Abstract
Objectives
The purpose of this study was to determine the prevalence, clinical signs and radiological features of breast lymphoma.
Methods
This is a retrospective review of 36 patients with breast lymphoma (22 primary and 14 secondary). 35 patients were female and 1 was male; their median age was 65 years (range 24–88 years). In all patients, the diagnosis was confirmed histopathologically.
Results
The prevalence of breast lymphoma was 1.6% of all identified cases with non-Hodgkin lymphoma and 0.5% of cases with breast cancer. B-cell lymphoma was found in 94% and T-cell lymphoma in 6%. 96 lesions were identified (2.7 per patient). The mean size was 15.8±8.3 mm. The number of intramammary lesions was higher in secondary than in primary lymphoma. The size of the identified intramammary lesions was larger in primary than in secondary lymphoma. Clinically, 86% of the patients presented with solitary or multiple breast lumps. In 14%, breast involvement was diagnosed incidentally during staging examinations.
Conclusion
On mammography, intramammary masses were the most commonly seen (27 patients, 82%). Architectural distortion occurred in three patients (9%). In three patients (9%), no abnormalities were found on mammography. On ultrasound, the identified lesions were homogeneously hypoechoic or heterogeneously mixed hypo- to hyperechoic. On MRI, the morphology of the lesions was variable. After intravenous administration of contrast medium, a marked inhomogeneous contrast enhancement was seen in most cases. On CT, most lesions presented as circumscribed round or oval masses with moderate or high enhancement.
Ductal and lobular carcinomas are the most frequent tumours of the breast. Breast involvement by lymphoma is very rare. It can occur as a primary breast tumour or as an extranodal manifestation in systemic disease [1-5]. According to the literature, the prevalence of breast lymphoma (BL) ranges from 0.04 to 0.5% of malignant breast neoplasms [1,2]. In addition, the prevalence of primary BL (PBL) varies from 0.85 to 2.2% of extranodal malignant lymphomas [3-5]. Secondary BL (SBL) is more common [6-8]. The rarity of BL can be attributed to the fact that the breast contains very little lymphoid tissue [9,10].
Only a few of the published studies focus on the radiological features of BL, and conflicting findings of BL have been reported [1,7,11-13]. Only in one investigation has the distinction between primary and secondary breast involvement been taken into consideration [1]. Therefore, the aim of this study was to determine the prevalence of BL in our population and to analyse its clinical and radiological characteristics.
Methods and materials
Patients
This retrospective study has been approved by the Institutional Ethics Committee of Martin Luther University Halle.
15 patients with BL were retrospectively identified in the pathological and radiological databases of the Martin Luther University Halle and 21 in the databases of 4 neighbouring hospitals in the time interval from 1997 to 2009 (keywords searched for in the reports). 35 patients were female and 1 was male. Their median age was 65 years (range 24–88 years).
In our institution, 2896 patients with breast carcinoma and 927 patients with non-Hodgkin lymphoma (NHL) were treated in the investigated time period. The corresponding numbers of patients in neighbouring hospitals was not available.
Imaging
Mammography
Mammography was performed in 33 patients. It was obtained on dedicated mammographic equipment: Mammomat 3000 Nova (Siemens AG, Erlangen, Germany), Mammomat Novation DR (Siemens AG), GE Seno essential (GE Medical Systems, Buc, France) and GE diamond (GE Medical Systems, Budaors, Hungary). Standard mediolateral oblique and craniocaudal mammograms were made, with additional views if necessary.
Ultrasound
Supplementary ultrasound images were available in 20 patients (transverse and sagittal views). Ultrasound imaging was performed using a 10.5 MHz linear array transducer Sonoline Elegra and Acuson Antares (Siemens AG), and a 7.5 MHz linear array transducer Voluson 530 MT (GE Medical Systems, Zipf, Austria) and GE Logiq P5 (GE Medical Systems, Seongnam, Republic of Korea). The presence or absence of tumoural vascularity was determined using colour Doppler ultrasound scanning in 11 patients.
MRI
In eight patients, MRI of the breast was performed using a dedicated receive-only breast coil on a 1.0 T scanner (Impact Expert; Siemens). Both breasts were imaged in the coronal plane with 2.5 mm slice thickness using a T1 weighted (T1W) three-dimensional fast low-angle shot sequence [repetition time/echo time (TR/TE), 14 ms/7 ms; flip angle 25°] with a 96×256 matrix, field of view 160×320 mm and 64 slices with phase encoding along the body axis. Measurement time was 87 s, once before and five times after intravenous bolus injection of 0.2 mmol gadolinium diethylenetriamine-pentaacid per kilogram of body weight (Bayer Schering Pharma, Leverkusen, Germany). The flow rate was 2 ml s–1 and the bolus was followed by a 20 ml saline flush. Central k-space data were acquired at 43, 130, 217, 304 and 391 s after injection. Automatic image subtraction was performed routinely.
T2 weighted (T2W) images included a turbo spin echo sequence (TR/TE, 4200/90 ms; flip angle 180°) with a 252×256 matrix and field of view 350 mm with a slice thickness of 5 mm.
In two patients, T2W images with fat saturation (TR/TE, 6242/70; flip angle 180°; field of view 350 mm) were additionally used.
Kinetic analysis of contrast enhancement was also performed. Time–signal intensity curves were drawn using operator-defined region of interest (ROI). The ROI was smaller than the lesion size. The time to peak (TTP) enhancement, i.e. the time between the administration of contrast agent and the maximum signal intensity value in the post-contrast phase, was estimated. The initial signal increase (Initial SI) from the pre-contrast value (SIp) to the maximum peak within the first 3 min after the administration of contrast medium (SI1–3 min) was calculated:
Initial SI (%)=(SI1–3 min–SIp)/Sip×100% (1)
The post-initial behaviour of the signal curve (post-initial SI) from the maximum peak (SIpeak) to the end of the examination (Siend) was also analysed:
Post-initial SI (%)=(SIpeak – Siend)/Sipeak×100% (2)
All signal intensity–time curves were classified according to shape into three types: Type I with a continuous increase in signal intensity on each successive contrast-enhanced image of more than 10% compared with initial enhancement; Type II with a plateau pattern, in which an initial increase in signal intensity is followed by a flattening of the enhancement curve (deviation of the signal curve between –10% and +10% compared with initial enhancement); Type III with a wash-out enhancement pattern, which involves an initial increase and subsequent decrease in signal intensity of more than 10% compared with initial enhancement [14-16].
All breast findings were classified according to the American College of Radiology Breast Imaging Reporting and Data Systems (BI-RADS) lexicon [17-19].
CT (Somatom Plus 4 VZ and Somatom Sensation 64; Siemens AG) of the thoracic region was performed as a staging investigation in seven patients. In all cases, 60–90 ml of iodinated intravenous contrast medium was given at a rate of 1.5–3.5 ml s–1 by a Medtron Power Injector (Medtronic GmbH, Meerbusch, Germany), with a scan delay of 30–90 s after onset of injection. Typical imaging parameters were 120 kVp, 150–300 mAs and 0.6–6 mm slice thickness with a pitch of 0.8–1.2.
All available images were reviewed by two radiologists (AS and KR, with 2 and 6 years' experience in breast imaging, respectively). Consensus of the investigators was obtained on the following features of the identified breast lesions: number, shape, localisation, size, margin, attenuation, homogeneity and contrast enhancement patterns. Lesion size was determined by measuring the maximum diameter.
Case histories were also reviewed retrospectively to determine clinical signs at presentation.
Histological analysis
In all patients, the diagnosis of BL was confirmed histopathologically. Ultrasound-guided biopsy was performed in most cases (n=27). Stereotactic biopsy was taken in eight instances and MR-guided biopsy in one.
Biopsy specimens were evaluated by one pathologist (HJH, 37 years' experience). All available histological materials (sections stained by haematoxylin/eosin or by immunohistochemistry) were analysed.
Statistics
For statistical analysis, the SPSS statistical software package was used (SPSS 17.0; IBM Corporation, Armonk, NY). Collected data were evaluated by means of descriptive statistics (absolute and relative frequencies). Continuous variables were expressed as mean±standard deviation, and categorical variables as percentages. Patient-specific outcomes were analysed by the Mann–Whitney U-test. Analyses of lesion-specific outcomes were performed by means of generalised linear mixed models.
A p-value<0.05 was taken to indicate statistical significance in all instances.
Results
Prevalence, localisation and clinical signs
The prevalence of BL in our institution (15 patients) was 1.6% of all NHL cases and 0.5% of breast cancer. Of the total identified 36 cases, PBL was diagnosed in 22 (61%) and SBL in 14 (39%). B-cell lymphoma was found in 34 patients (94%) and T-cell lymphoma in 2 patients (6%).
The left breast was involved in 14 patients (39%) and the right breast in 12 (33%), and bilateral lesions were identified in 10 patients (28%). 96 lesions were identified in 36 patients (2.7 lesions per patient). The mean size was 15.8±8.3 mm (median size 12 mm, range 3–60 mm). In 19 patients (53%), the lesions were multiple, ranging from 2 to 9, and in 17 patients (47%) they were solitary. The number of intramammary lesions was higher in SBL than in PBL (3.6 vs 2.0 lesions per patient, p=0.01). The size of the identified intramammary lesions was larger in PBL than in SBL (23.3 vs 10.8 mm, p=0.0001).
Clinically, 31 patients (86%) presented with solitary or multiple breast lumps. They were painful in 2 and painless in 29 cases. In 5 patients (14%), unilateral or bilateral axillary lymphadenopathy occurred too. Skin oedema was seen in two patients (6%). In 5 patients (14%), breast involvement was diagnosed incidentally during staging examination in CT.
Radiological features
Mammographic findings
3 mammographic patterns (85 findings) could be identified (Table 1):
Table 1. Mammographic findings of primary and secondary breast lymphoma.
| Characteristics | Patients | % |
| Patterns | ||
| Mass | 27 | 82 |
| Architectural distortion | 3 | 9 |
| No abnormalities | 3 | 9 |
| Mass features | Lesions (%) | |
| Shape | ||
| Round | 54 (68) | |
| Lobular | 2 (3) | |
| Oval | 23 (29) | |
| Margin | ||
| Circumscribed | 52 (66) | |
| Lobulated | 24 (30) | |
| Indistinct | 2 (3) | |
| Spiculated Density | 1 (1) | |
| High | 64 (81) | |
| Isodense | 15 (19) | |
breast masses (82%)
architectural distortion (9%)
no abnormalities (9%).
Intramammary masses were the most common mammographic appearance of BL (Figures 1–3). Most of them were round or oval in shape with circumscribed or microlobulated margins. There was no evidence of calcifications. There was no significant difference between PBL and SBL groups in the characteristics of the masses. Architectural distortion occurred in three patients (9%). In these patients, PBL was diagnosed (Figures 4 and 5).
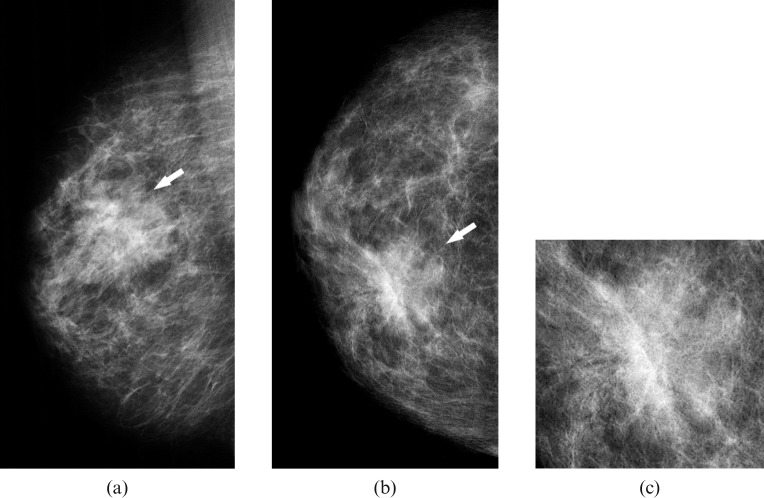
Figure 1.
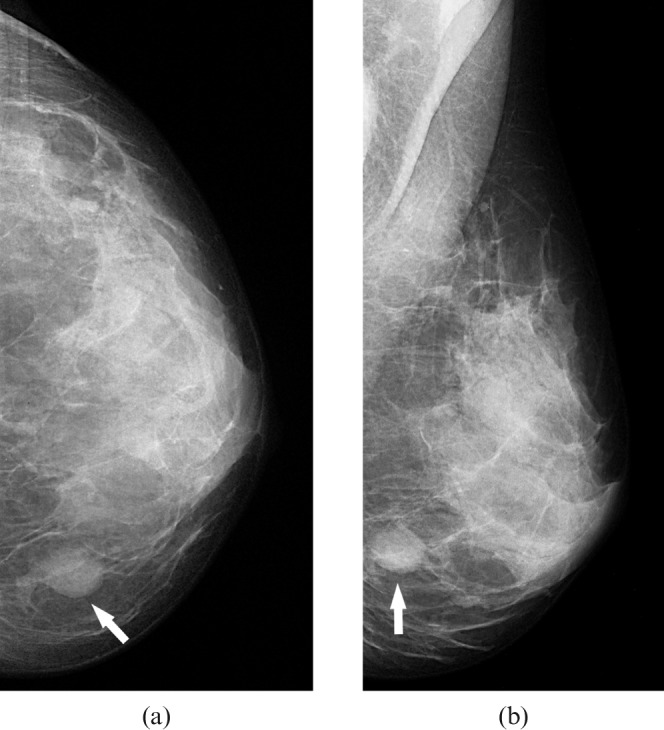
Craniocaudal (a) and mediolateral oblique mammograms (b) of the left breast in a patient with primary breast lymphoma showing a round, dense mass with circumscribed margins in the lower inner quadrant (arrows).
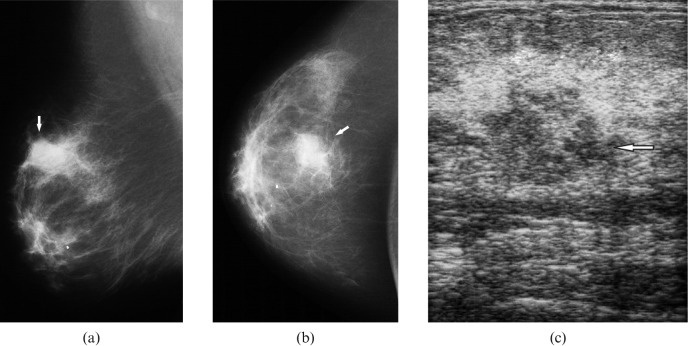
Figure 3.
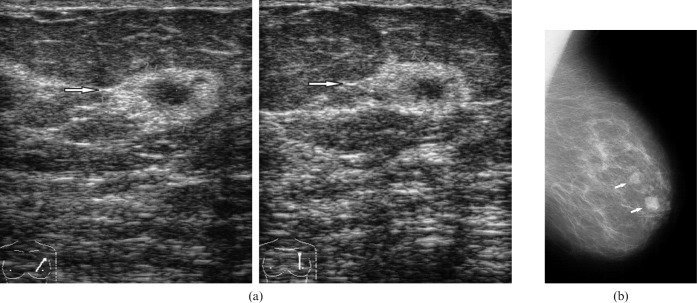
Radiological images in a 59-year-old patient with a right breast lump and known history of primary lymphoma of the left breast, 22 years before this presentation. Mediolateral oblique (a) and craniocaudal mammograms (b) of the right breast showing a microlobulated dense mass in the upper outer quadrant (arrows). (c) On ultrasound, the mass was mixed hypo- to hyperechoic with indistinct margins (arrow).
Figure 4.
Mediolateral oblique (a) and craniocaudal mammograms (b) of the right breast in a patient with primary breast lymphoma showing an asymmetric, poorly defined density containing architectural distortion (arrows) in the upper medial quadrant suspicious for a breast carcinoma. (c) Magnification view of the lesion.
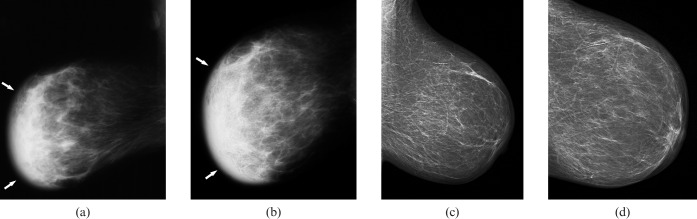
Figure 5.
Mammographic findings in a 68-year-old patient with secondary breast lymphoma. Mediolateral oblique (a) and craniocaudal mammograms (b) of the right breast showing a global asymmetry (diffuse opacity) of the parenchyma (arrows) with skin oedema compared with the left breast (c, d).
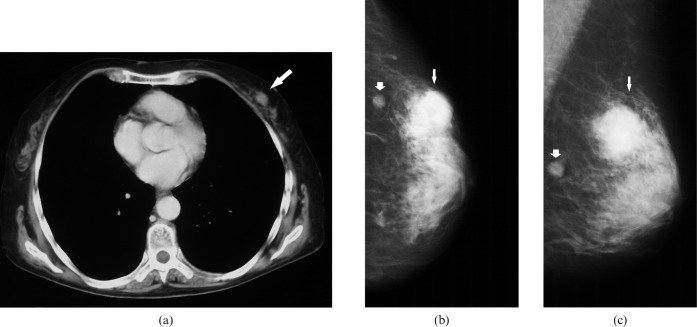
Figure 2.
Radiological findings in a 62-year-old patient with secondary breast lymphoma. (a) CT of the chest documenting an irregular mass in the left breast (arrow). Craniocaudal (b) and mediolateral oblique mammograms (c) of the left breast showing a round, dense mass with irregular defined margins in the upper outer quadrant (small arrows). A small, well-defined mass in this quadrant is also seen (fat arrows).
In three patients (9%), no abnormalities were found mammographically.
In 21 of 33 patients who underwent mammography, the identified masses were interpreted were classified as BI-RADS 3. Only in nine patients was a malignancy suspected (BI-RADS 4 or 5).
Ultrasound findings
Ultrasound images were available for 20 patients (33 lesions). The ultrasound features are shown in Table 2. Most of the identified lesions were homogeneously hypoechoic and oval or round in shape with circumscribed or microlobulated margins (Figures 6 and 7).
Table 2. Ultrasound findings of breast masses in primary and secondary breast lymphoma.
| Characteristics | n (%) |
| Shape | |
| Oval | 22 (67) |
| Round | 7 (21) |
| Lobular | 4 (12) |
| Echo pattern | |
| Anechoic | 5 (15) |
| Hypoechoic | 15 (46) |
| Mixed hypo- to hyperechoic | 13 (39) |
| Margin | |
| Circumscribed | 17 (52) |
| Microlobulated | 12 (36) |
| Indistinct | 4 (12) |
| Lesion boundary | |
| Echogenic halo | 1 (3) |
| None | 32 (97) |
| Posterior phenomenon | |
| Enhancement | 17 (52) |
| None | 12 (36) |
| Shadowing | 4 (12) |
Figure 6.
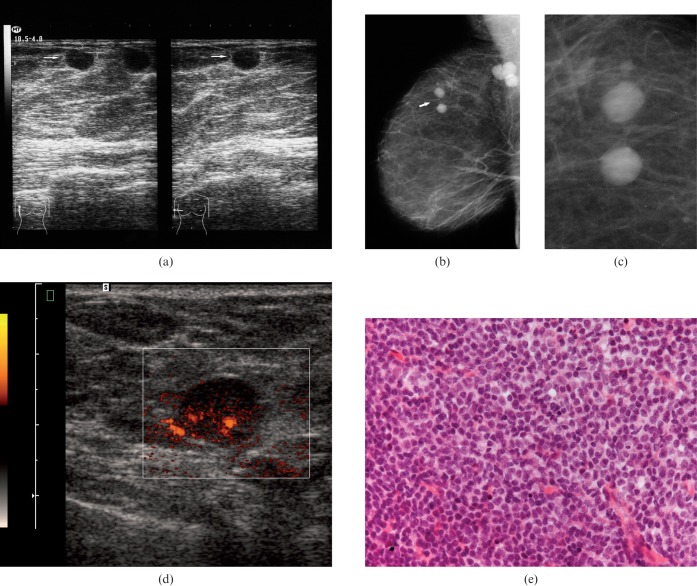
Breast images of a 64-year-old patient with secondary breast lymphoma. (a) Ultrasound showing circumscribed round anechoic lesions without posterior acoustic phenomena (arrows). (b) On mammography, the identified lesions were also well defined (arrow). Additionally, axillary lymph nodes were seen. (c) Magnification view of the lesion. (d) Power Doppler documenting the hypervascularity of one lesion. (e) Histological analysis after ultrasound-guided biopsy confirmed a B-cell lymphoma (Haematoxylin and eosin stain, ×100).
Figure 7.
Radiological findings in a 77-year-old female with a left breast lump. (a) Ultrasound demonstrating a hypoechoic lesion (arrows) with a broad hyperechoic boundary and indistinct margins without posterior acoustic phenomenon. (b) Mammography showing dense lesions with circumscribed margins (arrows).
There was no significant difference between the PBL and the SBL groups in the characteristics of the masses. Colour Doppler showed hypervascularity in 6 cases (55%). No vascularity was seen in 5 cases (45%). Ultrasound findings were categorised as BI-RADS 2 in 3 of 20 patients who underwent ultrasound, BI-RADS 3 in 3, BI-RADS 4 in 10 and BI-RADS 5 in 4.
MR findings
In 8 patients (23 lesions), MRI was performed. There were patients with high breast parenchymal density. MR features of the BL were variable (Table 3). The identified lesions showed a minimally hyperintense signal compared with normal breast parenchyma on T2W images. On T1W images, BLs were isointense to the normal breast tissue. After intravenous administration of contrast medium, a marked inhomogeneous contrast enhancement was seen in most lesions (Figures 8 and 9). Kinetic analysis of contrast enhancement was performed for 20 lesions. It showed in all cases a rapid Initial SI over 100% compared with pre-contrast signal intensity (median Initial SI 248%; range 102–441%). The median TTP was 270 s (range 180–270 s).
Table 3. MR findings of breast lymphoma.
| Characteristics | n (%) |
| Patterns | |
| Mass | 17 (81) |
| Non-mass enhancement | 4 (19) |
| Mass features | |
| Shape | |
| Oval | 6 (35) |
| Round | 8 (47) |
| Lobular | 3 (18) |
| Margin | |
| Smooth | 12 (71) |
| Irregular | 5 (29) |
| Homogeneity | |
| Homogeneous | 14 (82) |
| Inhomogeneous | 3 (18) |
| Non-mass enhancement | |
| Linear | 2 (50) |
| Regional | 2 (50) |
Figure 8.
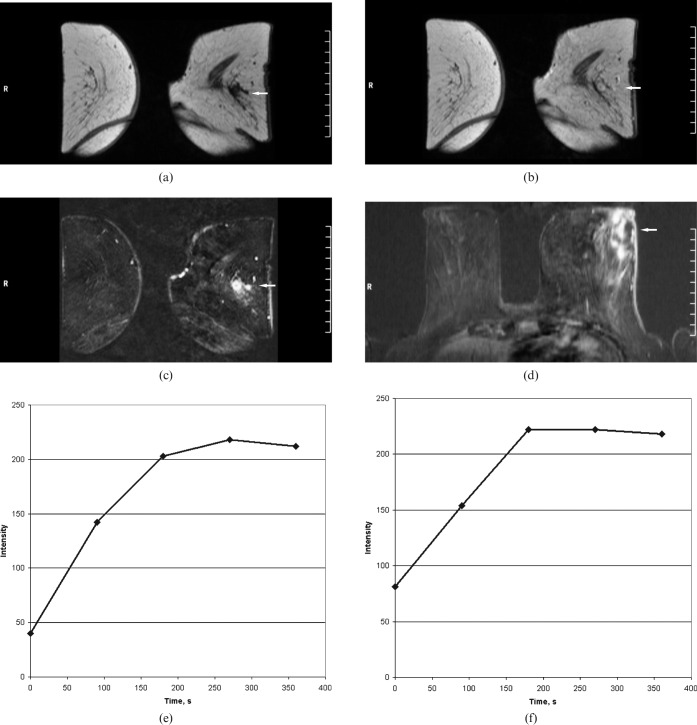
MRI of the breasts in a 61-year-old female with fever, weight loss and left breast swelling. (a) T1 weighted image before contrast administration confirmed masses in the left breast. These are isointense vs breast tissue (arrow). (b) First contrast-enhanced image showed a rapid contrast enhancement (arrow). (c) Subtracted images demonstrated multiple round lesions with marked enhancement. Additionally, a lateral cutaneous thickness of the left breast was seen (arrow). (d) Transverse reconstructions of a contrast-enhanced T1 weighted image showed additionally a moderate ductal enhancement between the lesions (arrow). (e, f) Time–intensity curves of the lesions. A strong rapid enhancement in the first minute associated with plateau (Type II curves). Time to peak 180 and 270 s; initial signal intensity, 174% and 407%.
Figure 9.
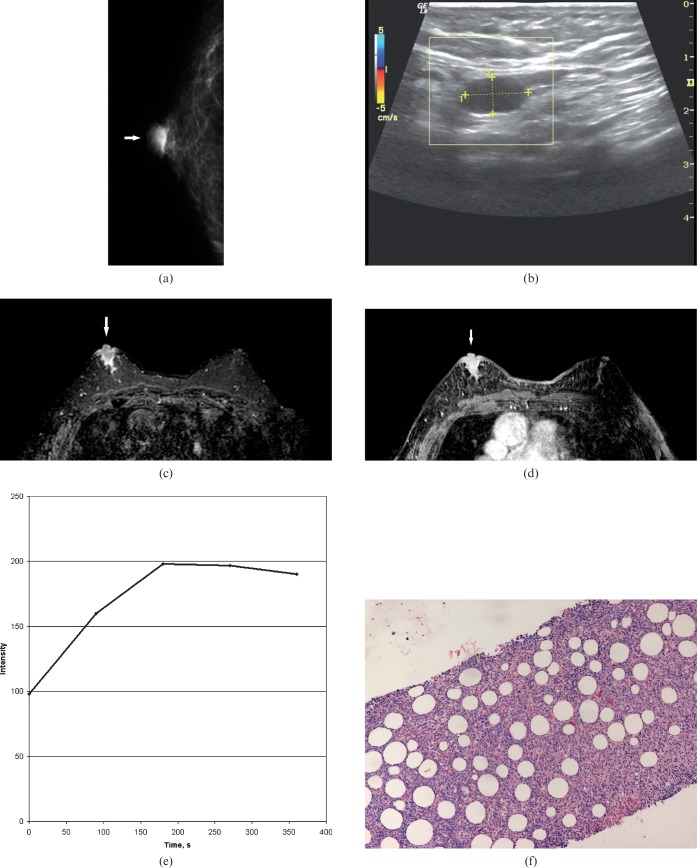
Imaging findings in an 85-year-old male with a right breast lump. (a) On mammography, the identified lesion was oval in shape with circumscribed margins (arrow). (b) On ultrasound with power Doppler, the lesion was homogeneously anechoic with circumscribed margins and posterior acoustic enhancement. There was no vascularity. (c) T2 weighted image with fat saturation demonstrating a hyperintense lobulated lesion in the right breast (arrow). (d) Subtracted image after intravenous contrast administration showing a marked enhancement of the lesion (arrow). (e) Time–intensity curve of the main lesion. A strong rapid enhancement in the first minute associated with plateau (Type II curve). Time to peak, 180 s; initial signal intensity, 102%. (f) Histological examination after ultrasound-guided biopsy revealed a B-cell lymphoma (haematoxylin and eosin stain, ×200).
Type 1 kinetic curve was seen in one lesion (5%). Type 2 kinetic curve was present in most cases (18 lesions, 90%). Type 3 kinetic curve was identified in one lesion (5%). The wash-out ratio was 12%. All MR findings were classified as BI-RADS 5.
CT findings
For 7 patients (18 lesions), CT images of the thorax were available. Most lesions were round or oval in shape with high or moderate enhancement.
Discussion
Prevalence, clinical signs and localisation of BL
Malignant lymphoma of the breast is an unusual diagnosis [1-5,20]. Lymphomas can become manifest as a primary breast tumour, or they can involve the breast secondarily as part of a metastatic process [7,11,20,21]. According to the literature, the true prevalence of breast lymphoma is difficult to determine [1,2]. In our series, the prevalence of BL was 1.6% of all identified cases with NHL and 0.5% of cases with breast cancer.
Clinically, BL can present with several symptoms. The symptoms are non-specific and may mimic those of breast carcinoma [1]. According to the literature, the most common presenting symptom was an enlarging, painless breast mass [1,22,23]. Pain has been reported to occur in 4–25% of patients [1,23]. Other local signs, such as nipple retraction or discharge and skin changes, are rare [1,2]. Likewise, systemic symptoms, such as sweating, weight loss and fever, are rare, and have been reported to occur in approximately 8–9% of the reported cases [2,21-23]. However, in the study of Ganjoo et al [24], 24% of BLs were clinically asymptomatic when identified incidentally on screening mammograms.
In agreement with previous reports in our series, most patients presented with painless solitary or multiple breast lumps. Other signs, such as skin oedema or local pain, were rare. 14% of BLs were diagnosed incidentally. Systemic symptoms occurred in only 8%. Some authors regard bilateral involvement as a possible specific feature of BL in contrast to breast cancer [11]. However, in the study of Liu and Clark [12] there were no instances of bilaterality. Most series documented that lymphomas tend to involve the right breast [1,2,25-29]. The ratio of right to left has been reported as 2:1 [6]. In addition, lesions have been reported to be located especially in the lower outer quadrant [2]. Ipsilateral axillary nodal involvement has been shown in more than 40% of patients with PBL [2].
In the present study, no side predominance of BL localisation was seen. Bilateral involvement occurred in 28%. Axillar lymphadenopathy was shown in 28% of patients. Most series have focused either on PBL or SBL [2-5,12,21,25]. Only a few studies compared the clinical and radiological features between PBL and SBL. It has been postulated that these were similar for both lymphomas [7,11]. Sabate et al [1], however, suggested that lesions in SBL presented with smaller diameters than those observed in primary cases. In our study, the number of intramammary lesions was higher in SBL than in PBL. In addition, the size of the identified intramammary lesions was significantly larger in PBL. Involvement of the left breast occurred more frequently in PBL than in SBL.
Radiological appearances
Mammographic finding
According to the literature, the radiological features of BL are not pathognomonic [1]. Radiological studies describe a relatively small number of lesions, ranging from 10 to 32 [1,7,11-14,27,28]. Therefore, our study with 96 findings in 36 patients is the largest to date. Previous reports suggested that on mammography most lesions are oval and high-density solitary or multiple masses with a well-circumscribed margin [13,14,28,30]. Typically, no masses had spiculated margins or calcifications [7,11,28,29]. However, in the study of Sabate et al [1], most masses presented with irregular or partially defined margins. In addition, well-defined contours mimicking benign lesions were depicted in only 12.5% of the identified cases [1]. Meyer et al [27] reported that in all cases with PBL, minimal and moderate spiculation was seen. Intramammary masses in BL are commonly solitary [13,30]. Other features such as diffuse increased opacity with or without skin thickening have also been described as mammographic manifestations of BL [1,7]. This pattern occurs in 9–33% of the cases [1,7,13]. Pameijer et al [31] reported miliary densities on mammography in a patient with NHL of the breast. 13% of patients with BL, however, showed no abnormalities on mammography [7,30].
In our study, intramammary masses were the most common mammographic pattern of BL (92.9% of the findings). Most of the masses were round or oval and had well-defined margins. This finding agrees with data presented in previous reports [1,7,11-14]. There was no significant difference in shape and margin characteristics of lesions between the PBL and SBL groups. In 3.6% of cases, BL presented on mammography as architectural distortion (focal or global asymmetry). In 3.5%, no mammographic abnormalities could be found.
Several reports have suggested a correlation between the histopathological type of BL and its imaging features [1,7,27]. High-grade lymphomas have been reported to manifest more commonly as diffuse breast enlargement, whereas low and intermediate grade NHL would preferentially display nodular patterns on imaging [1]. In our study, no such relationships between imaging and pathological findings could be identified.
Ultrasound finding
Although mammographic patterns in BL tend to vary, the sonographic appearance is typically uniform [11]. Usually, ultrasound demonstrates hypoechoic round or oval masses [11,13]. In addition, these lesions may be so significantly hypoechoic that they can be mistaken for simple cysts [13,30]. Lyou et al [28], however, described a hyperechoic mass in one BL patient. In the series by Yang et al [13], the most common ultrasound feature of BL was a solitary hypoechoic or mixed hypo- to hyperechoic mass with indistinct margins. In addition, masses with an echogenic centre and a surrounding hypoechoic zone have also been described [32]. Typically, no posterior attenuation is apparent [13]. However, Liberman et al [7] reported that posterior acoustical enhancement was present in 71% of cases. Mixed posterior attenuation with areas of shadowing and enhancement has been also described in BL [7,13,33].
In our series, 52% of the identified lesions were homogeneously hypoechoic and 48% were mixed hypo- to hyperechoic. In one case, the identified mixed lesions were homogeneously hypoechoic in the centre with a large hyperechoic margin. This feature has not been described in the literature. Concordant with previous reports, most lesions were oval or round in shape with circumscribed or ill-defined margins.
In addition, posterior acoustic phenomena were present in most cases.
Interestingly, ultrasound BI-RADS had a higher percentage of Category 4/5 lesions (70%) than mammography (18%). In one patient, the identified lesions were homogeneously hypo- or anechoic with circumscribed margins on ultrasound. This finding suggesting intramammary cysts has been described previously in the literature [13]. Further investigations, such as Doppler imaging, are needed here. However, according to the literature, colour Doppler imaging showed hypervascularity in 64% of BL lesions, and no vascularity in 9% [13]. Therefore, MR evaluation of masses that appear as probable cysts in patients with known lymphoma, or the appropriate history that might suggest lymphoma, can be helpful for correct diagnosis.
MRI findings
MRI findings of BL consist predominantly of isolated case reports [13,32,34]. Only one study analysed seven lesions in PBL [35]. Furthermore, in most reports only qualitative analysis of the enhancement kinetic was done.
In our series, 23 lesions were analysed in 8 patients. The identified lesions were hyperintense compared with breast parenchyma on T2W images and isointense on T1W images. After intravenous administration of contrast medium, homogeneous contrast enhancement was seen in most lesions.
Kinetic analysis of contrast enhancement showed in all cases a rapid initial signal increase over 100% compared with pre-contrast signal intensity. The median TTP was 270 s after administration of contrast medium. Type 1 kinetic curve was seen in one lesion (5%). Type 2 kinetic was present in majority of cases (18 lesions, 90%). Type 3 kinetic was identified in one lesion (5%).
CT and positron emission tomography findings
On CT, most lesions were round or oval with circumscribed margins. These findings of BL are non-specific, and could equally represent a cyst, fibroadenoma or breast carcinoma.
In a few reports, positron emission tomography (PET) was used in patients with BL [13,36-38]. Local and diffuse uptake has been described here. However, PET findings are non-specific and can mimic those of breast cancer or other malignant or benign breast diseases [13,39-41]. None of our patients underwent PET.
Histological diagnosis
Because the clinical signs and radiological features of BL are non-specific, a malignant lymphoma was suspected in 18% of patients only [2]. Other clinical diagnoses in previous reports before histopathological examination included carcinoma, inflammatory carcinoma, sarcoma, fibroadenoma and mammary dysplasia [1,2]. Stanton et al [42] reported one case of PBL masquerading clinically and radiologically as a breast abscess. In addition, PBL mimicking acute mastitis has also been described [43]. Therefore, the diagnosis should be established histopathologically. According to the literature, fine-needle aspiration cytology and/or excisional biopsy can differentiate NHL involvement of the breast from epithelial tumours [1,2]. In contrast, other authors have reported that the biopsy must be performed with a large needle because fine-needle puncture has a low diagnostic value [11]. Histologically, the majority of cases are B-cell lymphomas [1-7]. However, other types of BL, such as mucosa-associated lymphoma, Burkitt lymphoma and T-cell lymphoma have been described [44-46]. In our study, B-cell lymphoma was found in 94% and T-cell lymphoma in 6%.
There are several limitations to our study. First, this is a retrospective analysis. Second, not every patient underwent all investigations (mammography, ultrasound, Doppler and MRI). Third, our retrospective review was based on consensus and therefore the important aspect of observer variability could not be addressed.
In conclusion, our study shows that BL is a rare condition with a prevalence of 1.6% of all identified cases with NHL and 0.5% of cases with breast cancer. It has no specific clinical signs and can be an incidental finding.
Involvement of the left breast occurred more frequently in PBL than in SBL. The size of the identified intramammary lesions was larger in PBL. However, the number of intramammary lesions was higher in SBL than in PBL.
The most common mammographic patterns of BL are multiple intramammary round or oval masses with well-defined margins, mimicking benign breast disease. There was no significant difference in the mammographic characteristics of lesions between the PBL and the SBL groups. BL can manifest on mammography as architectural distortion or as diffuse breast opacity masquerading breast carcinoma.
On ultrasound, BL presents typically with hypoechoic or mixed hypo- to hyperechoic masses with posterior acoustic enhancement in most cases. A lobulated shape is more commonly seen in PBL than in SBL.
On MRI, BL lesions are variable in shape and show rapid contrast enhancement with post-initial plateau.
References
- 1.Sabate JM, Gomez A, Torruba S, Camins A, Roson N, De LasHeras P, et al. Lymphoma of the breast: clinical and radiological features with pathologic correlation in 28 patients. Breast J 2002;8:294–304 [DOI] [PubMed] [Google Scholar]
- 2.Giardini R, Piccolo C, Rilke F. Primary non-Hodgkin's lymphomas of the female breast. Cancer 1992;69:725–35 [DOI] [PubMed] [Google Scholar]
- 3.Ribrag V, Bibeau F, El Weshi A, Frayfer J, Fadel C, Cebotaru C, et al. Primary breast lymphoma: a report of 20 cases. Br J Haematol 2001;115:253–6 [DOI] [PubMed] [Google Scholar]
- 4.Hugh JC, Jackson FI, Hanson J, Poppema S. Primary breast lymphoma. An immunohistologic study of 20 new cases. Cancer 1990;66:2602–11 [DOI] [PubMed] [Google Scholar]
- 5.Avilés A, Delgado S, Nambo MJ, Neri N, Murillo E, Cleto S. Primary breast lymphoma: results of a controlled clinical trial. Oncology 2005;69:256–60 [DOI] [PubMed] [Google Scholar]
- 6.Schouten J, Weese JL, Carbone PP. Lymphoma of the breast. Ann Surg 1981;194:749–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liberman L, Giess CS, Dershaw DD, Louie DC, Deutch BM. Non-Hodgkin lymphoma of the breast: imaging characteristics and correlation with histopathologic findings. Radiology 1994;192:157–60 [DOI] [PubMed] [Google Scholar]
- 8.Slanetz PJ, Whitman GJ. Non-Hodgkin`s lymphoma of the breast causing multiple vague densities on mammography. AJR Am J Roentgenol 1996;167:537–8 [DOI] [PubMed] [Google Scholar]
- 9.Dao AH, Adkins RB, Glick AD. Malignant lymphoma of the breast. Am Surg 1992;58:792–6 [PubMed] [Google Scholar]
- 10.Brogi E, Harris NL. Lymphomas of the breast: pathology and clinical behaviour. Semin Oncol 1999;26:357–64 [PubMed] [Google Scholar]
- 11.Balu-Maestro C, Bruneton JN, Rogopoulos A, Marcy PY, Guidicelli T, Raffaelli C, et al. Mammographic and ultrasonographic appearances of lymphoma of the breast. Eur Radiol 1992;2:565–9 [Google Scholar]
- 12.Liu FF, Clark RM. Primary lymphoma of the breast. Clin Radiol 1986;37:567–70 [DOI] [PubMed] [Google Scholar]
- 13.Yang WT, Lane DL, Le-Petross HT, Abruzzo LV, Macapinlac HA. Breast lymphoma: imaging findings of 32 tumors in 27 patients. Radiology 2007;245:692–702 [DOI] [PubMed] [Google Scholar]
- 14.Kuhl CK, Mielcareck P, Leutner C, Wardelmann E, Gieseke J, Schild HH. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions. Radiology 1999;211:101–10 [DOI] [PubMed] [Google Scholar]
- 15.Szabo BK, Aspelin P, Wiberg MK, Bone B. Dynamic MR imaging of the breast: analysis of kinetic and morphologic diagnostic criteria. Acta Radiol 2003;44:379–86 [DOI] [PubMed] [Google Scholar]
- 16.Baum F, Fischer U, Vosshenrich R, Grabbe E. Classification of hypervascularized lesions in CE MR imaging of the breast. Eur Radiol 2002;12:1087–92 [DOI] [PubMed] [Google Scholar]
- 17.Lehman C, Holt S, Peacock S, White E, Urban N. Use of the American college of radiology BI-RADS guidelines by community radiologists: concordance of assessments and recommendations assigned to screening mammograms. AJR Am J Roentgenol 2002;179:15–20 [DOI] [PubMed] [Google Scholar]
- 18.Kim EK, Ko KH, Oh KK, Kwak JY, You JK, Kim MJ, et al. Clinical application of the BI-RADS final assessment to breast sonography in conjunction with mammography. AJR Am J Roentgenol 2008;190:1209–15 [DOI] [PubMed] [Google Scholar]
- 19.Erguvan-Dogan B, Whitman GJ, Kushwaha AC, Phelps MJ, Dempsey PJ. BI-RADS-MRI: a primer. AJR Am J Roentgenol 2005;187:152–60 [DOI] [PubMed] [Google Scholar]
- 20.Lee WK, Duddalwar VA, Rouse HC, Lau EW, Bekhit E, Hennessy OF. Extranodal lymphoma in the thorax: cross-sectional imaging findings. Clin Radiol 2009;64:542–9 [DOI] [PubMed] [Google Scholar]
- 21.DeCosse JJ, Berg JW, Fracchia AA, Farrow JH. Primary lymphosarcoma of the breast. A review of 14 cases. Cancer 1962;15:1264–8 [DOI] [PubMed] [Google Scholar]
- 22.Kuper-Hommel MJ, Snijder S, Janssen-Heijnen ML, Vrints LW, Kluin-Nelemans JC, Coebergh JW, et al. Treatment and survival of 38 female breast lymphomas: a population-based study with clinical and pathological reviews. Ann Hematol 2003;82:397–404 [DOI] [PubMed] [Google Scholar]
- 23.Brustein S, Filippa DA, Kimmel M, Lieberman PH, Rosen PP. Malignant lymphoma of the breast. Ann Surg 1987;205:144–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganjoo K, Advani R, Mariappan MR, McMillan A, Horning S. Non-Hodgkin lymphoma of the breast. Cancer 2007;110:25–30 [DOI] [PubMed] [Google Scholar]
- 25.Wiseman C, Liao K. Primary lymphoma of the breast. Cancer 1972;29:1705–12 [DOI] [PubMed] [Google Scholar]
- 26.Lawler MR, Jr, Richie RE. Reticulum cell sarcoma of the breast. Cancer 1967;20:1438–46 [DOI] [PubMed] [Google Scholar]
- 27.Meyer JE, Kopans DB, Long JC. Mammographic appearance of malignant lymphoma of the breast. Radiology 1980;135:623–6 [DOI] [PubMed] [Google Scholar]
- 28.Lyou CY, Yang SK, Choe DH, Lee BH, Kim KH. Mammographic and sonographic findings of primary breast lymphoma. Clin Imaging 2007;31:234–8 [DOI] [PubMed] [Google Scholar]
- 29.Paulus DD. Lymphoma of the breast. Radiol Clin North Am 1990;28:833–40 [PubMed] [Google Scholar]
- 30.Jackson FI, Lalani ZH. Breast lymphoma: radiologic imaging and clinical appearances. Can Assoc Radiol J 1991;42:48–54 [PubMed] [Google Scholar]
- 31.Pameijer FA, Beijerinck D, Hoogenbom HH, Deurenberg JJ, Nortier JW. Non-Hodgkin`s lymphoma of the breast causing miliary densities on mammography. AJR Am J Roentgenol 1994;164:609–10 [DOI] [PubMed] [Google Scholar]
- 32.Mussurakis S, Carleton PJ, Turnbull LW. MR imaging of primary non-Hodgkin's breast lymphoma. Acta Radiol 1997;38:104–7 [DOI] [PubMed] [Google Scholar]
- 33.Kiziltepe TT, Erden GA, Dingil G, Ince A. Breast metastasis from non-Hodgkin's lymphoma: evaluation with color Doppler sonography. AJR Am J Roentgenol 1996;167:1595–6 [DOI] [PubMed] [Google Scholar]
- 34.Demirkazik FB. MR imaging features of breast lymphoma. Eur Radiol 2002;42:62–4 [DOI] [PubMed] [Google Scholar]
- 35.Rizzo S, Preda L, Villa G, Brambilla S, Pruneri G, Alietti A, et al. magnetic resonance imaging of primary breast lymphoma. Radiol Med 2009;114:915–24 [DOI] [PubMed] [Google Scholar]
- 36.Nihashi T, Hayasaka K, Itou T, Ito K, Kato R, Okae T, et al. Findings of fluorine-18-FDG PET in extranodal origin lymphoma—in three cases of diffuse large B cell type lymphoma. Ann Nucl Med 2008;20:689–93 [DOI] [PubMed] [Google Scholar]
- 37.Kyoung Jung H, Kim EK, Yun M, Jung Kim M, Yonung Kwak J. Bilateral breasts involvement in Burkitt's lymphoma detected only by FDG-PET. Clin Imaging 2006;30:57–9 [DOI] [PubMed] [Google Scholar]
- 38.Kumar R, Xiu Y, Dhurairaj T, Yu JQ, Alavi A, Zhuang H. F-18 FDG positron emission tomography in non-Hodgkin lymphoma of the breast. Clin Nucl Med 2005;30:246–8 [DOI] [PubMed] [Google Scholar]
- 39.Kim MJ, Kim EK, Park SY, Yun M, Oh KK. Multiple nodular adenosis concurrent with primary breast lymphoma: pitfall in PET. Clin Radiol 2005;60:126–9 [DOI] [PubMed] [Google Scholar]
- 40.Castellucci P, Nanni C, Farsad M, Alinari L, Zinzani P, Stefoni V, et al. Potential pitfalls of 18F-FDG PET in a large series of patients treated for malignant lymphoma: prevalence and scan interpretation. Nucl Med Commun 2005;26:689–94 [DOI] [PubMed] [Google Scholar]
- 41.Moralidis E, Mandala E, Venizelos I, Arsos G, Zafiriadu E, Goutzioulis M. A breast fibroadenoma mimicking an extranodal deposit of Hodgkin's lymphoma in 67Ga imaging. Br J Radiol 2009;82:e58–62 [DOI] [PubMed] [Google Scholar]
- 42.Stanton MP, Cutress R, Royle GT. Primary non-Hodgkin's lymphoma of the female breast masquerading as a breast abscess. Eur J Surg Oncol 2000;26:429. [DOI] [PubMed] [Google Scholar]
- 43.Grubstein A, Givon-Madhala O, Morgenstern S, Cohen M. Extranodal primary B-cell non-Hodgkin lymphoma of the breast mimicking acute mastitis. J Clin Ultrasound 2005;33:140–2 [DOI] [PubMed] [Google Scholar]
- 44.Huber S, Vesely M, Medl M, Czembirek H. Low-grade mucosa-associated lymphoma of the breast: radiological-pathological correlation. Eur Radiol 2002;12:1093–6 [DOI] [PubMed] [Google Scholar]
- 45.Schramm N, Pfluger T, Reiser MF, Berger F. Subcutaneous panniculitis-like T-cell lymphoma with breast involvement: functional and morphological imaging findings. Br J Radiol 2010;83:e90–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DiPiro PJ, Lester S, Meyer JE, Denison CM, Takvorian T. Non-Hodgkin lymphoma of the breast: clinical and radiological presentations. Breast J 1996;6:380–4 [Google Scholar]