Abstract
Objectives
To investigate whether radiofrequency (RF) ablation with low power (LP) or maximal power (MP) for hepatocellular carcinoma (HCC) can achieve optimal ablation and fewer adverse effects.
Methods
RF ablation was performed with MP in 101 patients (129 tumours) and with LP in 46 patients (61 tumours). MP RF ablation used power of >120 W. RF power below this was designated as LP. Clinical outcomes were also analysed in subgroups of high-risk tumours near the bile duct and blood vessels.
Results
Primary effectiveness was achieved in 91.8% in the LP group and 89.9% in the MP group (p=0.795). 1 and 2-year local tumour progression rates were 28% and 30%, respectively, in the LP group, and 24% and 29%, respectively, in the MP group (p=0.70). 1 and 2-year survival rates were 98% and 98%, respectively, in the LP group, and 93% and 90%, respectively, in the MP group (p=0.216). The MP group had more adverse effects, with post-RF ablation syndrome, asymptomatic pleural effusion and ascites, than the LP group (20% vs 39% in the MP group; p=0.027); however, there was no significant difference in major complication rates (6% in the MP and LP groups; p=0.497). Among the patients with high-risk tumours, RF ablation using MP vs LP was comparable in primary effectiveness (91.7% vs 95.2%; p=0.618), local tumour progression (42.9% vs 29.2%; p=0.304) and overall complications (5% vs 8%; p=0.618).
Conclusion
RF ablation with LP and MP are comparable in clinical outcomes but considerably fewer adverse effects were encountered in the LP group.
Percutaneous image-guided radiofrequency (RF) ablation has gained approval as a minimally invasive strategy for focal liver malignancy [1-5]. During RF ablation, power is set to achieve temperatures that can adequately ablate tumour tissue. Current manufacturers' recommendations for internally cooled (supplied by a radionics generator) and LeVeen (supplied by a radiotherapeutics generator) electrodes are use of maximal power of up to 200 W. However, it has been reported that the higher the temperature, the greater the potential for inhibition of further power deposition into tissues. Previous studies have demonstrated that delivery of power >130 W can frequently induce rises in impedance that cause repeat impedance spiking and automatic power shut-off (reflected by the number of impedance spikes or cool-down cycles), which consequently diminishes the effectiveness of the treatment [6,7]. A study by Komorizono et al [8] demonstrated successful single-session ablation using a maximum RF power of 90 W. This would indicate that it is highly possible to effectively treat liver tumour using currents lower than formerly prescribed.
Aside from enhancing the effectiveness of RF treatment, improvements in technique-related variables have also been undertaken to increase patient safety and tolerance for RF procedures [9]. Raman et al [10] have successfully demonstrated less biliary tree thermal injury with an intraductal chilled saline infusion. Baker et al [11] and Yoko et al [12] demonstrated that there was a significant direct correlation between pain and the distance of the tumour from the hepatic capsule, and that this might require higher doses of narcotics. To address this, Hinshaw et al [13] showed that intraperitoneal instillation of 5% dextrose water prior to RF ablation can decrease post-procedural pain in the ablation of subcapsular hepatic tumours. Although these new innovations are very promising, their implementation may not always be successful and convenient for all operators. Thus, alternative techniques may be valuable. One proposed technique is to determine an optimal temperature that is high enough for sufficient ablation but low enough to avoid adverse effects.
The aims of this study were to find ways to balance adequate tissue ablation and patient tolerance for the RF procedure, especially in tumours in high-risk locations, and particularly to determine whether the use of low-power (LP) current would be comparable to ablation using maximal power (MP) in ablation of hepatocellular carcinomas (HCCs).
Methods and materials
Patients
A total of 366 patients underwent RF ablation in our institute from January 2006 to December 2007. This retrospective study included 147 eligible subjects: confirmed HCC patients with a cytological or pathological diagnosis, or with contrast-enhanced triphasic CT or MRI showing characteristic tumour enhancement in the arterial phase with washout in the portal venous or delay phase [14]; patients without vessel invasion or distant metastasis; index tumours with no prior RF treatment; no hydrodissection (creation of artificial ascites or pleural effusion) before RF ablation. Among these patients, 46 (with 61 tumours) had undergone LP RF ablation whereas 101 patients (with 129 tumours) had undergone MP RF ablation.
Sedation
All procedures were performed under moderate sedation. Patients were sedated initially with meperidine 30 mg and midazolam 3 mg. Additional doses were given as needed to achieve patient comfort during the procedure. 1% lidocaine hydrochloride was administered at the planned puncture site. Vital signs and oxygen saturation were monitored throughout the procedure.
Ablation techniques
The patients were treated by three operators, who each had at least 10 years’ experience in RF ablation. Ultrasound-guided percutaneous RF ablation was carried out using either a single 17 gauge, 20 cm long internally cooled electrode with a 3 cm uninsulated dispersive electrode (Valleylab Inc., Boulder, CO) or a 14 gauge, 15 cm long LeVeen® electrode with an expandable 4 cm array (RadioTherapeutics 200 watts, Mountain View, CA), depending on the operator's preference or availability of electrodes.
Ablations using a LeVeen electrode for the LP group were performed following the manufacturer's algorithm but with some modifications [15,16]. Power was initially set at 60 W with a fully deployed 4.0 cm LeVeen electrode and was increased by 10 W every 30 s. However, instead of increasing the power to 130 W then 190 W, the maximum power used for the LP group was only up to 90 W. This was maintained for 15 min or until impedance increased rapidly, shutting off the generator. A second phase was applied until either a second power shut-off was achieved or 10 min had elapsed. Each electrode was therefore applied for approximately 25 min. If the impedance failed to change within 8 min from the start of the first phase (or 5 min after the start of the second phase), the interactive algorithm was employed while maintaining power at 90 W. The tines were retracted 0.5–1 cm and the power was increased manually to achieve an increase in impedance. Once this was achieved, the tines were redeployed fully while maintaining maximum power until the generator shut off [15,16].
Similarly, ablations in the LP group using an internally cooled electrode were carried out with some alterations to the manufacturer's algorithm or the manual algorithm. Instead of using 200 W as the maximum power, it was set to <120 W. In the modified automated algorithm, ablation was set in the impedance control mode with a gradual increase in power output not to exceed 120 W. Power was maintained at this level until tissue impedance rose 20 Ω above the baseline impedance. Once the 20 Ω threshold was exceeded, the power output was automatically reduced to zero for 15 s then returned to the initial peak power setting until the tissue impedance rose again. Successive cycles were continued for 12 min until a single ablation was completed. For the manual algorithm, the power output was initially set at 60 W and then increased manually by 20 W min–1 to reach a maximum of 120 W. Power was maintained at this level until rapid tissue impedance was achieved. The power was then manually turned off for 15 s, then restarted with a 20 W reduction from the previous highest reading and again gradually increased by 20 W min–1 until rapid tissue impedance was observed. Again, successive cycles were continued for 12 min to complete a single ablation [7].
Ablations used for the MP group followed the standard algorithms for both the LeVeen and internally cooled electrodes. The decision to carry out the procedure using a modified LP algorithm or standard MP was determined by the operator's preference, based on the predicted tolerance of the patient, and projected injury to vital structures, based on tumour proximity.
Overlapping ablations were conducted as necessary for tumours >2 cm to achieve a 0.5–1.0 cm margin around the tumour area. Electrode tract thermocoagulation was performed after achieving the desired tumour ablation. The minimally deployed tines of the LeVeen electrode were withdrawn until they reached the liver capsule maintaining the power at 20 W, whereas the internally cooled electrode was withdrawn maintaining the temperature ≥70 °C [5,15].
Assessment of treatment efficacy
For tumours treated with the internally cooled electrode, the cool-down number (defined as the number of impedance spikes, i.e. impedance rising at least 20 Ω above baseline) and the cool-down temperature were recorded. The temperature of the electrode shown on the RF generator at 30 s to 1 min after completion of the RF procedure and the cooling system being turned off was used as the cool-down temperature. The cool-down number reflected the cycles of rise in impedance with resulting power shut-off during each ablation, whereas the cool-down temperature indicated the core temperature at the end of the RF ablation procedure.
Follow-up dynamic CT or MRI was carried out to evaluate the extent of coagulation 4 weeks after RF ablation. Complete ablation was defined as an area of low attenuation on CT or low signal intensity on T2 weighted MRI encompassing the area of ablation with no nodular peripheral tumour enhancement at 4 weeks after RF ablation [17]. Liver function tests, α-fetoprotein and dynamic imaging were repeated every 3 months after the first post-treatment study to monitor for progression or recurrence.
The primary endpoint was the primary technique effectiveness, which was defined as the complete ablation of the index tumour after one or more RF ablation sessions within a 3 month period [17]. The secondary endpoint included overall survival and local tumour progression, which was defined as the appearance of a newly enhancing tumour on CT during follow-up that was contiguous with the zone that had been considered completely ablated [17]. Other endpoints included complications, adverse effects and subanalysis for tumours near bile ducts or blood vessels.
Statistics
Continuous data were expressed as the mean±SD and were compared between groups using one-way analysis of variance. Categorical variables were compared using the χ2 test or Fisher's exact test. Local tumour progression and survival were calculated by the Kaplan–Meier method and were compared between groups using the log-rank test. All analyses were carried out using the SPSS v.13.0 statistical package for Windows (SPSS Inc., Chicago, IL). p<0.050 was considered statistically significant.
Results
Patients
Similar patient demographics and tumour characteristics were found between the LP and MP groups (Table 1).
Table 1. Patient demographics.
| Characteristics | MP (n=101) | LP (n=46) | p-value |
| Age (years)a | 64.65±10.10 | 65.39±9.90 | 0.680 |
| Sex | 0.104 | ||
| Male | 60 | 33 | |
| Female | 41 | 13 | |
| Aetiology | 0.831 | ||
| HBV | 42 | 18 | |
| HCV | 49 | 21 | |
| Dual HBV and HCV | 3 | 2 | |
| Non-B, non-C | 7 | 5 | |
| Child–Pugh score | 0.267 | ||
| Non-cirrhotic | 5 | 5 | |
| A | 81 | 32 | |
| B | 13 | 9 | |
| C | 2 | 0 | |
| Tumour number/patient | 0.704 | ||
| 1 | 82 | 35 | |
| 2 | 15 | 7 | |
| 3 | 3 | 3 | |
| 4 | 1 | 1 | |
| Mean numbera | 1.24±0.55 | 1.35±0.71 | 0.306 |
| Tumour size (cm)a | 2.38±0.94 | 2.14±0.75 | 0.067 |
HBV, hepatitis B virus; HCV, hepatitis C virus; LP, low power; MP, maximal power.
aData are expressed as mean±standard deviation.
Primary technique effectiveness
Primary technique effectiveness was achieved in 89.9% of the MP group and in 91.8% of the LP group (p=0.795). After a single session of RF ablation, complete necrosis was achieved in 98 (76%) of MP and 43 (70.5%) of LP tumours (p=0.478; Table 2). The mean total ablation time per patient was also comparable between the groups (LP vs MP=21.25 vs 25.99 min; p=0.051).
Table 2. Tumour and patient analyses for ablation effectiveness and local tumour progression.
| Factors | MP (n=101 patients with 129 tumours) | LP (n=46 patients with 61 tumours) | p-value |
| Follow-up (months)a | 21.30±8.30 | 19.78±6.53 | 0.277 |
| Hospital stay (days)a | 4.42±3.68 | 3.50±2.73 | 0.181 |
| Meperidine (mg dl–1)a | 51.63±20.58 | 57.07±18.31 | 0.127 |
| Midazolam (mg dl–1)a | 4.90±2.17 | 5.39±1.78 | 0.177 |
| Complete ablation after 1 session | 98 (76.0%) | 43 (70.5%) | 0.478 |
| Primary effectiveness | 116 (89.9%) | 56 (91.8%) | 0.795 |
| LTP | 38 (29.5%) | 19 (31.1%) | 0.866 |
| Time to LTP (months)a | 11.08±6.8 | 9.16±7.96 | 0.346 |
| Subanalysis for RF ablation using internally cooled electrode | |||
| MP (n=107 tumours) | LP (n=54 tumours) | ||
| Cool-down number | 16.61±15.57 | 7.59±7.94 | <0.001 |
| Cool-down temperature (°C)a | 74.72±7.51 | 74.49±9.42 | 0.870 |
LP, low power; LTP, local tumour progression; MP, maximal power.
aData expressed as mean±standard deviation.
Subanalysis of the data according to the type of electrode used showed that there were 161 tumours treated with the internally cooled electrode whereas only 29 patients were treated with the LeVeen electrode. The primary effectiveness of using LP or MP was similar to the analysis of the population taken together. There was still no statistical difference in terms of primary effectiveness even after the groups were subclassified (LeVeen, LP vs MP=90.6% vs 88.9%, p=1.00; internally cooled, LP vs MP=100% vs 95.2%, p=1.00).
Local tumour progression
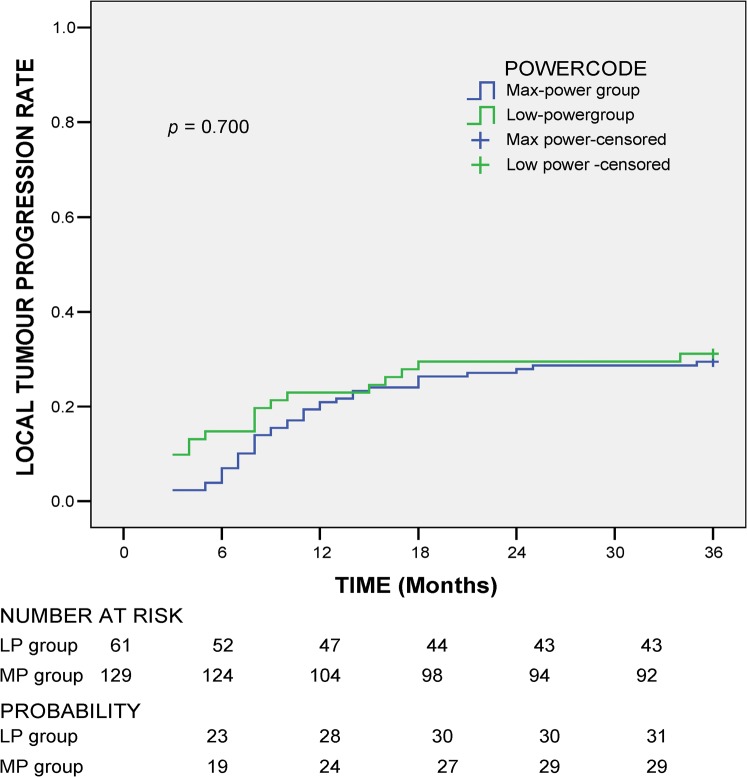
Local tumour progression (LTP) was comparable between 2 groups (p=0.866) with the rate of 38 (29.5%) in MP tumours and 19 (31.1%) in LP tumours. The mean time to local tumour progression was also comparable (MP vs LP=11.08 vs 9.16 months; p=0.346). The cumulative local tumour progression at 6, 12, 18 and 24 months was 19%, 24%, 27% and 29% in the MP group and 23%, 28%, 30% and 30% in the LP group (p=0.700; Figure 1). Univariate analysis for LTP with sex, age, aetiology, tumour size, complete ablation after one session and primary effectiveness showed no significant difference between LP and MP groups (all p>0.1). Multivariate analysis was no longer carried out.
Figure 1.
Local tumour progression rate.
When subclassified according to the electrode used, LTP was still comparable between the LP and the MP groups (LeVeen, LP vs MP=62.5% vs 23.8%, p=0.083; internally cooled, LP vs MP=26.4% vs 30.6%, p=0.713). The LTP rate for the LeVeen LP subgroup was 27% at 6, 12 and 18 months whereas the LTP rates for the LeVeen MP subgroup at 6, 12 and 18 months were 23%, 23% and 0%, respectively (p=0.973). For the internally cooled subset, the LTP rates for the LP group were 82%, 41% and 0% at 6, 12 and 18 months, respectively, whereas the LTP rates for the MP group were 91%, 37% and 10% at 6, 12 and 18 months, respectively (p=0.192).
Tumours in the MP group had significantly greater cool-down numbers than those in the LP group (MP vs LP=16.61 vs 7.59; p<0.001). Cool-down temperature was approximately 74 °C for both groups (p=0.87) (Table 2).
Complications and adverse effects
The MP group had more adverse effects, with post-RF ablation syndrome, asymptomatic pleural effusion and ascites, than the LP group (LP vs MP=20% vs 39%, p=0.027; Table 3). The overall complication rate for all the subjects was 12.2% and was similar between the 2 groups (MP vs LP=10% vs 6%; p=0.755). There were also comparable major complication rates (MP vs LP=6% vs 6%; p=0.497).
Table 3. Complications and adverse effects.
| Complications and adverse effects | MP patients (n=101) | LP patients (n=46) |
| Complications (p=0.755)a | ||
| Major (p=0.497)a | ||
| Pleural effusion needs intervention | 1 (1%) | 0 |
| Post-RF ablation bleeding | 1 (1%) (procedure-related mortality) | 1 (2%) (procedure-related mortality) |
| Sepsis | 1 (1%) | 0 |
| Abscess | 0 | 1 (2%) |
| Portal vein thrombosis | 2 (2%) | 0 |
| Bile duct injury | 1 (1%) | 1 (2%) |
| Total | 6 (6%) | 3 (6%) |
| Minor (p=0.497)a | ||
| Minimal haemothorax without intervention | 1 (1%) | 0 |
| Wound infection at RF site | 1 (1%) | 0 |
| Haematoma | 2 (2%) | 0 |
| Total | 4 (4%) | 0 |
| Adverse effects (p=0.027)a | ||
| Asymptomatic fever | 29 (29%) | 9 (18%) |
| Asymptomatic pleural effusion | 2 (2%) | 1 (2%) |
| Asymptomatic ascites | 5 (5%) | 0 |
| Fever + pleural effusion | 1 (1%) | 0 |
| Fever + ascites | 2 (2%) | 0 |
| Total | 39 (39%) | 10 (20%) |
ap-value, difference between occurrence of complication and side effects between maximal-power (MP) and low-power (LP) groups.
The overall procedure-related mortality rate for all the subjects was 1.36%. One patient in the LP group died of hypovolaemic shock from tumour rupture 5 days after RF ablation, and one patient in the MP group died of procedure-related intraperitoneal bleeding 23 days after treatment. Both of the patients also had hepatic decompensation.
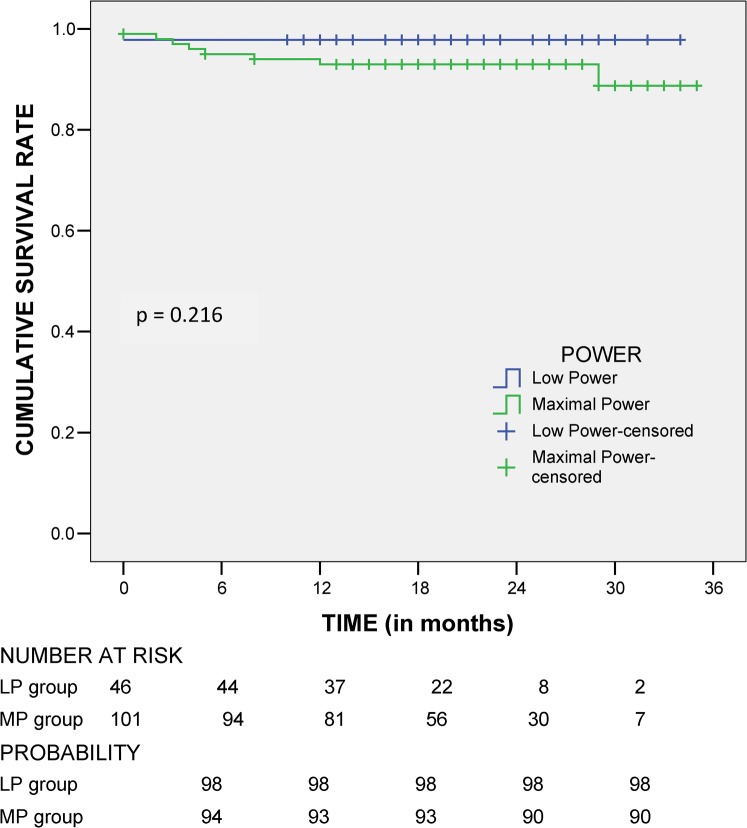
The cumulative survival rates at 6, 12, 18 and 24 months were 98%, 98%, 98% and 98%, respectively, in the LP group and 94%, 93%, 93% and 90%, respectively, in the MP group (p=0.216; Figure 2). Univariate analysis for survival with sex, age, aetiology, Child–Pugh score and tumour number showed no significant difference between the LP and MP groups (p>0.1). None of the variables were statistically significant; thus, multivariate study was no longer carried out.
Figure 2.
Survival rates.
Subanalysis of the patients according to the electrode used showed that there were 134 patients treated with the internally cooled electrode whereas only 13 patients were treated using the LeVeen electrode. When analysed using this subclassification, survival rates for the internally cooled electrode LP subgroup were 86%, 74%, 52% and 28% at 6, 12, 18 and 24 months, respectively, whereas survival rates for the MP group were 40%, 25%, 20% and 6% at 6, 12, 18 and 24 months, respectively (p=0.228). All of the patients in the LeVeen group were censored in the survival analysis, thus no clear Kaplan–Meier examination was completed. This may be because of the small number of patients included in this subgroup.
Subanalysis of 66 tumours located near major vessels (inferior vena cava, hepatic vein or portal vein) or bile ducts (42 in the MP group and 24 in the LP group) treated with internally cooled electrodes showed equivalent cool-down temperatures (MP vs LP=73.25±8.53 °C vs 72.65±8.97 °C; p=0.794).
RF ablation of high-risk tumours using MP vs LP were comparable in primary effectiveness (91.7% vs 95.2%; p=0.618) and local tumour progression (42.9% vs 29.2%; p=0.304). The mean time to local tumour progression was 11.67±5.95 months in the MP group and 13±4.47 months in the LP group (p=0.598). For the tumours in high-risk locations, there were only slightly more adverse effects in the MP group (MP vs LP=35.7% vs 25.0%; p=0.422). Complication rates were comparable (MP vs LP=5% vs 8%; p=0.618).
Discussion
This study shows that RF ablation using LP is comparable to MP in terms of primary effectiveness, local tumour progression and survival, with the former having notably fewer adverse effects. This could be another strategy to ensure safe ablation of tumours in patients with low tolerance to MP without compromising RF ablation effectiveness. According to the study carried out by Pereira et al [18], use of high-power output is not of primary importance in obtaining large volume coagulations as this may lead to overheating and tissue desiccation. This was confirmed in our study: complete ablation was satisfactorily achieved despite using power lower than the MP that has been conventionally used.
To our knowledge, a definitive value of LP or MP has never been elucidated in previous studies. However, previous studies have demonstrated that delivery of power >130 W in internally cooled electrodes can induce frequent rises in impedance that cause repeated impedance spiking and automatic power shut-off, which consequently diminishes the effect of the treatment [6,7]. Shibata et al [6] were also able to demonstrate that roll-off was obtainable in tumours using a maximum of 90 W power of the RF generator 2000 system. Furthermore, use of a maximal RF power of 90 W by Komorizono et al [8] and 140 W by Teratani et al [19] both achieved optimal ablation. In our study, LP was defined as tumour treatment with <120 W in the internally cooled electrode group and <90 W in the LeVeen group. Values above these limits were considered to be MP.
In our subset of tumours treated with internally cooled electrodes, the MP group had significantly more instances of cool-down (impedance spiking cycles) than the LP group. This was probably because the MP group reached impedance spiking faster than the LP group. A cool-down temperature of >70 °C is taken as evidence of satisfactory ablation [20]. The results of our study indicated that the two groups were equivalent in attaining the optimal target temperature despite different powers being used. These results confirm that optimal ablation does not necessarily require high RF power.
Initial complete ablation has better long-term outcomes [7,9]. Incomplete ablation of tumours after the local treatment is independently associated with tumour progression [21,22]. Our results showed that, despite using lower RF power, the complete ablation and primary effectiveness of index tumours was achieved similarly between both groups. Consequently, local tumour progression rates for both groups were comparable. This indicates that adequate ablation with a tumour-free margin can be achieved with use of powers lower than those conventionally used.
It is quite interesting to note that the mean total ablation time for the LP group, although not significant, was shorter than that in the MP group. This is possibly because lower temperatures used in the former allowed for gradual, more effective heat dissemination, meaning that less time was needed to achieve ablation than in the MP group. As the tumours in both groups had a mean size of <2.5 cm, an average of one pass was needed to achieve ablation of each tumour. Thus, the ablation time for each group coincided with the prescribed time of approximately 25 min in the algorithm.
Treatment of liver tumours close to major intrahepatic blood vessels by RF ablation may render the ablation incomplete owing to the “heat-sink” effect [23]. In our study, subanalysis of tumours near vessels showed that, despite the use of LP, optimal cool-down temperatures were achieved and the rates for complete necrosis and local tumour progression were similar to those in MP RF ablation. The efficacy of ablation was not compromised despite the use of power lower than that customarily used. This is in congruence with earlier studies showing that proximity to intrahepatic vessels is not a poor prognostic factor for local tumour progression [19].
Post-RF ablation adverse effects include asymptomatic pleural effusion, uncomplicated ascites formation and post-RF ablation syndrome, manifesting as fever with or without influenza-like symptoms [16,24]. Fever was directly proportional to both power applied during ablation and the total procedure time. RF ablation causes local heating of intrahepatic tissues and systemic heating through the intrahepatic blood flow [25]. This may explain the significantly fewer adverse effects observed in the LP group than in the MP group given the equivalent mean total ablation time per patient.
The incidence of complications in our study was 9% and was comparable to the incidence of 2–27.8% reported previously [26-28]. The overall bleeding rate was only 2% (three patients had bleeding: two patients had major complications and died as a result, and one had minor complications (haemothorax) that did not need intervention). Abscess formation was another common complication in earlier studies, occurring at between 8 days and 5 months [26,29,30]. In this series, only 1 (0.6%) patient had abscess after RF ablation. Abscess formation was not noted in the immediate post-RF surveillance but was detected 26 days after RF ablation. Early detection of abscess and a low incidence rate may be attributable to a better ablation technique. de Baère et al [26] reported that thrombosis may occur commonly in small vessels (<3 mm) but is quite rare in larger ones. In our series, only 2 (1.3%) patients in the MP group had post-RF thrombosis of major portal veins. A notable post-RF complication observed in other studies is peritoneal seeding with an incidence of 0.5–2.8%. Risk factors include subcapsular tumour location, multiple treatment sessions and multiple electrode placements [31]. In our study, none of the patients with subcapsular tumours had detectable RF-related tumour seeding. This may be due to routine electrode tract coagulation during withdrawal of the electrode that prevents tumour seeding.
66 tumours (42 in the MP group and 24 in the LP group) were located in high-risk locations near bile ducts or blood vessels. Post-RF ablation syndrome in these tumours was not significantly different between the MP and LP groups. Only one patient in the MP group and two patients in the LP group had partial portal vein thrombosis after RF ablation (p=0.459). This confirms the results of previous studies [28,32] that, in general, RF ablation of tumours in these areas is safe.
The overall mortality rate was 1.36% in our series, which was comparable to 0.2–2.1% in previous reports [29,33-36]. The cause of death was hypovolaemic shock from tumour bleeding combined with hepatic decompensation, similar to the most common cause of death in other studies [29,33-36].
The limitations of the current study include that, as it was a non-randomised study, there might be bias in determining the optimal RF power as LP or MP in achieving optimal ablation. However, there is no universal algorithm or optimal RF power defined by previous studies except manufacturer's algorithms using maximal RF power and automated pulsed RF ablation [2,7,8,17,23]. Further studies are required to compare different algorithms and to determine the values of RF power that may be useful in achieving optimal ablation and better tolerance of the RF procedure.
In conclusion, the present results showed that use of LP is comparable to MP during RF ablation in terms of equivalent primary effectiveness, local tumour progression and survival. However, LP RF ablation has fewer adverse effects. Among patients with tumours located in high-risk areas, RF ablation using MP versus LP was comparable in primary effectiveness, local tumour progression and overall complications.
References
- 1.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma ≤4 cm. Gastroenterology 2004;127:1714–23 [DOI] [PubMed] [Google Scholar]
- 2.Shiina S, Teratani T, Obi S. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005;129:122–30 [DOI] [PubMed] [Google Scholar]
- 3.Livraghi T, Meloni F, Di Stasi M. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology 2008;47:82–9 [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferro V, Battiston C, Perrone S. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg 2004;240:900–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandes ML, Lin CC, Lin CJ, Chen WT, Lin SM. Risk of tumour progression in early-stage hepatocellular carcinoma after radiofrequency ablation. Br J Surg 2009;96:756–62 [DOI] [PubMed] [Google Scholar]
- 6.Shibata T, Shibata T, Maetani Y, Isoda H, Hiraoka M. Radiofrequency ablation for small hepatocellular carcinoma: prospective comparison of internally cooled electrode and expandable electrode. Radiology 2006;38:346–53 [DOI] [PubMed] [Google Scholar]
- 7.Cua IH, Lin CC, Lin CJ, Chen WT, Hsu CW, Lin SM, et al. Treatment of hepatocellular carcinoma using internally cooled electrodes: a prospective comparison of modified automated vs. manual pulsed RF algorithms. Oncology 2007;72:76–82 [DOI] [PubMed] [Google Scholar]
- 8.Komorizono Y, Oketani M, Sako K, Yamasaki N, Shibatou T, Maeda M, et al. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer 2003;97:1253–62 [DOI] [PubMed] [Google Scholar]
- 9.Helton WS. Minimizing complications with radiofrequency ablation for liver cancer: the importance of properly controlled clinical trials and standardized reporting. Ann Surg 2004;239:459–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raman SS, Aziz D, Chang X, Ye M, Sayre J, Lassman C, et al. Minimizing central bile duct injury during radiofrequency ablation: use of intraductal chilled saline perfusion. Initial observations from a study in pigs. Radiology 2004;232:154–9 [DOI] [PubMed] [Google Scholar]
- 11.Baker M, Anderson JK, Jaffer O, Trimmer C, Cadeddu JA. Pain after percutaneous radiofrequency ablation of renal tumors. J Endourol 2007;21:606–9 [DOI] [PubMed] [Google Scholar]
- 12.Yoko S, Narutomo W, Michihisa K, Ritsuko G, Arifumi K, Ryozo S. Examination of pain relief methods during radiofrequency ablation for patients with hepatic tumors. Tokushima Red Cross Hosp Med J 2006;11:18–20 [Google Scholar]
- 13.Hinshaw JL, Laeseke PF, Winter TC, Kliewer MA, Fine JP, Lee FT. Radiofrequency ablation of peripheral liver tumors: intraperitoneal 5% dextrose in water decreases postprocedural pain. AJR Am J Roentgenol 2006;186:S306–10 [DOI] [PubMed] [Google Scholar]
- 14.Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR AM J Roentgenol 2000;174:323–31 [DOI] [PubMed] [Google Scholar]
- 15.Lin SM, Lin CC, Chen WT, Chen YC, Hsu CW. Radiofrequency ablation for hepatocellular carcinoma: a prospective comparison of four radiofrequency devices. J Vasc Interv Radiol 2007;18:1118–25 [DOI] [PubMed] [Google Scholar]
- 16.Lin SM, Lin CJ, Chung HJ, Hsu CW, Peng CY. Power roll-off during interactive radiofrequency ablation can enhance necrosis when treating hepatocellular carcinoma. AJR Am J Roentgenol 2003;180:151–7 [DOI] [PubMed] [Google Scholar]
- 17.Goldberg N, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol 2005;16:765–78 [DOI] [PubMed] [Google Scholar]
- 18.Pereira PL, Trubenbach J, Schenk M, Subke J, Kroeber S, Schaefer I, et al. Radiofrequency ablation: in vivo comparison of four commercially available devices in pig livers. Radiology 2004;232:482–90 [DOI] [PubMed] [Google Scholar]
- 19.Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, et al. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology 2006;43:1101–8 [DOI] [PubMed] [Google Scholar]
- 20.Strasberg I, Linehan DC. Radiofrequency ablation of liver tumors. Curr Probl Surg 2003;40:459–98 [DOI] [PubMed] [Google Scholar]
- 21.Wong SN, Lin CJ, Lin CC, Chen WT, Cua IHY, Lin SM. Combined percutaneous radiofrequency ablation and ethanol injection for hepatocellular carcinoma in high-risk location. AJR Am J Roentgenol 2008;190:W187–95 [DOI] [PubMed] [Google Scholar]
- 22.Harrison LE, Koneru B, Baramipour P, Fisher A, Barone A, Wilson D, et al. Locoregional recurrences are frequent after radiofrequency ablation for hepatocellular carcinoma. J Am Coll Surg 2003;197:759–64 [DOI] [PubMed] [Google Scholar]
- 23.Lu DS, Raman SS, Limanond P, Aziz D, Economou J, Busuttil R, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol 2003;14:1267–74 [DOI] [PubMed] [Google Scholar]
- 24.Wah TM, Arellano RS, Gervais DA, Saltalamacchia CA, Martino J, Halpern EF, et al. Image-guided percutaneous radiofrequency ablation and incidence of post-radiofrequency ablation syndrome: prospective survey. Radiology 2005;237:1097–102 [DOI] [PubMed] [Google Scholar]
- 25.Sawada M, Watanabe S, Tsuda H, Kano T. An increase in body temperature during radiofrequency ablation of liver tumors. Anesth Analg 2002;94:1416–20 [DOI] [PubMed] [Google Scholar]
- 26.de Baère T, Risse O, Kuoch V, Dromain C, Sengel C, Smayra T, et al. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol 2003;181:695–700 [DOI] [PubMed] [Google Scholar]
- 27.Choi H, Loyer EM, DuBrow RA, Kaur H, David CL, Huang S, et al. Radio-frequency ablation of liver tumors: assessment of therapeutic response and complications. Radiographics 2001;21:S41–54 [DOI] [PubMed] [Google Scholar]
- 28.Ng KK, Poon RT, Lam CM, Yuen J, Tso WK, Fan ST. Efficacy and safety of radiofrequency ablation for perivascular hepatocellular carcinoma without hepatic inflow occlusion. Br J Surg 2006;93:440–7 [DOI] [PubMed] [Google Scholar]
- 29.Wood TF, Rose M, Chung M, Allegra DP, Foshag LJ, Bilchik AJ. Radiofrequency ablation of 231 unresectable hepatic tumors: indications, limitations, and complications. Ann Surg Oncol 2000;7:593–600 [DOI] [PubMed] [Google Scholar]
- 30.Zagoria RJ, Chen MY, Shen P, Levine EA. Complications from radiofrequency ablation of liver metastases. Am Surg 2002;68:204–9 [PubMed] [Google Scholar]
- 31.Jaskolka JD, Asch MR, Kachura JR, Ho CS, Ossip M, Wong F, et al. Needle tract seeding after radiofrequency ablation of hepatic tumors. J Vasc Interv Radiol 2005;16:485–91 [DOI] [PubMed] [Google Scholar]
- 32.Curley SA, Izzo F, Ellis L, Vauthey N, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg 2000;232:381–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulier S, Mulier P, Ni Y. Complications of radiofrequency coagulation of liver tumors. Br J Surg 2002;89:1206–22 [DOI] [PubMed] [Google Scholar]
- 34.Livraghi T, Solbiati L, Meloni L, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 2003;226:441–51 [DOI] [PubMed] [Google Scholar]
- 35.Jansen MC, van Duijnhoven FH, van Hillegersberg R, Rijken A, van Coevorden F, van derSijp J, et al. Adverse effects of radiofrequency ablation of liver tumours in the Netherlands. Br J Surg 2005;92:1248–54 [DOI] [PubMed] [Google Scholar]
- 36.Poon RT, Ng KK, Lam C, Ai V, Yuen J, Fan S. Radiofrequency ablation for subcapsular hepatocellular carcinoma. Ann Surg Oncol 2004;11:281–9 [DOI] [PubMed] [Google Scholar]