal muscle fibres are hypoechoeic, but infiltration of skeletal muscles by fat results in increased muscular EI [15]. This may be due to a change in acoustic impedance at the surface of the fat cells and an increase in scattering of the ultrasound energy by the intramuscular fat. Therefore, the increased EI of the flexor muscles in our elderly subjects may in part be explained by the infiltration of the muscle by fat and connective tissue.
The EI of the FMs posterior to the MN was significantly lower than that in the anterior, lateral and medial sites in the young but not in the elderly. This is an expected finding and is due to attenuation of the ultrasound energy as it travels to depths. In contrast, the reason why comparable differences were not present in the elderly is not clear, but the generalised increase in musculoskeletal EI [4], increased inhomogeneity in skeletal muscle with age [4] and loss of contrast between the nerve and muscle that we have demonstrated in this study may be some of the reasons.
The EI of the MN was also significantly increased in the elderly group. There are no comparable data in the literature but we believe that the increased EI of the MN in our elderly subjects is also due to age-related changes in the MN. The MN, when examined in the transverse axis, using high-frequency ultrasound (13–15 MHz), shows multiple rounded hypoechoic areas in a homogeneous hyperechoic background [16]. The hypoechoic areas correspond to the neuronal fascicles and the hyperechoic background corresponds to the connective tissue layer that binds the neuronal fascicles together [16]. With advancing age, particularly after the sixth decade, there is a marked reduction in the number, diameter and average density of nerve fibres [17,18] and an increase in connective tissue elements [19] (hyperechoic) within a peripheral nerve, which may explain why the EI of the MN was higher in our elderly subjects.
There was also a reduction in contrast between the MN and the adjoining FMs in the elderly. This is not surprising considering that the EI of the MN and FMs was higher in the elderly subjects, and may also explain why it is often difficult to accurately delineate a peripheral nerve using ultrasound in this age group. Currently, there are no data on the success or failure rate or the incidence of needle-related complications after ultrasound-guided peripheral nerve blockade in the elderly, and further research in this area is warranted. There is also a need to develop new strategies to improve the quality of ultrasound images of musculoskeletal structures in the elderly.
In conclusion, we have demonstrated that the EIs of the median nerve and the flexor muscles of the forearm are significantly increased, and there is a reduction in contrast between the MN and the adjoining FMs in the elderly. Age-related changes in the nerve and muscle may explain why the EI of musculoskeletal structures is increased in the elderly.
Acknowledgment
The authors would like to thank Mr Thomas Lo Shek Fai, Technical Developer, Department of Anaesthesia and Intensive Care, The Chinese University of Hong Kong, for his assistance during this study.
References
- 1.Marhofer P, Greher M, Kapral S. Ultrasound guidance in regional anaesthesia. Br J Anaesth 2005;94:7–17 [DOI] [PubMed] [Google Scholar]
- 2.Karmakar MK, Ho AM, Li X, Kwok WH, Tsang K, Ngan Kee WD. Ultrasound-guided lumbar plexus block through the acoustic window of the lumbar ultrasound trident. Br J Anaesth 2008;100:533–7 [DOI] [PubMed] [Google Scholar]
- 3.Karmakar MK, Kwok WH, Ho AM, Tsang K, Chui PT, Gin T. Ultrasound-guided sciatic nerve block: description of a new approach at the subgluteal space. Br J Anaesth 2007;98:390–5 [DOI] [PubMed] [Google Scholar]
- 4.Maurits NM, Bollen AE, Windhausen A, De Jager AE, Van DerHoeven JH. Muscle ultrasound analysis: normal values and differentiation between myopathies and neuropathies. Ultrasound Med Biol 2003;29:215–25 [DOI] [PubMed] [Google Scholar]
- 5.Pillen S, van KM, Nievelstein RA, Verrips A, van Kruijsbergen-Raijmann W, Zwarts MJ. Skeletal muscle ultrasonography: visual versus quantitative evaluation. Ultrasound Med Biol 2006;32:1315–21 [DOI] [PubMed] [Google Scholar]
- 6.Nielsen PK, Jensen BR, Darvann T, Jorgensen K, Bakke M. Quantitative ultrasound image analysis of the supraspinatus muscle. Clin Biomech 2000;15:S13–16 [DOI] [PubMed] [Google Scholar]
- 7.Maurits NM, Beenakker EA, van Schaik DE, Fock JM, van derHoeven JH. Muscle ultrasound in children: normal values and application to neuromuscular disorders. Ultrasound Med Biol 2004;30:1017–27 [DOI] [PubMed] [Google Scholar]
- 8.Sipila S, Suominen H. Muscle ultrasonography and computed tomography in elderly trained and untrained women. Muscle Nerve 1993;16:294–300 [DOI] [PubMed] [Google Scholar]
- 9.Critchley LAH, Conway F. Hypotension during subarachnoid anaesthesia: haemodynamic effects of colloid and metaraminol. Br J Anaesth 1996;76:734–6 [DOI] [PubMed] [Google Scholar]
- 10.Soong J, Schafhalter-Zoppoth I, Gray AT. The importance of transducer angle to ultrasound visibility of the femoral nerve. Reg Anesth Pain Med 2005;30:505. [DOI] [PubMed] [Google Scholar]
- 11.Scholten RR, Pillen S, Verrips A, Zwarts MJ. Quantitative ultrasonography of skeletal muscles in children: normal values. Muscle Nerve 2003;27:693–8 [DOI] [PubMed] [Google Scholar]
- 12.Gallagher D, Visser M, De Meersman RE, Sepulveda D, Baumgartner RN, Pierson RN, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol 1997;83:229–39 [DOI] [PubMed] [Google Scholar]
- 13.Evans WJ. Exercise, nutrition and aging. J Nutr 1992;122:796–801 [DOI] [PubMed] [Google Scholar]
- 14.Tsubahara A, Chino N, Akaboshi K, Okajima Y, Takahashi H. Age-related changes of water and fat content in muscles estimated by magnetic resonance (MR) imaging. Disabil Rehabil 1995;17:298–304 [DOI] [PubMed] [Google Scholar]
- 15.Reimers K, Reimers CD, Wagner S, Paetzke I, Pongratz DE. Skeletal-muscle sonography: a correlative study of echogenicity and morphology. J Ultrasound Med 1993;12:73–7 [DOI] [PubMed] [Google Scholar]
- 16.Silvestri E, Martinoli C, Derchi LE, Bertolotto M, Chiaramondia M, Rosenberg I. Echotexture of peripheral nerves: correlation between US and histologic findings and criteria to differentiate tendons. Radiology 1995;197:291–6 [DOI] [PubMed] [Google Scholar]
- 17.Jacobs JM, Love S. Qualitative and quantitative morphology of human sural nerve at different ages. Brain 1985;108:897–924 [DOI] [PubMed] [Google Scholar]
- 18.Tohgi H, Tsukagoshi H, Toyokura Y. Quantitative changes with age in normal sural nerves. Acta Neuropathol 1977;38:213–20 [DOI] [PubMed] [Google Scholar]
- 19.Cottrell L. Histologic variations with age in apparently normal peripheral nerve trunks. Arch Neurol Psychiatry (Chic) 1940;43:1138–50 [Google Scholar]