Abstract
Stereotactic body radiotherapy for early stage non-small cell lung cancer is an emerging treatment option in the UK. Since relatively few high-dose ablative fractions are delivered to a small target volume, the consequences of a geometric miss are potentially severe. This paper presents the results of treatment delivery set-up data collected using Elekta Synergy (Elekta, Crawley, UK) cone-beam CT imaging for 17 patients immobilised using the Bodyfix system (Medical Intelligence, Schwabmuenchen, Germany). Images were acquired on the linear accelerator at initial patient treatment set-up, following any position correction adjustments, and post-treatment. These were matched to the localisation CT scan using the Elekta XVI software. In total, 71 fractions were analysed for patient set-up errors. The mean vector error at initial set-up was calculated as 5.3±2.7 mm, which was significantly reduced to 1.4±0.7 mm following image guided correction. Post-treatment the corresponding value was 2.1±1.2 mm. The use of the Bodyfix abdominal compression plate on 5 patients to reduce the range of tumour excursion during respiration produced mean longitudinal set-up corrections of −4.4±4.5 mm compared with −0.7±2.6 mm without compression for the remaining 12 patients. The use of abdominal compression led to a greater variation in set-up errors and a shift in the mean value.
Early stage non-small cell lung cancer in the UK is traditionally treated with surgical resection. This treatment modality is associated with a 60–70% 5-year overall survival rate [1]. However, there is a significant proportion of this patient group for whom surgery is not an option because the patients are either medically inoperable owing to comorbidity or choose not to undergo surgery. For these patients, conventional conformal radiotherapy is an alternative. However, long-term survival rates with long-course radiotherapy treatment are typically half those of surgery; local recurrence is the most prominent cause of failure [2]. In recent years, stereotactic body radiotherapy (SBRT) has gained favour in the USA, Japan and Western Europe as an alternative treatment. Several authors have published data indicating good local control, similar to that of surgery, with small numbers of high-dose ablative fractions [3,4]. The evidence, albeit from non-randomised studies, supports the use of SBRT as an enhancement to conventional radiotherapy with respect to local control, with the potential to improve the overall survival for these patients. This treatment technique was introduced into clinical practice in Middlesbrough, UK, in September 2009. Treatments were delivered on an Elekta Synergy linear accelerator (Elekta, Crawley, UK) employing the cone-beam CT (CBCT) facility and XVI software (Elekta) for image guidance at each treatment.
Methods and materials
Patient immobilisation and localisation
All patients were positioned in a flat and reproducible position: supine with both arms above their head. Each patient had a customised Bodyfix (Medical Intelligence, Schwabmuenchen, Germany) vacuum bag formed at initial localisation to provide immobilisation during subsequent planning and treatment. Patient tumour motion was initially assessed fluoroscopically under normal breathing conditions to estimate the range of travel in all three orthogonal directions. Where the maximum excursion of the tumour was greater than 1 cm from the mean position, abdominal compression was applied just below the level of the xiphisternum in an attempt to reduce this to within acceptable limits. If this was not possible, patients were offered conventional radiotherapy as an alternative. Patients suitable for SBRT had a full helical localisation CT scan with a Siemens Sensation open wide bore scanner (Siemens, Erlangen, Germany) with the patient breathing freely throughout. The slices were reconstructed contiguously with a separation of 3 mm. The scan extended from the upper cervical spine to the lower edge of the liver, which was sufficient to include all potential organs at risk. A treatment planning reference point was identified by the triangulation of three ball bearing markers on the patient's skin. Two further reduced length CT scans were acquired over a region centred around the tumour to aid outlining. One scan was acquired during light exhale and one during light inhale. The gross tumour volume (GTV) was defined as the radiologically visible “solid” tumour in the lung, which was contoured by a consultant clinical oncologist using a window of 150 HU centred at −300 HU on each of the three CT scans. An internal target volume (ITV) was generated as the union of the three defined GTV contours from the localisation, inhale and exhale scans as transferred onto the free breathing scan. The planning target volume (PTV) was defined as the ITV plus automatically generated 6 mm margins in the superior and inferior directions and 5 mm in all other directions by the ProSoma virtual simulation software (MedCom GmbH, Darmstadt, Germany).
A dose regime of either 54 Gy in 3 fractions or 55 Gy in 5 fractions for tumours with any part of the PTV in contact with the chest wall was prescribed to the 80% isodose surface. The shortened regime was delivered over 7 days and the longer fractionation over 14 days.
On-treatment imaging
Following dosimetric planning and verification, the localisation CT data and planning structures were transferred to the Elekta Synergy (Elekta) database in digital imaging and communications in medicine format to be available at the point of treatment. At each treatment fraction the patient was set up in their customised immobilisation device, which was indexed to the treatment couch. The marks for the treatment planning reference point were aligned to the sagittal and sidewall lasers. Relative moves were applied to place the centre of the ITV at the machine isocentre. Prior to treatment each day the patient underwent a full arc volumetric CBCT scan using the M20 and F1 filters to image the full longitudinal extent of the lungs. This will be referred to as the “set-up scan”. Anterior and peripheral tumours had a region of interest volume for image matching, defined or “clip box”, centred on the tumour that included part of the ipsilateral lung. Posterior tumours had a clip box defined that included part of the ipsilateral lung and across the midline to cover the whole spinal body. The set-up scan image was automatically volume registered with the localisation scan using a soft-tissue match initially. Manual refinements were made to the match by radiographers, primarily based on the overlay of the visible tumour, to create the final couch shifts required for patient alignment. The match was reviewed by two radiographers and the referring consultant clinical oncologist before being implemented. All shifts were recorded in terms of anteroposterior (vertical), supero-inferior (longitudinal) and mediolateral (lateral) directions and applied if greater than 2 mm. To confirm that any shifts had been applied correctly and that there had been no further patient movement, a second CBCT scan was acquired. This was automatically registered to the localisation scan and then manually fine tuned as before and will be referred to as the “post-correction scan”. The shifts were recorded in the three orthogonal couch movement directions. The tolerance for proceeding to treatment was that no set-up error should be greater than 2 mm. If this was the case, the adjustments would be applied and a further scan undertaken to reconfirm the efficacy of set-up. Following the patient's treatment, a third scan was acquired and matched to assess patient movement during the treatment, the post-treatment scan. Set-up correction data for all three scans in the three orthogonal directions were recorded for further analysis. For each scan the equivalent vector transformation was calculated from the three orthogonal displacement errors.
The interfraction random set-up error in each orthogonal direction (σ) was calculated as the mean of the individual random errors. The systematic set-up error in each orthogonal direction (Σ) for the population as a whole was calculated as the standard deviation of the mean corrections for each patient based on the formalism of the British Institute of Radiology Working Party [5]. From these two parameters, the ITV to PTV margin was calculated using the van Herk formula [6]: 2.5 Σ+0.7 σ; however, this will only account for patient set-up errors in this case.
Results
In total, 71 fractions were delivered to 17 patients, of whom 7 were treated with 3 fractions and 10 with 5 fractions. The PTVs ranged from 19.5 to 100.1 cm3 with a mean of value of 36.5 cm3. Each patient was imaged successfully before each treatment fraction to have an initial set-up error calculated. On 11 occasions no moves were required after the initial set-up scan; hence, no post-correction scan was acquired because the predicted adjustments were less than 2 mm in any orthogonal direction. A post-correction image acquisition was not possible on one patient fraction owing to an equipment malfunction, but treatment was delivered and authorised by a consultant clinical oncologist. Post-treatment scans were not recorded on two patient fractions owing to a malfunction of the imaging system in one case and patient non-compliance in the other.
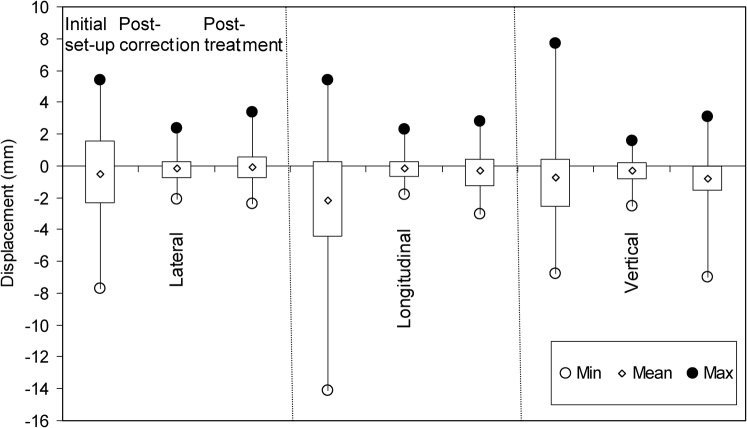
The data collected for all images acquired as part of SBRT treatment are summarised in Table 1 and presented in Figure 1 as a box-and-whisker plot. It can be seen from the tabulated data that the mean displacement correction in any one direction at initial patient set-up, post-correction or post-treatment is less than 1 mm except in the longitudinal direction for initial set-up, where it is 2.0 mm. Figure 1 clearly demonstrates the large range in set-up variation based on image matching the initial set-up CBCT scans. One patient required a movement correction of 14 mm in the longitudinal direction. The interquartile ranges for set-up corrections were 4.0 mm (lateral), 4.8 mm (longitudinal) and 3.0 mm (vertical).
Table 1. Data for patient set-up corrections in millimetres from initial set-up, post-correction and post-treatment cone beam CT scans.
| Initial set-up |
Post-correction |
Post-treatment |
|||||||
| Lat. | Long. | Vert. | Lat. | Long. | Vert. | Lat. | Long. | Vert. | |
| Number. of images | 71 | 59 | 69 | ||||||
| Mean error | −0.4 | −2.0 | −0.8 | −0.1 | −0.2 | −0.3 | −0.1 | −0.4 | −0.9 |
| σ | 3.0 | 3.9 | 2.6 | 0.9 | 0.8 | 0.8 | 1.1 | 1.3 | 1.5 |
| Maximum | 5.4 | 5.4 | 7.7 | 2.4 | 2.3 | 1.6 | 3.4 | 2.8 | 3.1 |
| Minimum | −7.7 | −14.1 | −6.8 | −2.1 | −1.8 | −2.5 | −2.4 | −3.0 | −7.0 |
| Σ | 2.6 | 3.0 | 2.1 | 0.6 | 0.3 | 0.5 | 0.8 | 1.0 | 1.0 |
| Any move >2.0 | 32 | 42 | 31 | 2 | 1 | 1 | 3 | 8 | 10 |
| Mean vector move | 5.3 | 1.4 | 2.1 | ||||||
| σvector | 2.7 | 0.7 | 1.2 | ||||||
| Vector move >3.5 | 52 | 0 | 6 | ||||||
| Margin | 8.5 | 10.3 | 7.2 | 2.1 | 1.4 | 1.8 | 2.8 | 3.3 | 3.6 |
Lat., lateral; long., longitudinal; vert., vertical; σ, interfraction random set-up error in each orthogonal direction; Σ, systematic set-up error for the whole population in each orthogonal direction; σvector, standard deviation of mean vector moves.
Figure 1.
Comparison of patient set-up errors in the three orthogonal directions from initial set-up, post-correction and post-treatment imaging. The box plots represent the interquartile range of each data set. The minimum value is shown as a hollow circle (○), maximum by a solid circle (•) and mean by a diamond (◊).
Discussion
The range of variation shown in the post-correction scan is much smaller than that at initial set-up; this was expected because initial patient set-up errors had been corrected and a tolerance of 2 mm is placed on these errors. There were four occurrences of displacements of 2−2.5 mm being sanctioned as acceptable by the attending consultant clinical oncologist without further set-up corrections being undertaken. The interquartile ranges for corrections based on the post-correction scans were 1.1 mm (lateral), 1.0 mm (longitudinal) and 1.1 mm (vertical). Post-treatment interquartile ranges were 1.4 mm (lateral), 1.7 mm (longitudinal) and 1.6 mm (vertical), indicating some patient movement during treatment.
The set-up deviations in the three orthogonal directions were combined into a vector displacement which is given in Table 1. The mean vector displacement for the initial set-up data is 5.3±2.7 mm. This was reduced to 1.4±0.7 mm for the post-correction imaging errors and slightly larger at 2.1±1.2 mm for the post-treatment imaging errors. A Student's t-test was used to compare initial set-up and post-correction mean vector shifts. There was a significant difference (p<0.05) between the means of the two samples; this was expected since we corrected patient set-up in between obtaining the two data sets. Of the 71 initial set-up scans, 52 (73%) produced a vector shift of 3.5 mm or more. There were no vector shifts of 3.5 mm or greater from the post-correction scans and 6 (9%) from the post-treatment scans, of which 4 were attributable to the first patient treated. A 3.5 mm vector shift corresponds to individual 2 mm corrections in each of the three orthogonal directions.
The values of σ calculated from the initial set-up images are 3.0, 3.9 and 2.6 mm in the lateral, longitudinal and vertical directions, respectively. Calculating the same parameters from the images taken post-correction gives values of less than 1 mm in all directions and post-treatment has a maximum σ in the vertical direction of 1.5 mm. These values compare favourably with data collected for patients immobilised in a stereotactic body frame (Elekta Oncology) or alpha cradle (KGF Enterprises, Chesterfield, MI) by Grills et al [7], also using the Elekta Synergy system. Similarly, Σ initial set-up values of 2.6, 3.0 and 2.1 mm in the lateral, longitudinal and vertical directions, respectively, compared with ≤1 mm for the post-correction and post-treatment set-up data. These are similar to the data of Grills et al [7] except for an Σ of 5.8 mm that was determined in the vertical direction for the alpha cradle. Sonke et al [8] reported Σ for post-correction errors of <1 mm in all three orthogonal directions determined using four-dimensional CBCT with patients immobilised by arm and knee support only. The calculated values of σ and Σ have been used to generate margin estimates in the three orthogonal directions for the three image sets. It can be seen, from the initial set-up data, that margins of 7−10 mm in the orthogonal directions would be required if no set-up correction were applied. Standard margins for lung SBRT at our institution are 6 mm margins in the superior and inferior directions and 5 mm in all other directions. Hence, the PTV would be expanded considerably from our current practice, which in turn would impact on the volume of normal tissue irradiated to a significant dose. The margin expansions calculated from the post-correction treatment image data are all 2 mm or less. These represent the ideal situation of a patient completely immobile during a treatment fraction and are representative of the residual accuracy of the image guidance process for this cohort. This value is consistent with our post-correction set-up tolerance of 2 mm.
The set-up data from the post-correction and post-treatment scans can be subtracted to generate additional information highlighting the patient movement during a treatment fraction. The mean move during treatment in any direction was no more than 0.5 mm with a range of 4.5, 3.9 and 6.6 mm in the lateral, longitudinal and vertical directions, respectively, for all patients. The largest four intrafraction shifts were all attributable to the first patient, who was seen to relax vertically after treatment had been delivered but before the post-treatment scan had been performed. The range of intrafraction motion in this direction would be reduced to 3.9 mm if this patient were excluded from the set. This small value shows that there is very little movement in any of the three orthogonal directions during a fraction of treatment. This lack of movement is despite the time between post-correction and post-treatment scans being of the order of 20 min and patients spending 30−40 min immobilised in total. We can use these values to generate σ and Σ for the motion during a treatment fraction and combine them with the values from the post-correction set-up image analysis. Application of the margin recipe [6] as before leads to ITV expansion margins in all directions of 4 mm to account for residual set-up error after image guidance correction and intrafraction motion. As previously mentioned, this margin does not account for target delineation and phantom transfer errors and is consistent with our current 5−6 mm margins.
7 of the 17 patients were treated with over 3 fractions and the remaining 10 were treated with 5 fractions. A significant difference was seen between the mean initial vertical set-up error (p<0.05) between the two groups of patients by comparison using the Student's t-test. The mean vertical displacement was –1.9 and −0.4 mm for the three and five fraction regime patients, respectively. There were no other significant differences between initial set-up, post-correction or post-treatment correction data between the two patient groups. The two groups are distinct in the fact that the five fraction regime is administered to patients with tumours close to the chest wall, whereas the three fraction regime is for tumours more central within the lung.
Abdominal compression was used to reduce tumour motion to <1 cm in 5 of the 17 patients after initial assessment using fluoroscopy. Means from the two groups were statistically compared for significant differences using the Student's t-test. Both means for the longitudinal and vertical initial set-up corrections were shown to be significantly different (p<0.05). The mean longitudinal corrections were −4.4 and −0.7 mm for the compressed and uncompressed groups, respectively, with standard deviations of 4.5 and 2.6 mm. A negative mean longitudinal correction for the compressed patient group implies that the patients have been set up more superiorly than planned and an inferior shift is required. The mean vertical corrections were 0.9 and −1.8 mm for the compressed and uncompressed cohorts, respectively, with standard deviations of 2.5 and 2.2 mm. Position reproducibility of patients with compression is worse than for those without compression in both longitudinal and vertical directions. The exact positioning of the compression device on the patient and the amount of compression applied will affect set-up in both of these directions. Misplacement too inferiorly on the patient is likely to push the sternum more superiorly and anteriorly than at localisation.
Conclusion
Lung patients treated using an SBRT technique are well immobilised in a Bodyfix vacuum bag. This was demonstrated with small intrafraction movements after initial set-up correction using image guidance. Differences between initial patient set-up in the longitudinal and vertical directions were seen between patients with abdominal compression applied and those without. Abdominal compression led to a greater variation in patient set-up errors and a shift in the mean set-up errors in these two directions. Our data suggest that lung SBRT should not be delivered without image guidance to correct initial set-up errors owing to the small size of the lesions treated and the large dose delivered each fraction. The risk of a geographical miss would certainly be increased unless larger margins were used to account for the increased set-up uncertainty leading in turn to irradiation of more healthy tissue.
References
- 1.Deslauriers J. Current surgical treatment of non small cell lung cancer 2001. Eur Respir J 2002;19:61S–70S [DOI] [PubMed] [Google Scholar]
- 2.Rowell NP, Williams CJ. Radical radiotherapy for stage I/II non-small cell lung cancer in patients not sufficiently fit for or declining surgery (medically inoperable). Cochrane Database Syst Rev 2001(2):CD002935. [DOI] [PubMed] [Google Scholar]
- 3.Timmerman R, Papiez L, McGarry R, Likes L, DesRosiers C, Frost S, et al. , Extracranial stereotactic radioablation: results of a Phase I study in medically inoperable Stage I non-small cell lung cancer. Chest 2003;124:1946. [DOI] [PubMed] [Google Scholar]
- 4.Nagata Y, Takayama K, Matsuo Y, Norihisa Y, Mizowaki T, Sakamoto T, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiation Oncology Biol Phys 2005;63:1427. [DOI] [PubMed] [Google Scholar]
- 5.The British Institute of Radiology Working Party Geometric uncertainties in radiotherapy: defining the planning target volume. London, UK: BIR; 2003 [Google Scholar]
- 6.van Herk MP, Remeijer P, Resch C, Lebesque JV. The probability of correct target dosage: dose-population histograms for the deriving of treatment margins in radiotherapy. Int J Radiation Oncology Biol Phys 2000;47:1121–35 [DOI] [PubMed] [Google Scholar]
- 7.Grills IA, Hugo G, Kestin L, Galerani AP, Chao KK, Wloch J, et al. Image guided radiotherapy via daily online cone beam CT substantially reduces margin requirements for stereotactic lung radiotherapy. Int J Radiation Oncol Biol Phys 2008;70:1045–56 [DOI] [PubMed] [Google Scholar]
- 8.Sonke JJ, Rossi M, Wolthaus J, van Herk M, Damen E, Belderbos J. Frameless stereotactic body radiotherapy for lung cancer using four dimensional cone beam CT guidance. Int J Radiation Oncol Biol Phys 2009;74:567–74 [DOI] [PubMed] [Google Scholar]