Abstract
Objectives
The aim of this study was to evaluate the role of three-dimensional transrectal ultrasound in the diagnosis of prostate cancer.
Methods
A total of 112 patients with elevated serum prostate-specific antigen (PSA) or a positive digital rectal examination were evaluated using three-dimensional greyscale transrectal ultrasound (3D-GS TRUS) and three-dimensional power Doppler sonography (3D-PDS). Target biopsies were obtained together with 12 core systematic biopsies. Pathological results were correlated with the imaging data.
Results
Cancers were detected in 269 biopsy sites from 41 patients. 229 sites of cancer were depicted by 3D-GS TRUS and 213 sites were depicted by 3D-PDS. 30 sites were missed by both 3D-GS TRUS and 3D-PDS. Abnormal prostate images depicted by 3D-GS TRUS and 3D-PDS were associated with lesions with a Gleason score of 6.9 or higher.
Conclusion
The detection rates of prostate cancer were significantly improved with 3D-GS TRUS and 3D-PDS on serum PSA levels >10 ng ml–1 or 20 ng ml–1. 3D-GS TRUS and 3D-PDS may improve the biopsy yield by determining appropriate sites for target and systematic biopsies. The abnormalities detected by 3D ultrasound were associated with moderate- and high-grade prostate cancers. However, based on the number of false-negative TRUS results, the use of systematic prostate biopsies should not be eliminated.
Prostate cancer is a common malignancy in older males. Previous autopsy studies have shown that one-third of males over 50 years old have latent cancer, yet only 10% develop clinically significant carcinomas during their lifetime [1]. The exact mechanism mediating the progression of microfocal cancers into symptomatic forms of the disease has not been elucidated. Since prostate cancers demonstrate remarkably heterogeneous behaviours ranging from slow-growing lesions to aggressive tumours that metastasise rapidly [2], the diagnosis and treatment of prostate cancers is very challenging. The current methods of screening for prostate cancer include measuring serum prostate-specific antigen (PSA) levels, digital rectal examination and transrectal ultrasound (TRUS) scanning and biopsy. However, controversy surrounds which screening method is the most clinically significant for detecting lesions.
Since approximately 20–50% of prostate cancers are invisible by greyscale (GS) TRUS [3], GS TRUS has limited value for detection of prostate cancer [4,5]. In addition, 35% of lesions missed by GS TRUS are moderate- or high-grade tumours [6]. Colour Doppler ultrasound, as an important adjunct to GS TRUS, could improve detection of prostate cancer, although in one study 16% of cases with clinically significant cancer were still missed by this method [7].
Three-dimensional (3D) TRUS is a relatively new imaging modality. Preliminary studies have shown improved cancer detection with 3D TRUS when compared with two-dimensional TRUS [8,9]. However, it is still unknown which malignant lesions may be detected by 3D TRUS. Furthermore, 3D TRUS has not been analysed in correlation with the site-specific biopsy pathological results.
The purpose of this study was to assess the role of 3D-GS TRUS and 3D power Doppler sonography (3D-PDS) in the diagnosis of prostate carcinoma. This study correlated 3D-GS TRUS and 3D-PDS data with biopsy pathological results using a site-by-site analysis that included target and systematic biopsies.
Methods and materials
Patients
A total of 112 consecutive patients (mean age, 73.4 years; range, 45–91 years) with suspected prostate cancer because of either an abnormal digital rectal examination or elevated serum PSA (>4.0 ng ml−1) were enrolled in this study between August 2007 and June 2009. Of these 112 males, the median PSA value was 9.4 ng ml−1 (range, 0.5–500), including 18 patients with PSA levels <4.0 ng ml−1, 40 patients with PSA levels between 4 and 10 ng ml−1, and 54 patients with PSA levels >10 ng ml−1.
Image acquisition and analysis
All patients were examined using a 6–10 MHz end-firing 3D TRUS volume probe on a GE Voluson 730 ultrasound (GE Medical System Kretz Ultrasound, Zipf, Austria). The patients were placed in the left lateral decubitus position with bent legs. The transducer probe was covered with a sterile condom and placed in the rectum.
The prostate was scanned from the apex to the base. The 3D-GS TRUS examination was followed by 3D-PDS. The 3D-GS TRUS examination was carried out using the surface-rendered mode. Prior to 3D-PDS, the Doppler gain was optimised to enable the maximum blood flow signal display in the sample box to be captured without background noise. On the final established image, only the 3D-PDS was displayed without GS information.
All 3D TRUS images were prospectively analysed. Assessment of the images was performed by scrolling through the prostate image in the three planes (transverse, coronal and sagittal). Results were considered to be abnormal when prostates were focal hypoechoic, echogenic or isoechoic with focal contour bulged lesions in the peripheral zone (PZ), had capsular irregularity or had ill-defined peripheral and transitional zone (TZ) demarcation on GS TRUS.
Vascular distribution patterns were evaluated according to the following grading system (1–3): 1, regular and strip-shaped flow distribution in the PZ and/or TZ (Figure 1); 2, asymmetric abundant flow with vascular disorganisation in the PZ; and 3, diffusely increased flow with vascular disorganisation in the PZ and TZ. Grades 2 and 3 were regarded as abnormal, and grade 1 was considered to be normal.
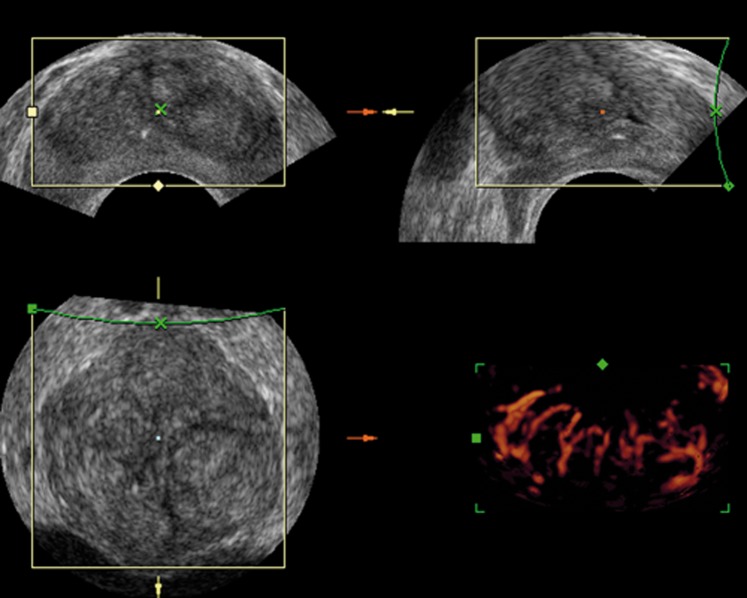
Figure 1.
A normal prostate image on three-dimensional (3D) transrectal ultrasound. 3D greyscale transrectal ultrasound shows well-defined peripheral and transitional zone demarcation. 3D power Doppler sonography shows a regular flow distribution in the prostate gland.
Prostate biopsy and pathological analysis
Prior to the biopsy, the patients were informed of the risks and benefits of the procedure and gave their written consent. After 3D-GS TRUS and 3D-PDS examination, a transrectal biopsy was performed with an 18 gauge needle powered by an automatic device (Pajunk biopsy needles and gun, Geisingen, Germany) on the same day.
One or two core biopsies were taken from each suspicious lesion detected by 3D-GS TRUS and/or 3D-PDS, followed by a systematic 12 core biopsy (6 cores from the base, mid and apex of the PZ bilaterally, 4 cores from the PZ in the bilateral margin and the remaining 2 cores from the TZ bilaterally), as illustrated in Figure 2. In the absence of suspicious 3D-GS TRUS and 3D-PDS images, patients only underwent the systematic 12 core biopsy. Biopsy samples were labelled according to the gland region from where they were obtained and fixed with formaldehyde in separate test tubes for pathological examination.
Figure 2.
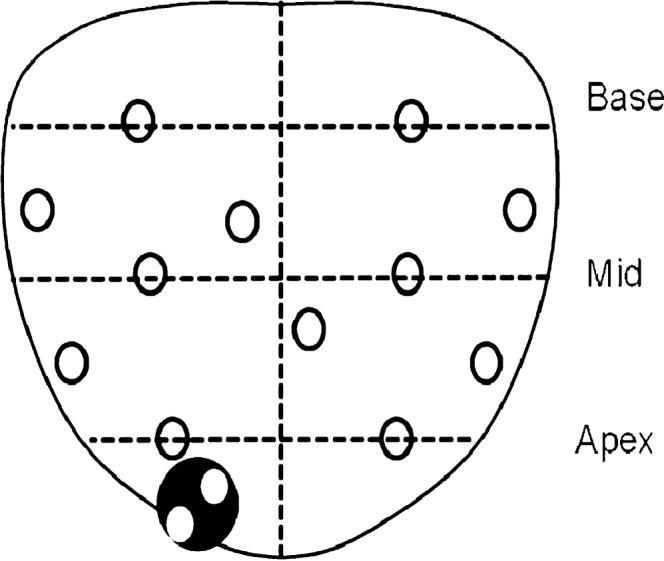
Coronal schematic representation of systematic 12 core and targeted biopsy techniques. Systematic biopsy (white circles) includes samples of both the peripheral and transitional zones. Targeted biopsy (black circle) samples from suspicious areas seen on three-dimensional greyscale transrectal or power Doppler sonography.
Histological analysis was performed by an experienced pathologist using a standard method for preparation and staining of the tissue slices [10]. The grade of the tumour was evaluated and given a standard Gleason score.
Data analysis
Data analysis was carried out according to the following. A true-positive 3D-GS TRUS or 3D-PDS finding was defined as an abnormality on GS or power Doppler ultrasound in the same location that the target and/or systematic biopsy specimen contained malignancy. A true-negative 3D-GS TRUS or 3D-PDS finding was defined as the absence of any abnormality on GS or power Doppler ultrasound and the absence of malignancy on all biopsy specimens. A false-positive finding was defined as the presence of abnormal GS or power Doppler imaging, but an absence of malignancy in that location.
Patients were stratified by serum PSA level and divided into four subgroups (<4 ng ml−1, 4–10 ng ml−1, >10 ng ml−1 and >20 ng ml−1). The cancer detection rates with the three modalities (systematic biopsy, 3D-GS TRUS and 3D-PDS) were calculated by means of the criteria described previously.
All data were analysed using the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL), version 16.0 for Windows. The χ2 test was used for non-parametric comparisons. The κ-test was applied to evaluate the degree of agreement between the 3D-PDS grade and Gleason score obtained from the biopsy samples. A p-value <0.05 was considered to be statistically significant.
Results
37 patients underwent 1 or 2 additional target biopsies besides the systematic 12 core biopsy for the presence of a PZ nodule on 3D-GS TRUS or for focal increased flow (grade 2) on 3D-PDS. 60 patients underwent only the systematic 12 core biopsy because of the absence of suspicious 3D-GS TRUS or 3D-PDS images. 10 patients underwent the systematic 12 core biopsy for diffusely increased flow (grade 3) or ill-defined PZ and TZ demarcation without any obvious PZ nodules on TRUS. The remaining five patients underwent only sextant biopsy because of their low pain tolerance. A total of 1356 core biopsy samples taken from 112 patients were sufficient for histological analysis. Pathological results showed 269 malignant foci and 1087 benign lesions (Table 1).
Table 1. 3D-GS TRUS and 3D-PDS detection of prostate cancer by biopsy site in 112 patients.
| TRUS result | Pathological finding |
|
| Benign | Malignant | |
| 3D-GS TRUS | ||
| Negative result | 791 | 40 |
| Positive result | 296 | 229 |
| 3D-PDS | ||
| Negative result | 846 | 56 |
| Positive result | 241 | 213 |
3D-GS TRUS, three-dimensional greyscale transrectal ultrasound; 3D-PDS, three-dimensional power Doppler sonography.
Transrectal ultrasound diagnostic capability
Of the 269 cancerous foci in 41 patients, 229 (85.1%) corresponded to the site of cancer shown by 3D-GS TRUS, 213 (79.2%) were indicated by abnormal flow on 3D-PDS, 10 were indicated by 3D-PDS only, and 30 were missed by both 3D-GS TRUS and 3D-PDS but were detected by the 12 core biopsy.
The mean Gleason score and median PSA value of the 30 cancerous foci missed by both 3D-GS TRUS and 3D-PDS were 5.1 (range, 3–8) and 8.1 ng ml−1 (range, 2.2–86.1 ng ml−1), respectively. Of these lesions, three or more positive biopsy cores were obtained from the bilateral PZ in five patients, two positive biopsy cores were acquired from the bilateral PZ in one patient, and only one positive biopsy core was obtained in three cases. Histological data revealed extensive lesions in two patients—one patient with one positive biopsy core and one patient with seven positive biopsy cores—who subsequently underwent radical prostatectomy.
37 patients with PZ nodules or focal increasing flow (grade 2) underwent further targeted biopsy sampling in 42 sites indicated as abnormal areas on 3D-GS TRUS or 3D-PDS. 5 of them underwent 2 extra biopsies, and 32 had 1 extra biopsy. Histological examination revealed carcinoma in 27 sites and benign lesions in 15 sites. Of the 15 benign foci, 10 sites revealed benign prostate hyperplasia and 5 sites showed focal chronic prostatitis.
Table 2 shows the systematic biopsy, 3D-GS TRUS and 3D-PDS detected rate of prostate cancer stratified by serum PSA level. The cancer detection rate improved significantly with a PSA level >10 ng ml−1 or 20 ng ml−1.
Table 2. Systematic biopsy, 3D-GS TRUS and 3D-PDS detection of prostate cancer by PSA level in 112 patients.
| PSA (ng ml−1) | Cancer detection rate |
||
| Systematic biopsy | 3D-GS TRUS | 3D-PDS | |
| <4 | 5.6% (1/18) | 0 | 0 (0/2) |
| 4–10 | 25% (10/40) | 45.5% (5/11) | 60% (3/5) |
| >10 | 55.6% (30/54) | 88.9% (24/27) | 80.6% (25/31) |
| >20 | 72.4% (21/29) | 85% (17/20) | 90% (18/20) |
3D-GS TRUS, three-dimensional greyscale transrectal ultrasound; 3D-PDS, three-dimensional power Doppler sonography; PSA, prostate-specific antigen.
Correlation with Gleason score
Figure 3 shows the relationship between 3D-GS TRUS images and the Gleason scores obtained from the biopsy tissue. The most common Gleason scores with positive GS TRUS were 6, 7 and 8, which accounted for 15.7% (36/229), 37.1% (85/229) and 24.5% (56/229) of the samples, respectively. The mean Gleason score of foci with positive GS TRUS images was 7.0, whereas the mean Gleason score of lesions with negative GS TRUS images was 5.0 (χ2=86.03, p<0.05).
Figure 3.
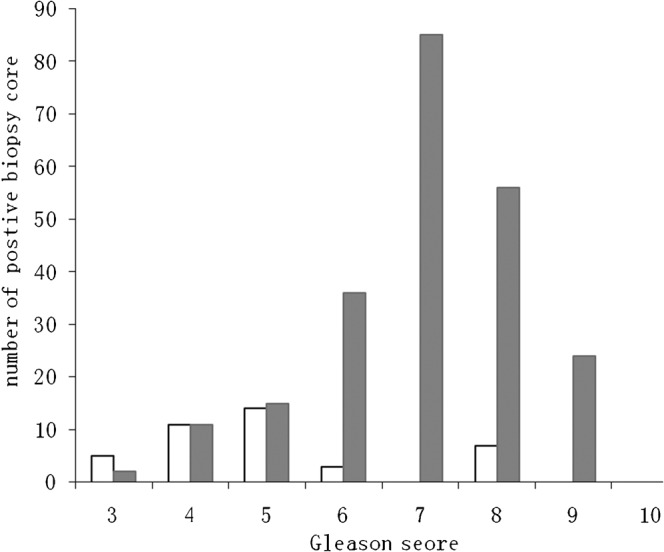
The correlation between three-dimensional (3D) greyscale transrectal ultrasound findings and Gleason score. White bars represent positive biopsy cores with negative results on 3D greyscale transrectal ultrasound; grey bars represent positive biopsy cores with positive results on 3D greyscale transrectal ultrasound.
Figure 4 shows the relationship between 3D-PDS grading and the Gleason scores obtained from the biopsy tissue. The predominant Gleason scores with abnormal 3D-PDS grading (2 or 3) were 7 and 8, representing 36.6% (78/213) and 25.8% (55/213) of the samples, respectively. The mean Gleason score of foci with abnormal flow grading was 6.9, whereas the mean Gleason score of lesions with normal flow grading was 5.9 (χ2=43.27, p<0.05).
Figure 4.
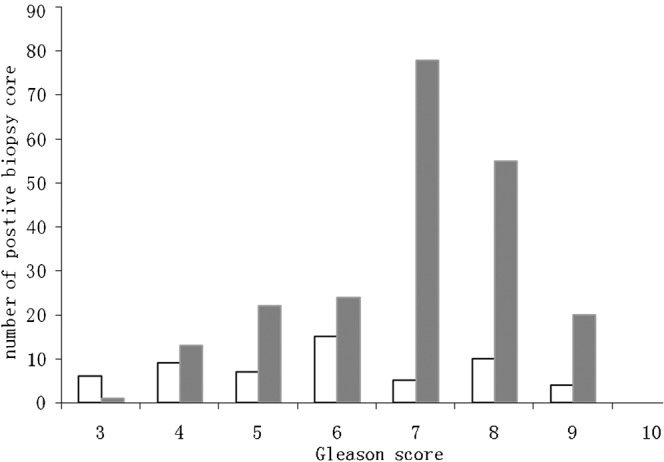
The correlation between three-dimensional (3D) power Doppler sonography findings and Gleason score. White bars represent positive biopsy cores with negative results on 3D power Doppler sonography; grey bars represent positive biopsy cores with positive results on 3D power Doppler sonography.
The relationship between 3D-PDS grading and Gleason scores was further evaluated and a poor correlation between the two was observed (κ=0.23, p<0.05), as summarised in Table 3.
Table 3. Relationship between 3D-PDS grading and histological data by biopsy site in 41 patients with prostate cancer.
| Histological finding | 3D-PDS grading |
Total | ||
| 1 | 2 | 3 | ||
| Gleason score | ||||
| 2–4 | 15 | 14 | 0 | 29 |
| 5–7 | 27 | 103 | 23 | 153 |
| 8–10 | 14 | 41 | 32 | 87 |
| Total | 56 | 158 | 55 | 269 |
3D-PDS, three-dimensional power Doppler sonography.
Discussion
A preliminary study has indicated that 3D TRUS should allow clearer imaging of the prostate anatomy and better visualisation of tumours [11]. Our results indicate that 3D-GS TRUS and 3D-PDS play an important role in guiding prostate biopsies, and in the detection of moderate- and high-grade prostate carcinomas.
In the present study, on the basis of site-by-site analysis, the majority of prostate cancers were detected by 3D-GS TRUS and 3D-PDS coupled with TRUS-guided biopsy. Moreover, with rising serum PSA level, 3D-GS TRUS increased the detection rate of cancer. When the PSA value was ≥20 ng ml−1, the cancer detection rates with 3D-GS TRUS and 3D-PDS were 85% (17/20) and 90% (18/20), respectively. However, we found that using 3D-GS TRUS or 3D-PDS alone could lead to false-negative or -positive results. 40 cancerous foci were missed by 3D-GS TRUS and 56 cancerous foci were missed by 3D-PDS, but detected by the 12 core biopsy. 15 benign foci were erroneously regarded as malignant lesions. Even when data from the two modalities were combined, 30 malignant lesions were still not detected. Moreover, 23 cancerous foci from 6 patients were clinically important cancers based on the PSA level, biopsy finding (Gleason score and positive core) and histological outcome of radical prostatectomy. These findings indicate that systematic biopsies should not be eliminated on the basis of negative 3D-GS TRUS and/or 3D-PDS data.
Difficulties in detecting some prostate carcinomas with 3D-GS TRUS and 3D-PDS may be the result of the pathological characteristics of prostate cancer. 85% of tumours originating from the PZ are present in a multifocal pattern [12]. Furthermore, malignant neoplasms are prone to growing along the capsule of the gland in an oblong shape [13]. On the other hand, the nodules of benign prostatic hyperplasia often distort the normal radial vascular pattern of the gland. If the tumour does not result in significant abnormalities in the contour, echogenicity or vascular patterns of the gland, it may be difficult to depict prostate carcinoma with GS TRUS or power Doppler imaging.
In our study, a significant trend was observed between increasing Gleason score and abnormal 3D-GS TRUS images. Tumours with Gleason scores of ≥7 (177/229) are likely to present abnormal GS TRUS images. This finding is consistent with findings by Toi et al [14], who reported that tumours with a Gleason score of ≥7 were more frequently observed in the TRUS-positive group than in the TRUS-negative group (62 vs 29%, p<0.001). These findings could affect the choice of treatment used for prostate cancer. Patients with moderate- and high-grade tumours have a shorter life expectancy than the general population [15], and would therefore require more aggressive treatment.
Our findings also demonstrated that focal or diffuse increased flow detected by 3D-PDS was associated with high Gleason scores (Figures 5 and 6). Cancers with 3D-PDS flow grades 2 and 3 had higher Gleason scores than grade 1 tumours (mean Gleason score, 6.9 vs 5.9, p<0.05). These findings are consistent with previous reports [5,16]. Increased intratumour flow seems to be a predictor of aggressive biological behaviour of prostate cancer. However, in our study, the poor correlation observed between the Gleason score and flow grading may be due to the heterogeneity in tumour grade within a specific neoplasm that could produce variability in the PDS appearance [17].
Figure 5.
A 69-year-old male suspected of having prostate cancer based on a high serum prostate-specific antigen (100.1 ng ml−1) level. All biopsy samples were positive in the 12 core systematic biopsies. Gleason score 4+4=8. Three-dimensional (3D) greyscale transrectal ultrasound shows ill-defined peripheral and transitional zone demarcation on the transverse, sagittal and coronal planes. 3D power Doppler sonography shows diffusely increased flow in the prostate gland.
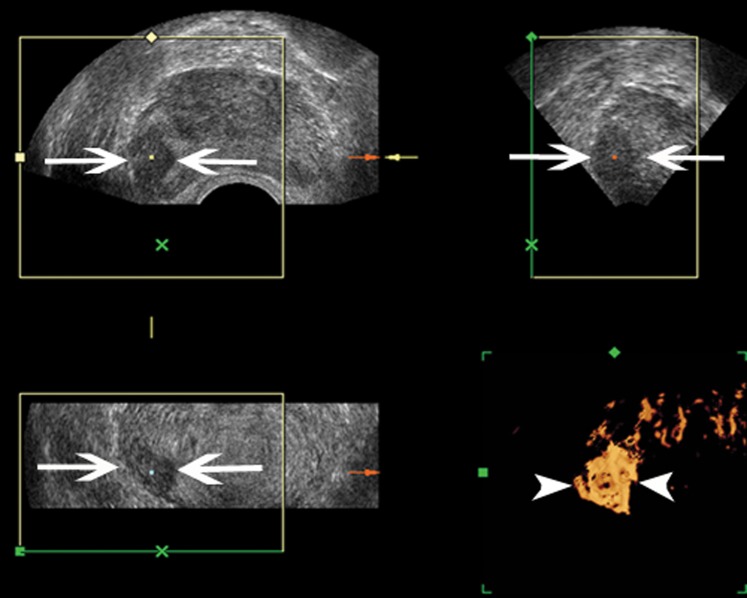
Figure 6.
An 80-year-old male suspected of having prostate cancer based on a borderline elevation in prostate-specific antigen (9.1 ng ml−1) level. Only two biopsy cores were positive in the right peripheral zone. Gleason score 4+4=8. Three-dimensional (3D) greyscale transrectal ultrasound shows an ill-defined hypoechoic nodule in the right peripheral zone on the transverse, sagittal and coronal planes (arrow). 3D power Doppler sonography shows distinct increased Doppler flow within the nodule (arrowhead).
One limitation of this study was that diagnosis and histological grading of prostate cancer were achieved using TRUS-guided needle core biopsies rather than thin-section, whole-mount prostatectomy specimens. It is known that TRUS-guided needle biopsies may produce some false-negative results [18]. In addition, this study was limited by the relatively small number of prostate cancer patients enrolled.
In conclusion, 3D-GS TRUS and 3D-PDS may improve biopsy yield by determining appropriate sites for target and systematic biopsies. Abnormal images obtained by 3D TRUS were strongly associated with moderate- and high-grade prostate cancers. In addition, our findings indicate that systematic biopsies should not be eliminated on the basis of negative 3D-GS TRUS and/or 3D-PDS data.
References
- 1.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA 1994;271:368–74 [PubMed] [Google Scholar]
- 2.Neal D, Donovan J. Prostate cancer: to screen or not to screen? Lancet Oncol 2000;1:17–24 [DOI] [PubMed] [Google Scholar]
- 3.Shinohara K, Wheeler TM, Scardino PT. The appearance of prostate cancer on transrectal ultrasonography: correlation of imaging and pathological examinations. J Urol 1989;142:76–82 [DOI] [PubMed] [Google Scholar]
- 4.Mettlin C, Lee F, Drago J, Murphy GP. The American Cancer Society National Prostate Cancer Detection Project. Findings on the detection of early prostate cancer in 2425 men. Cancer 1991;67:2949–58 [DOI] [PubMed] [Google Scholar]
- 5.Kuligowska E, Barish MA, Fenlon HM, Blake M. Predictors of prostate carcinoma: accuracy of gray-scale and color Doppler US and serum markers. Radiology 2001;220:757–64 [DOI] [PubMed] [Google Scholar]
- 6.Halpern EJ, Strup SE. Using gray-scale and color and power Doppler sonography to detect prostatic cancer. AJR Am J Roentgenol 2000;174:623–7 [DOI] [PubMed] [Google Scholar]
- 7.Lavoipierre AM, Snow RM, Frydenberg M, Gunter D, Reisner G, Royce PL, et al. Prostatic cancer: role of color Doppler imaging in transrectal sonography. AJR Am J Roentgenol 1998;171:205–10 [DOI] [PubMed] [Google Scholar]
- 8.Hamper UM, Trapanotto V, DeJong MR, Sheth S, Caskey CI. Three-dimensional US of the prostate: early experience. Radiology 1999;212:719–23 [DOI] [PubMed] [Google Scholar]
- 9.Sauvain JL, Palascak P, Bourscheid D, Chabi C, Atassi A, Bremon JM, et al. Value of power Doppler and 3D vascular sonography as a method for diagnosis and staging of prostate cancer. Eur Urol 2003;44:21–31 [DOI] [PubMed] [Google Scholar]
- 10.Norberg M, Egevad L, Holmberg L, Sparen P, Norlen BJ, Busch C. The sextant protocol for ultrasound-guided core biopsies of the prostate underestimates the presence of cancer. Urology 1997;50:562–6 [DOI] [PubMed] [Google Scholar]
- 11.Strasser H, Janetschek G, Reissigl A, Bartsch G. Prostate zones in three-dimensional transrectal ultrasound. Urology 1996;47:485–90 [DOI] [PubMed] [Google Scholar]
- 12.Byar D, Mostofi F. Carcinoma of the prostate: prognostic evaluation of certain pathologic features in 208 radical prostatectomies. Cancer 1972;30:5–13 [DOI] [PubMed] [Google Scholar]
- 13.McNeal JE. Origin and development of carcinoma in the prostate. Cancer 1969;23:24–34 [DOI] [PubMed] [Google Scholar]
- 14.Toi A, Neill MG, Lockwood GA, Sweet JM, Tammsalu LA, Fleshner NE. The continuing importance of transrectal ultrasound identification of prostatic lesions. J Urol 2007;177:516–20 [DOI] [PubMed] [Google Scholar]
- 15.Albertsen PC, Fryback DG, Storer BE, Kolon TF, Fine J. Long-term survival among men with conservatively treated localized prostate cancer. JAMA 1995;274:626–31 [PubMed] [Google Scholar]
- 16.Wilson NM, Masoud AM, Barsoum HB, Refaat MM, Moustafa MI, Kamal TA. Correlation of power Doppler with microvessel density in assessing prostate needle biopsy. Clin Radiol 2004;59:946–50 [DOI] [PubMed] [Google Scholar]
- 17.Newman JS, Bree RL, Rubin JM. Prostate cancer: diagnosis with color Doppler sonography with histologic correlation of each biopsy site. Radiology 1995;195:86–90 [DOI] [PubMed] [Google Scholar]
- 18.Rabbani F, Stroumbakis N, Kava BR, Cookson MS, Fair WR. Incidence and clinical significance of false-negative sextant prostate biopsies. J Urol 1998;159:1247–50 [PubMed] [Google Scholar]