Abstract
Carcinoma of unknown primary origin (CUP) accounts for 3–5% of cancer cases and is the fourth most common cause of cancer death in the UK. CUP management is challenging, partly owing to the heterogeneity of the condition and its presentation, but also owing to the lack of dedicated clinical services for these patients. The recent National Institute for Health and Clinical Excellence (NICE) guidelines on metastatic malignancy of unknown primary origin were developed to improve the co-ordination of diagnostic and clinical services at hospitals treating cancer patients in England and Wales, in particular by the setting up of CUP teams to manage these patients. Radiologists have a vital role in the diagnosis of these patients and should work closely with the CUP team to streamline the diagnostic pathway. This article summarises areas of the NICE guidelines relevant to radiology and discusses the radiological management of patients with CUP, including initial investigation, the importance of biopsy, the management of specific presentations, special investigations and organisational issues.
Approximately 10% of newly diagnosed cancer patients present with symptoms secondary to metastases. These presentations are diverse, ranging from palpable lymphadenopathy as the only sign of disease to abdominal distension with ascites or bone pain due to extensive skeletal metastases. In many of these cases, a primary tumour site will be immediately suspected based upon the clinical presentation. These patients will be investigated according to their symptoms and the primary tumour will often be identified. However, in some patients there is no immediately suspected primary site and in a proportion no primary is ever identified, even at post-mortem. The proportion of patients with no identified primary will depend upon the number and type of investigations used, but in most series is estimated at 3–5% of cancer diagnoses [1,2]. The length of the investigative pathway varies greatly and is dependent upon many factors, not least the wishes of the patient.
The management of these patients has generally fallen to specialist clinicians and multidisciplinary teams (MDTs) which deal with cancers with a similar clinical picture, but there has been a lack of standardisation of investigative pathways and a tendency to pass the patient to another MDT when initial investigations are negative. This unco-ordinated approach leads to delays and frustration for the patient and carers. In view of these difficulties, the National Collaborating Centre for Cancer (NCC-C) was commissioned by the National Institute for Health and Clinical Excellence (NICE) to produce clinical guidelines on the diagnosis and management of metastatic malignant disease of unknown primary origin. The guidelines were developed by a guideline development group, incorporating health professionals from a range of specialties and lay representatives, with the support of the NCC-C, and were published in July 2010 [3].
Definitions
When a patient initially presents with a clinical, radiological or pathological diagnosis of malignancy, with no immediately apparent primary site, they can be classified as having metastatic malignancy of undefined primary origin (MUO). In many of these patients, a primary tumour site will be identified following initial simple investigations, such as chest radiograph, serum tumour markers, histological evaluation of a biopsy specimen or CT of the thorax and abdomen. Those patients with no primary identified following initial investigation can be classified as having carcinoma of unknown primary origin (CUP). This group of patients can be subdivided into those who have not (yet) undergone more extensive investigation, provisional CUP, and those patients who have no primary identified despite extensive investigation, confirmed CUP [3]. Although this classification system is somewhat imprecise and artificial, it does help to differentiate between patients at different stages of the investigative pathway and also defines a group of patients who have reached the end of the diagnostic pathway (confirmed CUP). While there is considerable heterogeneity within the confirmed CUP group, there may be a common approach to the management of these patients and opportunities for evaluation of therapies within clinical trials. These terms and their definitions are summarised in Table 1.
Table 1. Terms used in the National Institute for Health and Clinical Excellence (NICE) guidelines [3] (reproduced with the permission of NICE).
| Term | Definition |
| Malignancy of undefined primary origin | Metastatic malignancy identified on the basis of a limited number of tests, without an obvious primary site, before comprehensive investigation |
| Provisional carcinoma of unknown primary origin | Metastatic epithelial or neuroendocrine malignancy identified on the basis of histology/cytology, with no primary site detected despite a selected initial screen of investigations, before specialist review and possible further specialised investigations |
| Confirmed carcinoma of unknown primary origin | Metastatic epithelial or neuroendocrine malignancy identified on the basis of final histology, with no primary site detected despite a selected initial screen of investigations, specialist review, and further specialised investigations as appropriate |
Pathology
The majority of patients presenting with MUO will have tumours of epithelial origin (i.e. carcinomas). However a minority of patients will have non-epithelial tumours, consisting of lymphomas, melanomas and sarcomas, and recognition of these tumours is important as their management is distinct from that of metastatic carcinomas [1,2]. Approximately 80–85% of epithelial tumours are adenocarcinomas or poorly differentiated adenocarcinomas, 5–10% are squamous carcinomas, 5% are neuroendocrine tumours and a small number are germ cell tumours (not truly carcinomas, but often broadly included in this group). In the past, a proportion of tumours would have been classified as “undifferentiated carcinoma”; however, with the widespread use of immunohistochemistry (IHC) this classification is now rarely used as most of these cases are now categorised as poorly differentiated adenocarcinomas [4].
Epidemiology
Data collection on the incidence of CUP is imprecise. This is partially because of the heterogeneity of the disease and difficulties with diagnosis, but also because of the lack of a single discrete code for the condition within the International Classification of Disease (ICD) nomenclature. According to Cancer Registry data, there were 9778 new cases of CUP in England in 2006, accounting for 2.7% of new cancer diagnoses [5]. The number of annual registrations fell over the period 1998–2006; the reasons for this are unclear, but may be related to improvements in diagnosis, changes in cancer coding practice or the emphasis on management of patients within site specific multidisciplinary teams (MDTs). There is a similar incidence between the sexes (46.3% males, 53.7% females according to 2006 data). There is a steady increase in incidence of CUP with age, with the highest incidence in those over 75 years of age.
Mortality data are also likely to be inaccurate, with probable inclusion of patients with a known primary site, but widespread metastases at death. According to the Office for National Statistics, there were 11 018 CUP deaths in England and Wales in 2006; this represents 2.2% of all deaths and approximately 8% of cancer deaths for that year [6]. Survival from CUP is poor, with 1-year survival of approximately 16% and 5-year survival of 8% (using survival data from Thames Cancer Registry, 1992-2006). In contrast with overall cancer survival rates [7], which have significantly improved, there appears to have been no improvement in CUP survival over the past 15 years.
The role of the radiology department in the initial diagnosis
A proportion of cancer patients' first presentation is with symptoms due to metastases. In many this will be an unexpected finding on a radiological examination; examples include multiple lung nodules on a chest radiograph, a destructive lesion on a bone radiograph, multiple bone lesions on a lumbar spine MRI (Figure 1) and multiple liver metastases on an abdominal ultrasound. Radiology departments have a responsibility to immediately inform the referring doctor of such findings and the Cancer Network has a responsibility that hospitals have robust systems in place to ensure that this happens. Once the patient has been identified as having a high suspicion of cancer, they should be referred by their GP to a hospital consultant via the 2-week wait, urgent referral system or, if they are already within the secondary care system, their investigations should be upgraded within the existing cancer tracking system.
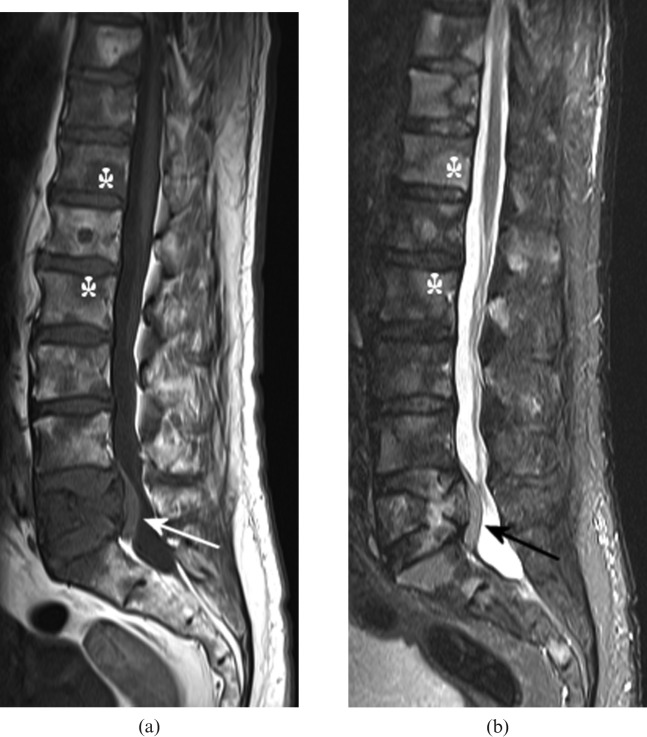
Figure 1.
(a) T1 weighted and (b) short tau inversion-recovery (STIR) MRI sequences of the lumbar spine in a 74-year-old male who presented with back pain. There are multiple vertebral lesions, which are of low signal on T1 weighting and high signal on STIR, consistent with tumour (asterisks indicate examples). L5 vertebral body is partially collapsed with a posterior soft tissue mass, which narrows the spinal canal and indents the thecal sac (arrows). Biopsy of an enlarged neck node showed non-Hodgkin lymphoma; the patient responded well to chemotherapy.
Initial imaging investigations
When a patient first presents with MUO, the initial diagnostic process must be geared towards high-yield tests which can be carried out promptly. It is also important that certain highly treatable malignancies are identified at an early stage.
The following imaging investigations are recommended in the initial diagnostic phase, as clinically appropriate [3]:
chest X-ray (CXR)
CT of the chest, abdomen and pelvis
testicular ultrasound in males with a presentation compatible with germ cell tumour
biopsy and standard histological examination, with immunohistochemistry where necessary, to distinguish carcinoma from other malignant diagnoses.
Chest X-ray
All patients should have a CXR. Bronchial carcinoma is one of the most common causes of MUO and in the majority of cases will be detected or strongly suspected on CXR. CXR is also a cheap test that can be very rapidly performed and evaluated. It should be recognised, however, that benign abnormalities, particularly infection, may be misinterpreted as malignancy. Also, in patients with air space opacification or ill-defined opacities attributed to infection it is important to perform a 6-week follow-up CXR in those patients with persistent symptoms or signs or in those with a high risk of malignancy (particularly smokers and patients over the age of 50), to exclude an underlying bronchial tumour [8].
Blood and urine tests will be performed by the investigating clinician during the initial diagnostic process. As a radiologist, it is worthwhile reminding clinicians of any particularly relevant laboratory investigations, such as by including myeloma in the differential diagnosis of lytic bone lesions, suggesting correlation with prostate-specific antigen (PSA) levels in an elderly male with sclerotic bone lesions or by suggesting serum germ cell tumour markers (and testicular ultrasound) in a young male presenting with a midline nodal mass or with widespread metastases.
CT
CT of the thorax, abdomen and pelvis is an extremely valuable investigation in the initial diagnostic process. CT is sensitive in the detection of many of the primary cancers which commonly present with metastatic disease, particularly bronchial, pancreatic, colonic and renal carcinomas. CT may also determine the extent of metastatic disease (i.e. stages the patient), particularly with regard to the lung, liver, lymph nodes and bones, and potentially identifies the most suitable site for biopsy.
At this stage, the radiologist should also be alert to signs suggesting a non-malignant diagnosis or a specific, more treatable tumour type, such as lymphoma or germ cell tumour. For instance, consider lymphoma in a patient with multiple lung and liver lesions if there is also upper abdominal lymphadenopathy and splenic enlargement. Metastatic germ cell tumours commonly present in young males with large mediastinal and/or retroperitoneal masses (midline disease), or with widespread metastases (often involving lung, liver, bone and sometimes brain). It is important to emphasise germ cell in the differential diagnosis and to perform testicular ultrasound to detect an occult testicular primary in these patients (Figure 2).
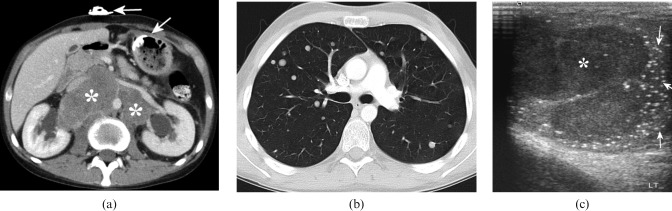
Figure 2.
21-year-old male who presented with muscle weakness, weight loss and swallowing difficulties, and was initially diagnosed with dermatomyositis. He subsequently developed severe back pain. (a) Contrast-enhanced CT through the upper abdomen showing a large retroperitoneal mass (asterisk), displacing the aorta and inferior vena cava, and causing bilateral hydronephrosis. There is also a gastrostomy tube in situ (arrows), which had been inserted because of the swallowing difficulties. (b) CT through the mid-thorax showing multiple well-defined rounded nodules consistent with metastases. (c) Ultrasound image of the left testis showing a solid hypoechoic mass (asterisk) and multiple tiny echogenic foci within the adjacent testis (arrows), consistent with microlithiasis. CT-guided biopsy of the retroperitoneal mass was non-diagnostic, but in view of the clinical and imaging findings a presumptive diagnosis of germ cell tumour was made; the patient responded well to platinum-based systemic chemotherapy.
Despite the widespread acceptance of the value of CT in MUO, there have been few studies to assess its sensitivity or impact on management. In an early study from 1982, in which CT was limited to the abdomen in the majority of patients, a primary tumour was identified in 34.8% (16/46) of cases [9]. In a study published in 2002 by Losa Gaspa et al [10], 221 patients presenting with MUO over a 5-year period were initially assessed by clinical examination, blood test (including tumour markers) and chest X-ray. In 138 patients (62.4%) a diagnosis was made on these basic tests. The remaining 83 patients were investigated according to a protocol which included abdominal CT and mammography (in females), and a primary diagnosis was made by CT in 20 patients and with mammography in 4 patients. The remaining 59 patients underwent exhaustive investigations and a primary was eventually identified in 13 patients. This study was carried out before the modern CT era, but suggests that CT identifies a primary tumour in approximately 25% of patients with negative initial basic investigations.
Evidence is also lacking as to which body areas should be included on CT examinations. The Losa Gaspa imaging protocol only included abdominal CT, but it is recognised that chest CT is superior to chest radiography in the detection of bronchial carcinoma [11], and it is therefore logical to perform CT of the thorax and abdomen as a minimum. Inclusion of the pelvis is likely to have a lower yield in terms of primary tumour identification, but in this difficult group of patients it is justified to include the pelvis to optimise CT staging and to identify occasional pelvic primaries.
Use of intravenous contrast is important, to maximise sensitivity, and in most cases the examination should consist of an arterial-phase acquisition of the thorax and a portal phase acquisition of the abdomen and pelvis. Arterial-phase liver imaging should be added for unusual liver lesions, particularly in those cases with tumours apparently confined to the liver, or cases where there is clinical or pathological suspicion of neuroendocrine tumour.
Non-malignant diagnoses that may mimic metastatic disease are numerous; examples include hypodense liver lesions on CT being interpreted as malignant, without proper consideration of a benign diagnosis, and Paget's disease misinterpreted as sclerotic (or occasionally lytic) bone metastases. Multiple lung nodules may have a benign aetiology and may occur either in the context of acute infection or as an incidental finding, presumably secondary to previous infection. Such nodules should be evaluated within the clinical context and may require further diagnostic work-up or follow-up, according tothe Fleischner Society guidelines [12]. Peritoneal tuberculosis can be radiologically indistinguishable from peritoneal malignancy, but there may be an atypical clinical feature which suggests the diagnosis (Figure 3a). Sarcoidosis occasionally presents with disseminated liver, bone and splenic lesions or with widespread lymphadenopathy (Figure 3b); the characteristic lung changes may be absent or extremely subtle.
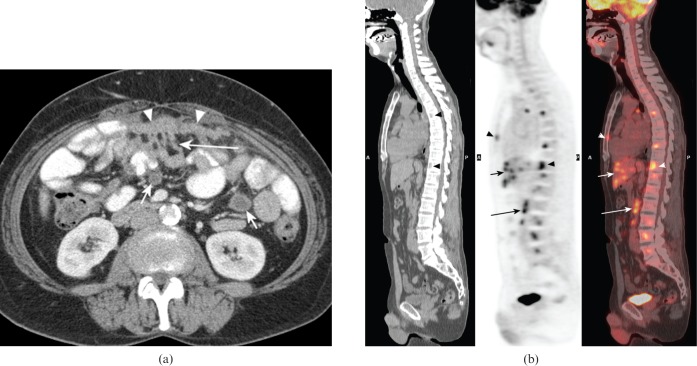
Figure 3.
Non-malignant conditions presenting as malignancy of undefined primary origin. (a) Contrast-enhanced abdominal CT in a 53-year-old female with a history of resected mature ovarian teratoma who presented with abdominal pain. There is a plaque of soft tissue (“omental cake”) within the anterior omental fat (arrowheads), with beading extending to the adjacent small bowel (long arrow) and well-defined, thick-walled cystic lesions within the small bowel mesentry (short arrows). These were initially felt to represent manifestations of disseminated peritoneal malignancy, but biopsies showed granulomatous inflammation and a diagnosis of tuberculosis was eventually confirmed. (b) Sagittal fluorine-18 fludeoxyglucose (18F-FDG) positron emission tomography (PET), CT and fused PET/CT images showing multiple foci of increased FDG uptake within the liver (short arrows), bones (arrowheads) and retroperitoneal nodes (long arrows) in a 49-year-old male who presented with hip pain and was found to have multiple bone lesions and hypodense liver lesions, which were thought to represent metastases. Liver and bone biopsies showed no evidence of malignancy, but showed multiple granulomata, consistent with sarcoidosis.
An important role of CT is in the identification of lesions amenable to biopsy. Always consider that there may be a more accessible lesion not included on the CT examination (e.g. a superficial neck mass), or that a lesion may be better accessed by bronchoscopy or endoscopy. Also, there are situations where a radiological biopsy is either too hazardous or unlikely to provide diagnostic tissue, in which case a surgical biopsy should be suggested, in order not to delay diagnosis.
The choice of imaging guidance modality for biopsy is dependent upon the nature and site of the lesion, as well as upon local expertise and availability (Figure 4), but in many cases ultrasound guidance will be appropriate. Even if a lesion is readily accessible to ultrasound-guided biopsy, the diagnostic CT provides a useful roadmap and may help to target the biopsy towards more solid or vascular areas of the tumour.
Figure 4.
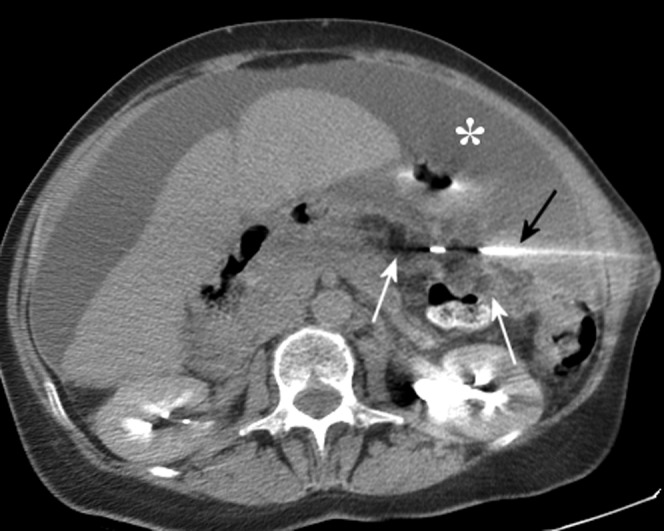
CT-guided omental biopsy in a 63-year-old female who presented with abdominal distension and ascites (asterisk). The tip of the core biopsy needle (black arrow) is seen within the omentum of the left upper quadrant (white arrows), which shows soft tissue stranding consistent with tumour infiltration. In this case CT guidance was preferred to ultrasound guidance, owing to the deep position of the abnormality and the presence of overlying small bowel. Histology showed serous carcinoma with immunohistochemistry highly suggestive of an ovarian origin.
The importance of biopsy
It is recommended in the NICE guidelines that, wherever possible, needle core biopsy or surgical biopsy is obtained for histological evaluation. This is because histological assessment provides information on the architecture of the tumour that is not available with cytology, and also because IHC is more easily performed on histological biopsies. However, cytology can also provide an accurate diagnosis in many cases and IHC can also be performed on cytology specimens (immunocytochemistry) [13]. It is particularly important to perform core biopsy (or surgical excision biopsy) for histology in cases with a possible diagnosis of lymphoma, as it is well recognised that this diagnosis is problematic using cytology. In cases with a potential infective cause, including tuberculosis, it is important to send aspirated fluid or a separate fresh tissue sample for microbiological assessment.
The initial histological assessment, using basic haematoxylin and eosin staining, is focused upon differentiating carcinoma from the less common non-epithelial malignancies, namely sarcomas, melanomas and lymphomas. In the majority of cases the diagnosis is confidently made on haematoxylin and eosin staining; however, in a minority of cases this distinction is impossible on basic histology. In these cases, IHC is mandatory to accurately identify the non-epithelial malignancies. The initial panel of IHC antibodies would typically include a marker for lymphoma (e.g. common leukocyte antigen; CLA), a marker for melanoma (e.g. S100), a marker for carcinoma (usually a pan-cytokeratin) and sometimes a marker for sarcoma (e.g. vimentin) if this is suspected on initial histology. Squamous carcinomas and neuroendocrine tumours are usually distinctive on standard histology, but poorly differentiated tumours may cause difficulty and markers are helpful in diagnosis (CK5/6 and p63 for squamous carcinoma, and chromogranin A and others for neuroendocrine tumours). At this point it is also important to consider the possibility of a germ cell tumour, which commonly closely resembles carcinoma histologically and may also be positive for cytokeratin. Fortunately, there is a highly sensitive marker for germ cell tumours, namely placental alkaline phosphatase (PLAP), which should be used within the IHC standard panel in CUP. Other important diagnoses to make, because of their potential good response to therapy, are of metastatic carcinomas of the breast and prostate. For this reason, PSA in males and oestrogen receptor (ER) and progesterone receptor (PR) in females are also included in the standard IHC panel.
However, after initial histological and IHC analysis, the majority of cases will be identified as adenocarcinoma, but will have no specific features to confidently identify the primary site. In some of these cases the histological morphology of the tumour will suggest the organ of origin and further IHC may support this diagnosis. Cytokeratin 7 (CK7), cytokeratin 20 (CK20) and thyroid transcription factor (TTF1) are recommended as useful factors. TTF1 is relatively specific in the diagnosis of lung cancer and combined CK7/CK20 immunoreactivity are helpful in suggesting groups of likely primary sites [3,4].
In the future, gene-expression-based profiling, performed on biopsy material, may provide additional information to identify primary tumours or to guide specific anticancer therapy, but this technology is currently unproven in CUP [3,4].
The role of mammography and breast MRI
Do not offer mammography routinely to females presenting with MUO, unless clinical or pathological features are compatible with breast cancer [3].
Mammography is a commonly performed investigation in females with MUO and provisional CUP, but its value has not been clearly established. There are only a small number of published studies of mammography in females with CUP.
Kirsten et al [14] studied 286 patients with CUP; a primary cancer was eventually identified in 88 patients (30 of which were diagnosed post-mortem). Of the 143 females, 40 had mammography; of these cases, there were no true-positives, 4 false-positives, 4 true-negatives, 9 false-negatives and 23 equivocal or unevaluable results.
Stevens et al [15] described 31 females, with a variety of presentations of metastatic carcinoma. A confident primary diagnosis was eventually made in 27 of these patients and was of breast cancer (based upon histological and immunohistochemical characteristics) in 5 patients (16%). Mammography was negative in all 5 cases of breast cancer, there were 3 false-positive mammograms and 1 inconclusive case.
Losa Gaspa et al [10] prospectively evaluated 83 patients presenting with metastatic cancer with no primary identified on initial basic investigations. Of these patients, a primary breast cancer was identified in 4 of 29 females undergoing mammography (diagnostic yield 14%), numbers of false-positives were not recorded.
These studies suggest that mammography has limited value in the routine investigation of females with MUO. However, a diagnosis of breast cancer has “high value” as these patients often respond to targeted therapies and have a better outcome than other CUP groups. Therefore, mammography should be performed if initial histology or cytology suggests a possible diagnosis of breast cancer. Also, mammography should be performed if the clinical presentation suggests a possible diagnosis of breast cancer; this is particularly important if there is no readily accessible site for biopsy (e.g. a patient with widespread bone metastases at presentation).
Females presenting with apparently isolated unilateral axillary adenopathy
Refer patients with adenocarcinoma involving the axillary nodes to a breast cancer MDT for evaluation and treatment. If no breast primary tumour is identified after standard breast investigations, consider dynamic contrast-enhanced breast MRI to identify lesions suitable for targeted biopsy [3].
Females presenting with isolated axillary adenopathy, with no breast mass on clinical examination, are an important group of CUP patients. It is recognised that this presentation accounts for approximately 0.3–0.5% of breast cancer diagnoses [16]. It is suggested from surgical series that around two-thirds of these patients will have an occult breast primary within the ipsilateral breast [17]. If the tumour has not metastasised beyond the axilla then these patients may be suitable for radical treatment, which may result in long-term survival. Indeed, there is some evidence that these patients have a better prognosis than those presenting with a palpable breast mass and axillary adenopathy [18]. These patients should be assessed by a specialist breast MDT and should undergo a standard diagnostic work-up, including clinical examination, breast ultrasound and mammography. Should a breast primary be identified then, these patients should be managed in accordance with the current NICE guidance on the management of early breast cancer [19]. There are only a small number of studies evaluating mammography in the investigation of patients with malignant axillary adenopathy. In one of the largest series, including 50 patients, a breast primary was eventually identified in 12 patients, but only 4 were identified on mammography and there was a false-positive rate of 16% (sensitivity 33%) [20].
If initial breast evaluation is negative then dynamic contrast-enhanced breast MRI should be considered. This has been shown, across a number of small series, to have a high sensitivity for detection of occult breast primary. The true sensitivity is difficult to determine, as in most series, the majority of patients with negative MRI do not undergo further biopsy or surgery. However, in two of the larger studies [21,22], a proportion of the MRI-negative patients did undergo mastectomy and the combined sensitivity for detection of a breast primary was 91%. Nevertheless, it is recognised that breast MRI has a low specificity (combined specificity 42% in these studies) and suspicious lesions should therefore be biopsied prior to surgery. The theoretical advantage of detecting a breast primary in this situation is that patients with a discrete primary can then be treated with more conservative surgery and can avoid the morbidity of mastectomy. Conversely, if MRI reveals widespread or multifocal tumour then these patients are likely to require a more radical surgery and may also benefit from neoadjuvant chemotherapy prior to surgery. In the largest series [21], breast MRI was considered helpful in deciding the extent of surgery in 26/55 (47%) of patients, and in another series [23] MRI influenced the decision to give neoadjuvant chemotherapy in 3/10 patients (30%).
The role of fluorine-18 fludeoxyglucose positron emission tomography/CT
Consider fluorine-18 fludeoxyglucose (18F-FDG) positron emission tomography (PET)/CT in patients with provisional CUP with extra cervical presentations after discussion with the CUP team or CUP network MDT [3].
18F-FDG PET/CT is an attractive modality for investigation of CUP. It has a high sensitivity for detection of malignancy and could therefore be expected to be effective in identifying occult primary tumours. However, in practice, primary sites often cannot be distinguished from metastases, particularly in patients with widespread metastases. It may also be difficult to distinguish tumour from physiological activity within the oesophagus or colon, or from excreted FDG within the urinary tract. Also, 18F-FDG is a non-specific tracer and there may be false-positive uptake within inflammatory lesions.
However, these are almost exclusively retrospective series with relatively small patient numbers. The largest body of evidence is for patients presenting with cervical lymphadenopathy; this group of patients will be discussed separately below. Of the studies evaluating patients with extra cervical presentations, most have a heterogeneous patient population, with only a small number of patients with any particular presentation.
In the review carried out by the NCC-C for the NICE guideline development group, a total of 47 primary studies were analysed (35 PET, 12 PET/CT), and in 12 of these studies data for extracervical presentations could be separately assessed. The pooled data show a sensitivity of 0.80 (95% confidence interval, CI: 0.72–0.86) and specificity of 0.81 (95% CI: 0.75–0.86) for either PET or PET/CT, with PET/CT having higher sensitivity and specificity than PET. The results of the individual studies, however, were significantly heterogeneous [3].
Two other recent systematic reviews considered PET/CT for the identification of unknown primary tumours, and reported similar results. In 2009, Kwee and Kwee [24] reported pooled sensitivity and specificity of PET/CT as 84% (95% CI: 78–88%) and 84% (95% CI: 78–89%), respectively. Dong et al [25] estimated the pooled sensitivity and specificity of PET/CT as 81% (95% CI: 74–87%) and 83% (95% CI: 78–87%), respectively. Both meta-analyses identified significant heterogeneity among the individual study results. The estimated tumour detection rate for PET/CT was 37% for Kwee and Kwee [24] and 31% for Dong et al [25].
While these figures are initially impressive, it should be considered that sensitivity and specificity are likely to be overestimated for a number of reasons. The extent of subsequent investigations is highly variable, even within a single study, and is dependent upon the general fitness and wishes of the patient. The results of the PET examinations are likely to have influenced which subsequent investigations were performed or whether they were performed at all; also, those patients with suggested primary sites on PET were more likely to have biopsies of those sites than patients with negative examinations. Overall, from a total of 317 patients in this group, 99 were considered true-positive (31.2%), 36 false-positive (11.4%), 25 false-negative (7.9%) and 157 true-negative (49.5%). Although numbers in individual groups are low, it is interesting to note the true-positive rates for specific presentations, these are 38% (8/21) for patients with liver metastases, 41% (9/22) for patients with peritoneal metastases, 61% (58/95) for brain metastases, 48% (16/33) for presentations with axillary lymphadenopathy and 13% (6/47) for presentations with other sites of lymphadenopathy (non-cervical or axillary). The rate of indeterminate results was recorded in only five studies; the pooled rate of indeterminate results was 16% (95% CI: 11–23%). An additional potential advantage of PET–PET/CT is the detection of previously unsuspected sites of metastases; this information may result in a change of management—in particular the avoidance of a more aggressive treatment, which may have been inappropriate.
Of course, PET/CT is only beneficial in CUP if it either results in a favourable change in patient management or shortens the diagnostic pathway, thereby reducing the number of unnecessary investigations. Unfortunately, there are no prospective studies which address these factors. Some studies have reported on change of management attributed to PET; however, this has mainly been assessed by retrospective questioning of clinicians' subjective opinions as to the value of PET.
The optimal timing of PET/CT in CUP is also unknown. If it is used early in the investigative pathway, when a patient is first identified as having malignancy (MUO), then other, less sensitive tests could be avoided. However, with this protocol, many patients may undergo PET/CT when their tumours could have been readily identified by standard investigations such as CT, leading to unnecessary expense. Alternatively, PET/CT could be used at the end of the investigative pathway, when all other investigations have been negative. Further research is required to compare investigative protocols that include early and late PET/CT.
Presentations of carcinoma of unknown primary origin with specific management issues
Patients presenting with squamous cell carcinoma in cervical nodes
Refer patients presenting with upper- or mid-neck squamous cell carcinoma and an unidentified primary tumour to a head and neck MDT for evaluation and treatment [3].
Offer 18F-FDG PET/CT to patients with provisional CUP presenting with cervical lymphadenopathy with no primary tumour identified on ear, nose and throat (ENT) panendoscopy if radical treatment is considered to be an option [3].
There is a well-recognised subset of patients who present with squamous cell carcinoma in cervical nodes, but no primary identified on endoscopy of the upper aerodigestive tract (ENT panendoscopy), cross-sectional imaging or biopsy of possible primary sites in the head and neck. In these cases, the cancer is assumed to have arisen from an occult upper aerodigestive tract primary (Figure 5). Experience suggests that these patients may have a good long-term prognosis if treated with curative intent. Standard treatment would include ipsilateral neck dissection, with post-operative radiotherapy to the ipsilateral neck and to the entire upper aerodigestive tract mucosa. The rationale for the use of PET/CT in these patients is that if a primary tumour can be identified in the upper aerodigestive tract, then radiotherapy can be directed at this site, thereby reducing radiation toxicity to the rest of the mucosa, possibly increasing treatment effectiveness and also allowing the possibility of further radiotherapy in the event of relapse within the head and neck.
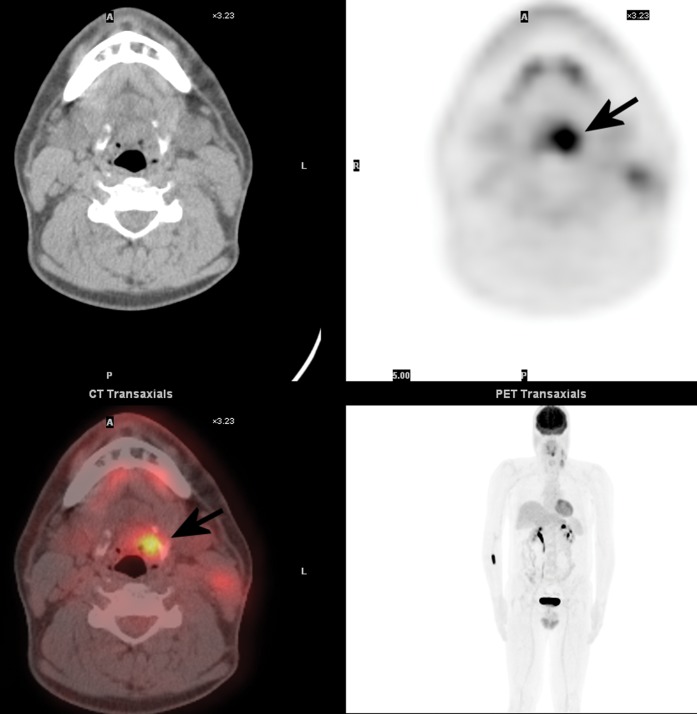
Figure 5.
Axial-fused fluorine-18 fludeoxyglucose (18F-FDG) positron emission tomography (PET)/CT images in a 44-year-old male who presented with left cervical lymphadenopathy with biopsy showing squamous carcinoma. Initial investigations showed no definite primary within the upper aerodigestive tract, but PET/CT showed a focus of intense FDG uptake in the left vallecula, and subsequent biopsy of this area confirmed primary carcinoma.
In a 2004 review of 16 FDG PET studies between 1994 and 2003, involving 302 patients presenting with cervical lymphadenopathy, Rusthoven et al [26] found that PET identified primary tumours in 24.5% of cases unidentified on conventional work-up, with a sensitivity of 88.3% and a specificity of 74.9%. PET also identified previously unrecognised sites of metastases in 27.1% of cases. In a subset of six of the reviewed studies, PET was considered to have led to a change in treatment in 24.7% of cases. PET had the highest accuracy for detection of primaries in the hypopharynx and larynx. However, there was a notably high false-positive rate in the tonsils, and a relatively low sensitivity for detection of tumours at the base of the tongue. One of the difficulties of PET in the head and neck is the localisation of areas of abnormality, and PET/CT would be expected to have a higher accuracy than PET; the pooled data from more recent studies suggest a slightly higher sensitivity for PET/CT, but this is not statistically significant.
Patients presenting with peritoneal carcinomatosis
Obtain a tissue sample for histological examination in patients with MUO who present with ascites, if technically possible [3].
MUO patients with peritoneal carcinomatosis usually present with abdominal distension due to ascites. This presentation is more common in females and in the majority of cases will be due to peritoneal dissemination of a gynaecological primary tumour. A markedly elevated serum CA125 tumour marker supports the likelihood of a gynaecological primary, but is a non-specific test, and further investigation with imaging and histology or cytology is required. Cytology of aspirated ascites will usually demonstrate adenocarcinoma cells. Immunocytochemistry can be performed on these samples and shows promise in predicting primary tumour site. In a study of patients with serous effusions (not only ascites, but also pleural and pericardial effusions), Pomjanski et al [27] were able to predict the primary tumour site in 85.1% of cases using immunocytochemistry with a panel of six antibodies. However, core biopsy with standard histological analysis combined with immunohistochemistry is considered the “gold standard” for diagnosis. In a large study of 149 patients presenting with peritoneal malignancy, Hewitt et al [28] reported that image-guided biopsy with histology and IHC was diagnostic in 93% of cases, with repeat biopsy required in 7% of cases. This study only included females and contained a large proportion of patients with gynaecological malignancy (81%). Nevertheless, this and other studies have shown that image-guided core biopsy is safe and effective in diagnosis of these patients, and should be performed in preference to surgical or laparoscopic biopsy. Fine-needle biopsy with cytology and immunocytochemistry is a valid alternative in difficult cases or where transgression of the bowel is likely; there are no studies directly comparing the effectiveness of core biopsy with fine-needle cytology or fluid cytology. Biopsy can be performed using either CT or ultrasound guidance; Hewitt's series showed no difference in diagnostic rates between the two, and the initial diagnostic CT should be assessed to determine the optimal modality for guidance (Figure 4).
The importance of diagnosis of a gynaecological primary in these females is that these patients typically have a good response to platinum containing chemotherapy. Breast cancer is a well-recognised, but rare (3% of cases in Hewitt's series), cause of peritoneal malignancy, and can also respond well to targeted therapy. There are also occasional cases of lymphoma presenting with peritoneal malignancy, enlarged mesenteric nodes are often present and may suggest the diagnosis; however, core biopsy is particularly important in these patients.
Radical surgery to remove peritoneal tumour (cytoreduction) with intraoperative hyperthermic intraperitoneal chemotherapy is becoming a more established treatment and may result in long-term survival in selected patients with peritoneal malignancy [29].
Patients presenting with brain metastases
Refer patients presenting with apparent brain metastases as the first sign of malignant disease to a neuro-oncology MDT for evaluation and treatment [3].
Presentation with brain metastases as the first sign of malignancy is an uncommon event and assessment of these patients by a specialist neuro-oncological team is recommended. This is particularly important in patients with apparently solitary brain lesions for two reasons. First, there may be an alternative diagnosis that has not been fully considered; this differential might include a primary brain tumour, central nervous system lymphoma or even an ischaemic, inflammatory or infective diagnosis (Figure 6). These patients should undergo brain MRI to characterise the lesion and to exclude small lesions elsewhere within the brain (diffusion-weighted sequences are often helpful in diagnosis). A proportion of these patients will require surgical biopsy to clarify the diagnosis. Second, there is a small group of patients who may be suitable for resection of a brain metastasis. This should particularly be considered in fit patients with no primary identified elsewhere, or with a suspected primary site that may be suitable for radical treatment. Examples would include patients with localised renal or lung carcinomas, in whom occasional long-term survival is well recognised. Patients with multiple brain metastases and poor performance status, and those with disseminated extracerebral metastases, have an extremely poor prognosis and are generally managed with supportive care. Early oncological and palliative care input is required in these patients and specialist neuro-oncological review is unlikely to be beneficial.
Figure 6.
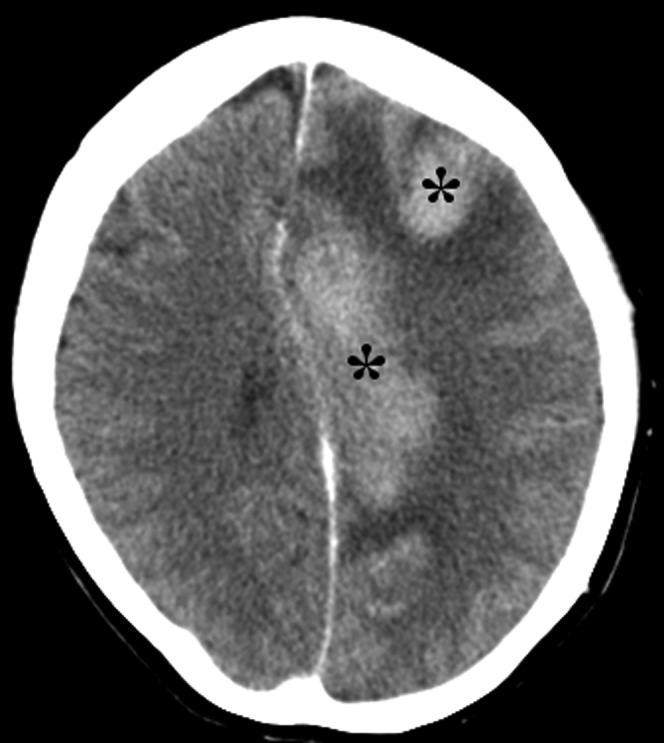
Contrast-enhanced CT brain in a 61-year-old female who presented with right hemiplegia, dizziness, vertigo and nystagmus. There are uniformly enhancing masses within the left frontal and frontoparietal lesions with surrounding oedema and mass effect. These were initially considered to represent metastases, but after specialist neuro-oncological review, biopsy was performed and showed high-grade non-Hodgkin lymphoma.
The most likely primary site in patients presenting with brain metastases is the lung. CXR should therefore be performed as an initial investigation, but there is a well-recognised false-negative rate and patients with negative or non-specific CXR should proceed to chest CT. In a study of 32 patients presenting with brain metastases and no apparent primary, 31 had lung cancer, which was identified on CXR in 19 cases (61%) and on CT in all 31 cases (100%) [11]. Although CT of the chest is likely to have the highest yield, it is logical to include the abdomen in the CT examination, given the frequency of abdominal primaries in CUP and the staging information it provides. The use of FDG PET has also been investigated in this patient group, but given the high sensitivity of CT, its use should be considered only in those fit patients with a negative CT.
Organisational issues affecting radiology
The NICE guidelines [3] recommend that each cancer unit and centre should set up a CUP team that includes an oncologist, palliative care physician and CUP specialist nurse or key worker as a minimum. The CUP team will co-ordinate the investigation and management of patients presenting with MUO. Each unit or centre should have a nominated radiologist and pathologist for CUP with whom these patients are discussed and investigations reviewed. The guidelines fall short of recommending a full MDT for each unit, partially because in many units patient numbers would not justify this, but in many hospitals a CUP MDT could be “tagged onto” an existing MDT. Many patients with particular presentations are effectively managed within existing MDTs and in most cases this should continue. However, it is hoped that the CUP team (and possibly the CUP MDT) will streamline the investigation and co-ordinate the management of patients whose presentation does not fall into an obvious category.
In addition to the CUP teams at cancer unit level, there should be a CUP network MDT, which would normally be based at the cancer centre. This MDT should work with cancer unit CUP teams to advise on patients with difficult diagnostic or management issues. Patients with confirmed CUP should be referred to the network MDT and may be offered systemic chemotherapy, if appropriate. It is hoped that this centralisation of patients with true-CUP will facilitate the set-up of clinical trials, which are currently lacking. In addition, each cancer network should form a clinical subgroup for CUP, to oversee the organisation of CUP services within the network, in line with the NICE guidelines.
Conclusions
The recent NICE guidelines address variations in practice and areas of clinical uncertainty in the current management of CUP, particularly the lack of co-ordinated investigation and specific services for these patients. Radiology has a vital role in the management of patients presenting with MUO and CUP. Radiologists should work with the newly formed CUP teams to streamline diagnostic pathways for CUP.
Acknowledgments
The authors would like to recognise staff of the National Collaborating Centre for Cancer, in particular Dr Andrew Champion, Angela Bennett, Victoria Titshall, Helen Pearson, Dr Fergus Macbeth and Dr John Graham, and members of the Guideline Development Group, for their work in the development of the guidelines. This work was undertaken by the National Collaborating Centre for Cancer, which received funding from the National Institute for Health and Clinical Excellence. The views expressed in this publication are those of the authors and not necessarily those of the institute.
Thanks also to Professor Archibald Malcolm, consultant histopathologist, Royal Shrewsbury Hospital, for reviewing the sections on pathology, and to Dr Karen Francis, senior researcher, National Collaborating Centre for Cancer, for manuscript review.
Figure 6 is courtesy of Dr Carolyn Allen, Pennine Acute NHS Trust, Manchester, UK.
References
- 1.Greco FA, Hainsworth JD. Cancer of unknown primary site. DeVita TV, Hellman S, Rosenberg SA, Cancer: principles and practice of oncology. 8th edn Philadelphia, PA: Lippincott, Williams & Wilkins; 2008. pp. 2363–88 [Google Scholar]
- 2.Pavlidis N, Briasoulis E, Hainsworth J, Greco FA. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer 2003;39:1990–2005 [DOI] [PubMed] [Google Scholar]
- 3.National Institute for Health and Clinical Excellence Diagnosis and management of metastatic malignant disease of unknown primary origin (clinical guideline 104). London, UK: National Institute for Health and Clinical Excellence; 2010. Available from: www.nice.org.uk/CG104 [Google Scholar]
- 4.Oien KA. Pathologic evaluation of unknown primary cancer. Semin Oncol 2009;36:8–37 [DOI] [PubMed] [Google Scholar]
- 5.Office for National Statistics Cancer statistics registrations: registrations of cancer diagnosed in 2006, England. Series MB1, no. 37. London, UK: HMSO; 2008 [Google Scholar]
- 6.Office for National Statistics Mortality statistics: deaths registered in 2006. Series DR. London, UK: HMSO; 2008 [Google Scholar]
- 7.Office for National Statistics Health Statistics Quarterly 38 (Summer). London, UK: HMSO; 2008 [Google Scholar]
- 8.Royal College of Radiologists Making the best use of clinical radiology services. 6th edn London, UK: The Royal College of Radiologists; 2007 [DOI] [PubMed] [Google Scholar]
- 9.McMillan J, Levine E, Stephens R. Computed tomography in the evaluation of metastatic adenocarcinoma from an unknown primary site. A retrospective study. Radiology 1983;143:143–6 [DOI] [PubMed] [Google Scholar]
- 10.Losa Gaspa F, Germa JR, Albareda JM, Fernandez-Ortega A, Sanjose S, Fernandez TV. Metastatic cancer presentation. Validation of a diagnostic algorithm with 221 consecutive patients. [In Spanish.] Rev Clin Esp 2002;202:313–19 [DOI] [PubMed] [Google Scholar]
- 11.Latief KH, White CS, Protopapas Z, Attar S, Krasna MJ. Search for a primary lung neoplasm in patients with brain metastasis: is the chest radiograph sufficient? AJR Am J Roentgenol 1997;168:1339–44 [DOI] [PubMed] [Google Scholar]
- 12.MacMahon H, Austin JHM, Gamsu G, Herold CJ, Jett JR, Naidich DP, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395–400 [DOI] [PubMed] [Google Scholar]
- 13.Fowler LJ, Lachar WA. Application of immunohistochemistry to cytology. Arch Pathol Lab Med 2008;132:373–83 [DOI] [PubMed] [Google Scholar]
- 14.Kirsten F, Chi CH, Leary JA, Ng AB, Hedley DW, Tattersall MH. Metastatic adeno or undifferentiated carcinoma from an unknown primary site–natural history and guidelines for identification of treatable subsets. Q J Med 1987;62:143–61 [PubMed] [Google Scholar]
- 15.Stevens KJ, Smith SL, Denley H, Pinder SE, Evans AJ, Chan SY. Is mammography of value in women with disseminated cancer of unknown origin? Clin Oncol (R Coll Radiol) 1999;11:90–2 [DOI] [PubMed] [Google Scholar]
- 16.Kemeny MM, Rivera DE, Terz JJ, Benfield JR. Occult primary adenocarcinoma with axillary metastases. Am J Surg 1986;152:43–7 [DOI] [PubMed] [Google Scholar]
- 17.Merson M, Andreola S, Galimberti V, Bufalino R, Marchini S, Veronesi U. Breast carcinoma presenting as axillary metastases without evidence of a primary tumor. Cancer 1992;70:504–8 [DOI] [PubMed] [Google Scholar]
- 18.Rosen PP, Kimmel M. Occult breast carcinoma presenting with axillary lymph node metastases: a follow-up study of 48 patients. Hum Pathol 1990;21:518–23 [DOI] [PubMed] [Google Scholar]
- 19.National Institute for Health and Clinical Excellence Early and locally advanced breast cancer: diagnosis and treatment (clinical guideline 80). London, UK: National Institute for Health and Clinical Excellence; 2009. Available from: www.nice.org.uk/CG80 [Google Scholar]
- 20.Galimberti V, Bassani G, Monti S, Simsek S, Villa G, Renne G, et al. Clinical experience with axillary presentation breast cancer. Breast Cancer Res Treat 2004;88:43–7 [DOI] [PubMed] [Google Scholar]
- 21.Buchanan CL, Morris EA, Dorn PL, Borgen PI, Van Zee KJ. Utility of breast magnetic resonance imaging in patients with occult primary breast cancer. Ann Surg Oncol 2005;12:1045–53 [DOI] [PubMed] [Google Scholar]
- 22.Orel SG, Weinstein SP, Schnall MD, Reynolds CA, Schuchter LM, Fraker DL, et al. Breast MR imaging in patients with axillary node metastases and unknown primary malignancy. Radiology 1999;212:543–9 [DOI] [PubMed] [Google Scholar]
- 23.Henry-Tillman RS, Harms SE, Westbrook KC, Korourian S, Klimberg VS. Role of breast magnetic resonance imaging in determining breast as a source of unknown metastatic lymphadenopathy. Am J Surg 1999;178:496–500 [DOI] [PubMed] [Google Scholar]
- 24.Kwee TC, Kwee RM. Combined FDG-PET/CT for the detection of unknown primary tumors: systematic review and meta-analysis. Eur Radiol 2009;19:731–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong MJ, Zhao K, Lin XT, Zhao J, Ruan LX, Liu ZF. Role of fluorodeoxyglucose-PET versus fluorodeoxyglucose-PET/computed tomography in detection of unknown primary tumor: a meta-analysis of the literature. Nucl Med Commun 2008;29:791–802 [DOI] [PubMed] [Google Scholar]
- 26.Rusthoven KE, Koshy M, Paulino AC. The role of fluorodeoxyglucose positron emission tomography in cervical lymph node metastases from an unknown primary tumor. Cancer 2004;101:2641–9 [DOI] [PubMed] [Google Scholar]
- 27.Pomjanski N, Grote HJ, Doganay P, Schmiemann V, Buckstegge B, Bocking A. Immunocytochemical identification of carcinomas of unknown primary in serous effusions. Diagn Cytopathol 2005;33:309–15 [DOI] [PubMed] [Google Scholar]
- 28.Hewitt MJ, Hall GD, Wilkinson N, Perren TJ, Lane G, Spencer JA. Image-guided biopsy in women with breast cancer presenting with peritoneal carcinomatosis. Int J Gynecol Cancer 2006;16:108–10 [DOI] [PubMed] [Google Scholar]
- 29.Hainsworth JD, Fizazi K. Treatment for patients with unknown primary cancer and favorable prognostic factors. Semin Oncol 2009;36:44–51 [DOI] [PubMed] [Google Scholar]