Abstract
Objective
The aim of this study was to diagnose microvascular invasion in patients with solitary hepatocellular carcinoma (HCC) from pre-operative CT imaging.
Methods
102 patients with solitary HCC who underwent curative hepatectomy were retrospectively included in our study. The pre-operative 3-phase CT imaging and laboratory data for the 102 patients were reviewed. Tumour size, tumour margin, peritumoral enhancement and α-fetoprotein level were assessed. Surgical pathology was reviewed; tumour differentiation, liver fibrosis score and microvascular invasion were recorded.
Results
The histopathological results revealed that 50 HCCs were positive and the other 52 were negative for microvascular invasion. Univariate analysis revealed that tumour size (p=0.036), higher Edmondson–Steiner grade (p=0.047) and non-smooth tumour margin (p<0.001) showed statistically significant associations with microvascular invasion. Multivariate logistic regression analysis showed that non-smooth tumour margin had a statistically significant association with microvascular invasion only (p<0.001). The sensitivity, specificity, positive predictive value and negative predictive value of the non-smooth tumour margin in the prediction of microvascular invasion were 66%, 86.5%, 82.5% and 72.6%, respectively.
Conclusion
Non-smooth tumour margin in pre-operative CT had a statistically significant association with microvascular invasion. More aggressive treatment should be considered in HCC patients with suspected positive microvascular invasion.
Hepatic resection is a potentially curative treatment modality for patients with hepatocellular carcinoma (HCC) [1-4]. Histopathological vascular tumour invasion is a well-known major prognostic factor for patients with HCC who have undergone hepatic resection or liver transplantation [5-8]. Iwatsuki et al [9] reported that microvascular and macrovascular invasions were associated with a 4.4- and 15-fold increased risk of recurrence, respectively, for patients who had undergone liver transplantation. Because microvascular tumour invasion has a significant impact on recurrence and prognosis, pre-operative diagnosis of microvascular invasion is needed.
Radiological detection of microvascular tumour invasion may facilitate the pre-operative prediction of a patient's prognosis. Many researchers have tried to elucidate microvascular invasion based on pre-operative imaging studies, including CT during hepatic angiography, dynamic MRI and superparamagnetic iron oxide-enhanced MRI [10-13]. However, radiological findings suggestive of microvascular invasion in pre-operative CT have not yet been well established. The purpose of our study was to diagnose microvascular invasion in patients with solitary HCC from pre-operative triphasic CT findings.
Methods and materials
Patients
Approval for retrospective study was obtained from our institutional review board. Between January 2007 and December 2009, 153 patients with HCC who underwent elective curative hepatectomy in our institution were retrospectively identified from medical records. All CT images were retrieved from the picture archiving and communication system (Centricity™ PACS-IW; GE Healthcare, Waukesha, WI) and were reviewed by one of the authors (JWL, who was not involved in the original imaging analysis) to select patients by the following criteria: (a) presence of a solitary HCC without macrovascular thrombosis on pre-operative imaging evaluation; and (b) a time interval between pre-operative CT study and surgery of less than 1 month. 37 patients with more than 1 HCC (2 nodules, n=16; 3 nodules, n=21), 5 patients with macrovascular thrombosis of portal vein on pre-operative CT, 6 patients who had a pre-operative CT more than 1 month before surgery and 3 patients with a pre-operative MRI instead of CT study were excluded from the study. Finally, 102 patients with solitary HCC were included for our study (75 males, 27 females; mean age, 60.4 years, range, 32–83 years).
The underlying hepatic disease was hepatitis B in 55 patients, hepatitis C in 34 patients, both hepatitis B and C in 6 patients, alcoholic cirrhosis in 1 patient and cryptogenic cirrhosis in 6 patients. 99 patients had Child–Pugh Class A disease and 3 patients had Child–Pugh Class B disease. 39 patients had liver cirrhosis.
The surgical pathology report for each patient was reviewed, and the presence or absence of microvascular invasion was recorded. The degree of tumour differentiation was categorised according to the Edmondson–Steiner classification. The degree of liver fibrosis was categorised according to the Metavir fibrosis scoring system.
CT imaging acquisition
CT images of the liver were obtained with a 16 slice multidetector CT scanner (Lightspeed Ultra 16; GE Healthcare) by using the following parameters: gantry rotation times of 0.6 s for non-enhanced study and for the hepatic arterial and portovenous phases, with 0.8 s for the equilibrium phase; a 5 mm section thickness; 27.5 mm s–1 table speed; 120 kVp; and 160–440 mA. Patients were imaged with a CT scanner in a craniocaudal direction. Non-ionic contrast medium (Omnipaque 350; GE Healthcare) was administered at a total dose of 100–120 ml with an injection rate of 3 ml s–1 through a 20 gauge venous cannula placed in the antecubital vein. For triphasic acquisitions, scanning was started with a 10 s scan delay (about 25–30 s after injection of the contrast agent) for the hepatic arterial phase after the attenuation value of the aorta reached 120 HU. 15 s after the end point of the hepatic arterial phase (about 50–55 s after injection of the contrast agent), the scans for the portovenous phase were acquired. Equilibrium-phase images were acquired 120 s (about 180–200 s after injection of the contrast agent) after the end of the acquisition of the portovenous phase. Whole-liver scanning was completed in 4–8 s with the patients holding their breath.
Imaging analysis
The imaging analysis was performed on a dual-screen diagnostic workstation (GE Healthcare). In each image assessment, liver maps were completed by drawing each individual liver lesion on a corresponding map according to the Couinaud system of liver anatomy. This was to be done as accurately as possible by one investigator. Two observers were blinded to the clinical information and final diagnosis. Tumour size, tumour margins, tumour capsule and peritumoral enhancement were assessed. Before starting the evaluation, the two readers discussed the definition of tumour margin, tumour capsule and peritumoral enhancement. Coronary and sagittal reformatted images of the whole liver additional to transverse plane images were provided for the two reviewers, and the observers independently reviewed the CT images of all patients. The reviewers recorded the location and size of the tumour for correlation with the pathological report. Tumour margins were categorised as: (a) smooth margin (Figure 1), presenting as a nodular-shaped tumour on all axial, coronary and sagittal imaging, and (b) non-smooth margin (Figure 2), presenting as a single nodule with extranodular extension, multinodular confluence or infiltrative margin [14]. The radiological tumour capsules were assessed in the venous phase by identifying a thin linear-enhancing structure encasing the tumour. We categorised the tumour capsules into two groups as follows: (a) presenting tumour capsule, a radiological capsule that completely or incompletely surrounded the tumour circumference, and (b) absent tumour capsule, no radiological capsule could be identified. Peritumoral enhancement was defined as the existence of a detectable arterial-enhancing portion adjacent to the tumour border on arterial-phase images that became isodense with the liver parenchyma on equilibrium-phase images. The pattern of peritumoral enhancement was categorised as absent or present (wedge shaped, or irregular circumferential enhancement).
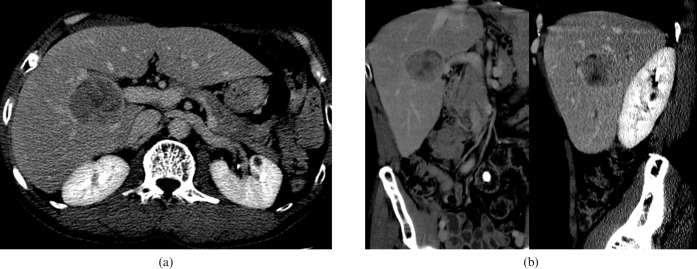
Figure 1.
A 57-year-old male with a moderately differentiated hepatocellular carcinoma within segment 5 underwent right hemihepatectomy. (a) The tumour with smooth margin on axial imaging. (b) The tumour depicted smooth margin on both coronary and sagittal reformatted images.
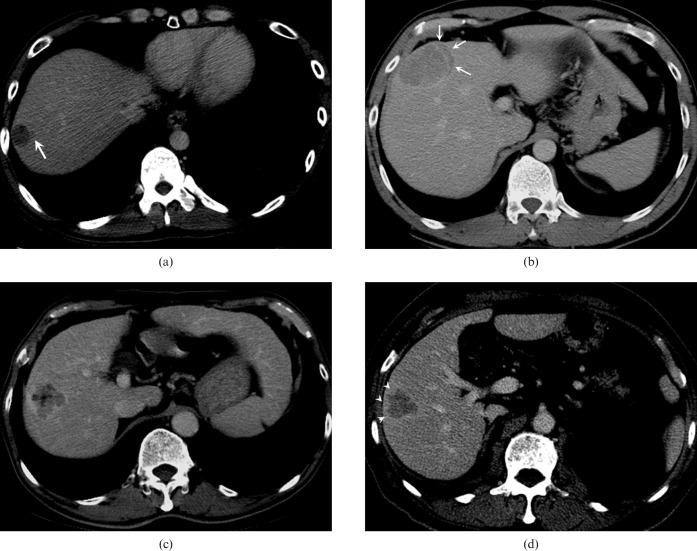
Figure 2.
Illustration of the patterns of non-smooth tumour margin. (a) A tumour with focal extranodular extension (arrow). (b) A tumour with crescent extranodular extension (arrows) beyond the tumour capsule. (c) A tumour with multinodular confluent appearance. (d) A tumour with focal infiltrative margin (arrowheads).
Statistical analysis
The interobserver difference between the initial two observers was evaluated with the κ test. An independent t-test was used to compare tumour size between the positive and negative microvascular invasion groups. Categorical variables, such as fibre score of underlying liver, Edmondson–Steiner grade of tumour, peritumoral enhancement, tumour margin and tumour capsule, were analysed with the χ2 test. The parameters found to have statistical significance by univariate analysis were entered into a multiple logistic regression model to elucidate the independent predictors of microvascular invasion. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated for the parameters that showed statistical significance by multivariate analysis. A p-value <0.05 was considered to indicate a statistically significant difference.
Results
The histopathological results revealed that 50 HCC lesions were positive for microvascular invasion, whereas 52 lesions were negative for microvascular invasion. The results of the univariate analysis for patient clinical characteristics and histopathological findings in patients with and without microvascular invasion are presented in Table 1. Tumour size (microvascular invasion-positive group: 4.6±2.6 cm; microvascular invasion-negative group: 3.6±2.1 cm; p=0.036) and higher Edmondson–Steiner grade (p=0.047) showed statistically significant associations with microvascular invasion. With regard to age, sex, liver fibrosis, Child–Pugh class and α-fetoprotein (AFP), there were no statistically significant differences between the groups with and without microvascular invasion.
Table 1. Univariate analysis of patient clinical characteristics and histopathological findings in patients with and without microvascular invasion.
| Histopathological microvascular invasion |
|||
| Risk factors | Negative (n=52) | Positive (n=50) | p-value |
| Age, years (mean±SD) | 60.2±12.3 | 60.6±11.5 | 0.876 |
| Sex | |||
| Male | 35 | 40 | 0.146 |
| Female | 17 | 10 | |
| Underlying liver disease | |||
| HBV | 29 | 26 | 0.966 |
| HCV | 17 | 17 | |
| HBV+HCV | 3 | 3 | |
| Other | 3 | 4 | |
| Child–Pugh class | |||
| A | 50 | 49 | 0.581 |
| B | 2 | 1 | |
| C | 0 | 0 | |
| Size, cm (mean±SD) | 3.6±2.1 | 4.6±2.6 | 0.036 |
| AFP (mean±SD) | 2241±15 459 | 2754±15 536 | 0.876 |
| Tumour differentiation (Edmondson–Steiner grade) | |||
| 1 | 5 | 2 | 0.047 |
| 2 | 26 | 15 | |
| 3 | 21 | 32 | |
| 4 | 0 | 1 | |
| Liver fibrosis (Metavir fibrosis score) | |||
| 0 | 1 | 1 | 0.958 |
| 1 | 8 | 9 | |
| 2 | 16 | 12 | |
| 3 | 9 | 10 | |
| 4 | 18 | 18 | |
AFP, α-fetoprotein; HBV, hepatitis B virus; HCV, hepatitis C virus; SD, standard deviation.
Univariate analyses of radiological findings for patients with and without microvascular invasion are shown in Table 2. Multivariate logistic regression analysis of radiological findings in patients with and without microvascular invasion is shown in Table 3. Only non-smooth tumour margin had a statistically significant association with microvascular invasion in both univariate and multivariate analysis (p<0.001). The sensitivity, specificity, PPV and NPV of the non-smooth tumour margin in the prediction of microvascular invasion were 66% [95% confidence interval (CI) 51.2–78.8%], 86.5% (95% CI 74.2–94.4%), 82.5% (95% CI 67.2–92.7%) and 72.6% (95% CI 59.8–83.1%), respectively.
Table 2. Univariate analysis of radiological findings for patients with and without microvascular invasion.
| Histopathological microvascular invasion |
||||
| CT findings | Total (n=102) | Negative (n=52) | Positive (n=50) | p-value |
| Radiological capsule | ||||
| Negative | 55 | 28 | 27 | 0.988 |
| Positive | 47 | 24 | 23 | |
| Peritumoral enhancement | ||||
| Negative | 86 | 45 | 41 | 0.528 |
| Positive | 16 | 7 | 9 | |
| Non-smooth tumour margin | ||||
| Negative | 62 | 45 | 17 | <0.001 |
| Positive | 40 | 7 | 33 | |
Table 3. Multivariate logistic regression analysis of radiological findings in patients with and without microvascular invasion.
| CT findings | Standard error | Significance | Odds ratio | 95% confidence interval |
|
| Lower | Upper | ||||
| Radiological capsule | 0.625 | 0.648 | 0.752 | 0.221 | 2.561 |
| Peritumoral enhancement | 0.788 | 0.575 | 1.556 | 0.332 | 7.288 |
| Non-smooth tumour margin | 0.677 | <0.001 | 13.79 | 3.66 | 51.958 |
Discussion
Multiphasic CT is being widely applied for pre-operative evaluation of patients with HCC [15]. This study was designed to assess the usefulness of pre-operative CT findings with axial, coronary and sagittal planes in the prediction of microvascular invasion in HCC.
In our results, non-smooth tumour margin was the only significant risk factor for microvascular invasion in both univariate and multivariate analysis. Some investigators have reported that the pathological gross category was an important predictor of portal vein invasion and intrahepatic metastasis in HCC [14,16,17]. They reported that “single nodule type with extranodular growth” and the “confluent multinodular type” showed higher frequencies of vessel invasion than the “single nodular type”. However, differentiation between the confluent multinodular type and the single nodular type with extranodular growth by pre-operative CT imaging can be difficult. We hypothesised that vascular invasion of HCC may occur when the tumour margin is invaded. Therefore, we assessed the tumour margins by simply categorising the shape as having either a smooth or a non-smooth margin instead of following the pathological gross categories.
In the present study, tumour size and histopathological differentiation were also the factors that showed statistical significance and predicted the risk of microvascular invasion. In our results, mean tumour size and higher Edmondson–Steiner grades were greater in the microvascular invasion group. Kim et al [18] also reported that tumour size, number and Edmonson–Steiner grade were major pre-operative predictors of microvascular invasion. Previous reports on the relationship between tumour size and histopathological grade demonstrated that the larger the tumour becomes, the higher the histopathological grade, and therefore tumour size is strongly related to histopathological grade [19]. Large HCCs were also reported to have a higher rate of vascular invasion [5,20].
Recently, several reports have described peritumoral enhancement as a parameter that is suggestive of an increased risk of microvascular tumour invasion [10-12]. Kim et al [12] stated that irregular circumferential peritumoral enhancement is a risk factor for microvascular invasion of HCC. Miyata et al [11] reported that distortion of corona enhancement and a tumorous arterioportal shunt on CT hepatic arteriography could be significant predictors of portal vein tumour invasion. Nishie et al [10] reported that the size of the peritumoral enhancement was a significant risk factor for microvascular invasion. In our study, peritumoral enhancement in triphasic CT study was not a statistically significant risk factor for microvascular tumour invasion. This might be due to a discrepancy between different imaging modalities and the lower percentage of HCCs showing peritumoral enhancement on dynamic CT images in our study.
Whether the presence or absence of a tumour capsule is related to the post-operative recurrence remains unclear [21,22]. A fibrous capsule in HCC has been considered a favourable prognostic factor, because the capsule may prevent invasion of HCC to the adjacent liver parenchyma [23-25]. However, Adachi et al [26] reported that the blood vessels of the fibrous capsule were frequently invaded by cancer cells and stated that the presence of a fibrous capsule is a predictor of portal venous invasion. In our study, a radiological capsule of tumour did not show significant correlation with microvascular invasion. This might be due to combination of the favourable and the unfavourable effects of the tumour capsule in microvascular invasion.
Serum AFP is one of the most common diagnostic tumour markers for HCC. Eguchi et al [16] reported that the AFP level could be used as a predictor of latent microscopic vascular invasion and early recurrence. However, the utility of AFP is restricted by the existence of non-AFP-secreting tumours. In our study, the elevation of serum AFP levels (>20 ng ml–1) was noted in 23 of 52 patients with negative microvascular invasion and in 31 of 50 patients with positive microvascular invasion. There was no significant difference between negative and positive microvascular invasion groups.
In the western world, alcoholism is the leading cause of chronic liver disease. Although the risk of HCC development was lower in alcoholic cirrhosis than in hepatitis C virus-related cirrhosis [27], the combination of alcohol and viral hepatitis results in a more rapid progression of liver disease [28]. An HCC that develops in the presence of chronic liver disease with underlying alcoholic aetiology may have a different clinical course. However, only one patient with alcoholic liver disease was enrolled in our study. Further studies are required to investigate microvascular invasion characteristics of HCCs that develop in the setting of alcoholic liver disease.
One limitation of our study is that it was a retrospective study, and we could not correlate non-smooth tumour margin with pathological microvascular invasion on a site-by-site basis. A prospective study with site-by-site histological correlation is needed in the future.
In conclusion, non-smooth tumour margin in pre-operative CT had a statistically significant association with microvascular invasion in both univariate and multivariate analysis. The non-smooth tumour margin may serve as a radiological sign in the prediction of microvascular invasion in patients with HCC. HCC patients with suspected positive microvascular invasion might need a more aggressive treatment, such as surgery of a wider extent or in combination with adjuvant therapy.
References
- 1.Lau WY, Lai EC. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat Dis Int 2008;7:237–57 [PubMed] [Google Scholar]
- 2.Clark HP, Carson WF, Kavanagh PV, Ho CP, Shen P, Zagoria RJ. Staging and current treatment of hepatocellular carcinoma. Radiographics 2005;25:S3–23 [DOI] [PubMed] [Google Scholar]
- 3.Ng KK, Vauthey JN, Pawlik TM, Lauwers GY, Regimbeau JM, Belghiti J, et al. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi-institutional database. Ann Surg Oncol 2005;12:364–73 [DOI] [PubMed] [Google Scholar]
- 4.Mor E, Kaspa RT, Sheiner P, Schwartz M. Treatment of hepatocellular carcinoma associated with cirrhosis in the era of liver transplantation. Ann Intern Med 1998;129:643–53 [DOI] [PubMed] [Google Scholar]
- 5.Shah SA, Cleary SP, Wei AC, Yang I, Taylor BR, Hemming AW, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery 2007;141:330–9 [DOI] [PubMed] [Google Scholar]
- 6.Ikai I, Arii S, Kojiro M, Ichida T, Makuuchi M, Matsuyama Y, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer 2004;101:796–802 [DOI] [PubMed] [Google Scholar]
- 7.Lauwers GY, Terris B, Balis UJ, Batts KP, Regimbeau JM, Chang Y, et al. Prognostic histologic indicators of curatively resected hepatocellular carcinomas: a multi-institutional analysis of 425 patients with definition of a histologic prognostic index. Am J Surg Pathol 2002;26:25–34 [DOI] [PubMed] [Google Scholar]
- 8.Hemming AW, Cattral MS, Reed AI, Van DerWerf WJ, Greig PD, Howard RJ. Liver transplantation for hepatocellular carcinoma. Ann Surg 2001;233:652–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwatsuki S, Dvorchik I, Marsh JW, Madariaga JR, Carr B, Fung JJ, et al. Liver transplantation for hepatocellular carcinoma: a proposal of a prognostic scoring system. J Am Coll Surg 2000;191:389–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishie A, Yoshimitsu K, Asayama Y, Irie H, Tajima T, Hirakawa M, et al. Radiologic detectability of minute portal venous invasion in hepatocellular carcinoma. AJR Am J Roentgenol 2008;190:81–7 [DOI] [PubMed] [Google Scholar]
- 11.Miyata R, Tanimoto A, Wakabayashi G, Shimazu M, Nakatsuka S, Mukai M, et al. Accuracy of preoperative prediction of microinvasion of portal vein in hepatocellular carcinoma using superparamagnetic iron oxide-enhanced magnetic resonance imaging and computed tomography during hepatic angiography. J Gastroenterol 2006;41:987–95 [DOI] [PubMed] [Google Scholar]
- 12.Kim H, Park MS, Choi JY, Park YN, Kim MJ, Kim KS, et al. Can microvascular invasion of hepatocellular carcinoma be predicted by pre-operative MRI? Eur Radiol 2009;19:1744–51 [DOI] [PubMed] [Google Scholar]
- 13.Kim H, Park MS, Park YN, Kim H, Kim KS, Choi JS, et al. Preoperative radiologic and postoperative pathologic risk factors for early intra-hepatic recurrence in hepatocellular carcinoma patients who underwent curative resection. Yonsei Med J 2009;50:789–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakashima Y, Nakashima O, Tanaka M, Okuda K, Nakashima M, Kojiro M. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatol Res 2003;26:142–7 [DOI] [PubMed] [Google Scholar]
- 15.Valls C, Cos M, Figueras J, Andía E, Ramos E, Sánchez A, et al. Pretransplantation diagnosis and staging of hepatocellular carcinoma in patients with cirrhosis: value of dual-phase helical CT. AJR Am J Roentgenol 2004;182:1011–17 [DOI] [PubMed] [Google Scholar]
- 16.Eguchi S, Takatsuki M, Hidaka M, Soyama A, Tomonaga T, Muraoka I, et al. Predictor for histological microvascular invasion of hepatocellular carcinoma: a lesson from 229 consecutive cases of curative liver resection. World J Surg 2010;34:1034–8 [DOI] [PubMed] [Google Scholar]
- 17.Nagano Y, Shimada H, Takeda K, Ueda M, Matsuo K, Tanaka K, et al. Predictive factors of microvascular invasion in patients with hepatocellular carcinoma larger than 5 cm. World J Surg 2008;32:2218–22 [DOI] [PubMed] [Google Scholar]
- 18.Kim BK, Han KH, Park YN, Park MS, Kim KS, Choi JS, et al. Prediction of microvascular invasion before curative resection of hepatocellular carcinoma. J Surg Oncol 2008;97:246–52 [DOI] [PubMed] [Google Scholar]
- 19.Kenmochi K, Sugihara S, Kojiro M. Relationship of histologic grade of hepatocellular carcinoma (HCC) to tumor size, and demonstration of tumor cells of multiple different grades in single small HCC. Liver 1987;7:18–26 [DOI] [PubMed] [Google Scholar]
- 20.Kim SJ, Lee KK, Kim DG. Tumor size predicts the biological behavior and influence of operative modalities in hepatocellular carcinoma. Hepatogastroenterology 2010;57:121–6 [PubMed] [Google Scholar]
- 21.Shirabe K, Kanematsu T, Matsumata T, Adachi E, Akazawa K, Sugimachi K. Factors linked to early recurrence of small hepatocellular carcinoma after hepatectomy: univariate and multivariate analysis. Hepatology 1991;14:802–5 [DOI] [PubMed] [Google Scholar]
- 22.Nagasue N, Uchida M, Makino Y, Takemoto Y, Yamanoi A, Hayashi T, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology 1993;105:488–94 [DOI] [PubMed] [Google Scholar]
- 23.Ng IO, Lai EC, Ng MM, Fan ST. Tumor encapsulation in hepatocellular carcinoma: a pathologic study of 189 cases. Cancer 1992;70:45–9 [DOI] [PubMed] [Google Scholar]
- 24.Ishizaki M, Ashida K, Higashi T, Nakatsukasa H, Kaneyoshi T, Fujiwara K, et al. The formation of capsule and septum in human hepatocellular carcinoma. Virchows Arch 2001;438:574–80 [DOI] [PubMed] [Google Scholar]
- 25.Torimura T, Ueno T, Inuzuka S, Tanaka M, Abe H, Tanikawa K. Mechanism of fibrous capsule formation surrounding hepatocellular carcinoma: immunohistochemical study. Arch Pathol Lab Med 1991;115:365–71 [PubMed] [Google Scholar]
- 26.Adachi E, Maeda T, Kajiyama K, Kinukawa N, Matsumata T, Sugimachi K, et al. Factors correlated with portal venous invasion by hepatocellular carcinoma: univariate and multivariate analyses of 232 resected cases without preoperative treatments. Cancer 1996;77:2022–31 [DOI] [PubMed] [Google Scholar]
- 27.Toshikuni N, Izumi A, Nishino K, Inada N, Sakanoue R, Yamato R, et al. Comparison of outcomes between patients with alcoholic cirrhosis and those with hepatitis C virus-related cirrhosis. J Gastroenterol Hepatol 2009;24:1276–83 [DOI] [PubMed] [Google Scholar]
- 28.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997;349:825–32 [DOI] [PubMed] [Google Scholar]