Abstract
Objective
The purpose of our study was to evaluate the diagnostic accuracy of transthoracic fine-needle aspiration biopsy (TFNAB) using a C-arm cone-beam CT (CBCT) system and to assess risk factors for immediate post-procedural complications in patients with lung lesions.
Methods
From October 2007 to April 2009, 94 TFNAB procedures using a C-arm system were studied in 91 patients with pulmonary lesions a chest CT scans. We retrospectively reviewed the patients' radiological and histopathological findings. We evaluated the lesion size, lesion abutted to pleura and presence or absence of emphysema along the needle path, lesion depth, visibility of target lesion and patient's position. Pneumothorax and pulmonary haemorrhage were assessed after TFNAB. Overall diagnostic accuracy, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were analysed.
Results
In 94 TFNAB procedures, 58 lesions were malignant and 36 were benign. The sensitivity, specificity, PPV, NPV and overall diagnostic accuracy rate of TFNAB were 93.1%, 100%, 100%, 90% and 97.9%, respectively. Pneumothorax was developed in 24 procedures. None of the parameters showed significant impact on the frequency of the pneumothorax. Overall haemorrhage occurred in 43 procedures. The incidence of overall haemorrhage was higher in patients with smaller lesions, longer pleural distance and pleural abutted lesions (p<0.05). Differences in visibility at projection radiographs were statistically significant between patients with or without perilesional haemorrhage (p<0.05).
Conclusion
Transthoracic fine-needle aspiration biopsy using a C-arm CBCT system is feasible for imaging guidance of lung lesion and early detection of the procedural-related complications.
Introduction
Transthoracic fine-needle aspiration biopsy (TFNAB) of the lungs has been performed under conventional fluoroscopy, conventional CT and CT fluoroscopy [1-6]. Conventional fluoroscopic guidance allows for real-time processing with smaller radiation doses to patients and operators, but has limitations when applied to small or invisible lesions in radiography [1]. Conventional CT guidance provides superior contrast and spatial resolution, but does not allow for real-time capability [1]. CT fluoroscopic guidance enables real-time visualisation, but has the disadvantage of significant radiation exposure to patients and operators [7].
Variable clinical applications and ongoing investigations have been performed using a C-arm cone-beam CT (CBCT) system [8,9]. The C-arm CBCT system provides projection radiography, fluoroscopy, digital subtraction angiography and CT capabilities in a single patient set-up [10]. In an initial trial of TFNAB using a C-arm CBCT system, Jin et al [7] suggested that CBCT could reduce the radiation exposure to both patients and operators by lowering the number and lessening the imaging coverage of CBCT image acquisition.
The complications of TFNAB in lungs include pneumothorax, haemoptysis, air embolism and needle tract metastasis [1,3,5,11,12]. In TFNAB using a C-arm CBCT system by Jin et al [7], the overall complication rate was reported as 38%, which comprised pneumothorax (25.4%), haemoptysis (14.1%) and chest pain (1.4%). There were no serious complications such as air embolism or procedure-related death [7]. To our knowledge, however, there has been no study in the evaluation of risk factors for post-procedural complications after TFNAB using a C-arm CBCT system. We assess the diagnostic accuracy of TFNAB using a C-arm CBCT system and evaluate risk factors for immediate post-procedural complications in patients with lung lesions.
Methods and materials
Our institutional review board approved this study. Patients' informed consent was waived for the retrospective study, but written informed consent was obtained from all patients for TFNAB using a flat-panel-based C-arm system and chest CT.
From October 2007 to April 2009, 119 TFNAB procedures using a flat-panel-based C-arm system (Axiom Artis dBA; Siemens Healthcare, Malvern, PA) were performed on 116 consecutive patients with a pulmonary nodule or mass suggesting primary malignancy or metastasis at chest CT scans (Brilliance iCT; Philips Healthcare, Eindhoven, the Netherlands; LightSpeed16, GE Healthcare, Milwaukee, WI). No patients with a bleeding tendency were included in our study. 25 patients were excluded owing to data loss from the file archives (n=19) and follow-up loss (n=6). Of 91 patients (27 females and 64 males; mean age, 62±12 years; age range, 26–85 years), 3 underwent TFNAB twice on separate days. We retrospectively reviewed the patients' radiological and histopathological findings of 94 TFNAB procedures.
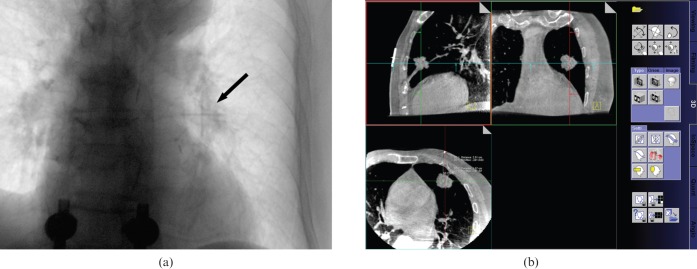
Each patient was positioned prone or supine according to the location of the lesion. The X-ray tube angle was set to 90°. After placing the patient on the examination table, a cross-shaped radio-opaque skin marker made of a 5.0-Fr angiographic catheter (KMP; Cook Medical Incorporated, Bloomington, IN) was applied to determine the needle entry point under fluoroscopy (Figure 1a). Subsequently, a rotational radiography using a flat-panel-based C-arm system (30×40 cm2 flat panel detector; 0.5° increment per image; 210° rotation angle; 480 images in total) was taken to obtain a 3D data set. These imaging data were transferred to a workstation (Leonardo; Siemens Healthcare) and CBCT images in axial, coronal, and sagittal orientations with 1.5-mm thicknesses were obtained by 3D software (DynaCT; Siemens Healthcare) (Figure 1b). Imaging acquisition and reconstruction times were approximately 80 s.
Figure 1.
A 77-year-old male with squamous cell carcinoma in left upper lobe. (a) Projection radiograph under real-time fluoroscopy shows a cross-shaped radio-opaque skin marker (arrow) on the target lesion of left upper lobe. (b) On a workstation, pre-procedural three-dimensional CT images obtained from C-arm cone-beam CT system show a 25-mm spiculated nodular lesion in left upper lobe below the radio-opaque skin marker of the needle entry site.
When drawing a trajectory from the intended biopsy site to the skin surface, the vertical line to the detector plane was chosen. Lesion depth from the skin was measured at axial CBCT image on the scanner console (Figure 1b). After preparation of the skin and sterilisation in a standard aseptic fashion, the needle entry site was infiltrated with 1% lidocaine (Xylocaine; AstraZeneca, London, UK) for local anaesthesia. The biopsy needle was a 21-gauge aspiration needle (Chiba; MI Tech, Pyeongtaek-si, Republic of Korea) and a 21-gauge automated core biopsy tool (Autovac; Bard Angiomed, Mississauga, Canada) was used. The needle was inserted and advanced towards the lesion with a vertical approach under fluoroscopy with the patient's breathing suspended. After successful completion of the aspiration or biopsy, the needle was carefully retracted. Specimens were immediately fixed in 99% ethyl alcohol or formalin and transferred for histopathological diagnosis. Immediately after the biopsy procedure, projection radiography and post-biopsy CBCT were performed to detect possible complications. Follow-up posteroanterior chest radiographs were routinely obtained within 4 h of each biopsy. All biopsy procedures were performed by one thoracic radiologist with 7 years' experience.
We evaluated the lesion size (defined as the longest diameter of the lesion), lesion abutted to pleura, presence or absence of emphysema along the needle path at chest CT image, lesion depth (measured from the pleura to the lesion along the planned needle path) at CBCT image, visibility of target lesion at projection radiograph and patient's position (prone or supine). Presence or absence of immediate complications (such as pneumothorax and haemorrhage) were assessed at projection radiograph, post-biopsy CBCT and follow-up chest radiograph (Figures 2–4). At post-biopsy CBCT, we classified haemorrhage into two groups: perilesional and needle tract haemorrhage. Perilesional haemorrhage was defined as increased attenuation around the lesion after biopsy (Figure 3). Needle tract haemorrhage was defined as increased attenuation along the needle path (Figure 4). We compared the complication detection rates of projection radiography, CBCT and follow-up chest radiography. All images were analysed by one thoracic radiologist with 7 years' experience and one senior resident in consensus.
Figure 2.
A 70-year-old female with pulmonary metastasis from breast cancer. Post-procedural coronal cone-beam CT images show small pneumothorax (arrow) and perilesional haemorrhage (arrowhead).
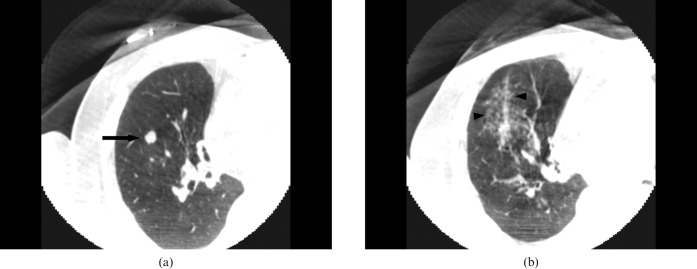
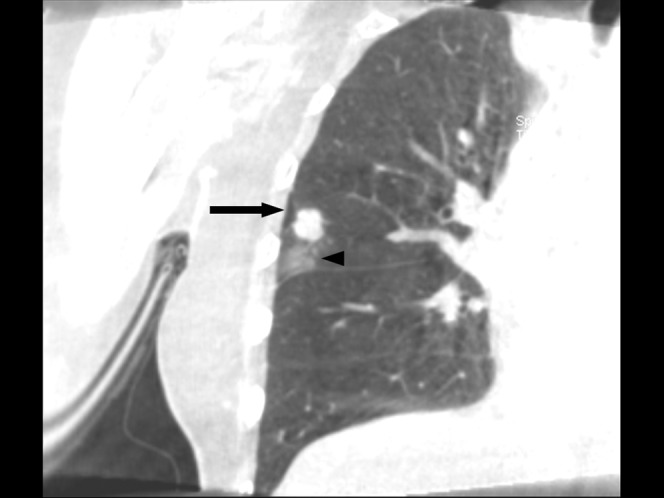
Figure 4.
A 75-year-old male with lung-to-lung metastasis in right lower lobe. (a) Pre-procedural axial cone-beam CT (CBCT) image shows an 8-mm nodular lesion (arrow) in right lower lobe below the radio-opaque marker on the skin area. (b) Post-procedural axial CBCT image shows ground-glass opacity along the needle path, which is suggestive of needle tract haemorrhage (arrowheads).
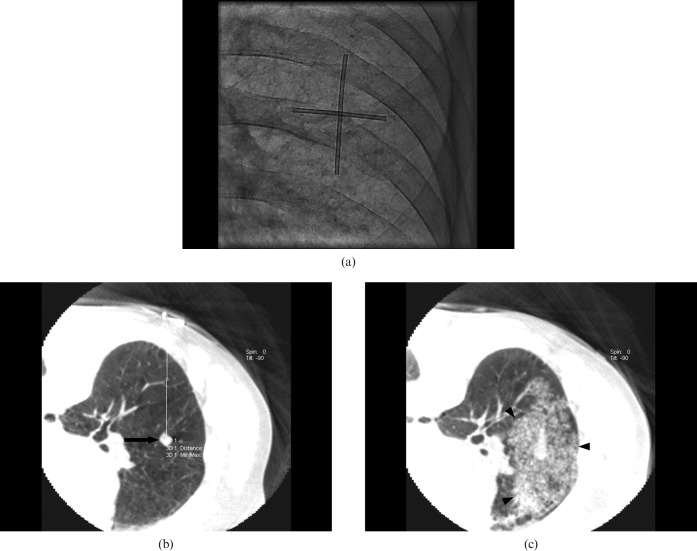
Figure 3.
A 70-year-old male with squamous cell carcinoma in right middle lobe. (a) Projection radiograph under real-time fluoroscopy shows a cross-shaped radio-opaque skin marker on the intended biopsy site of right middle lobe on a prone position. (b) Pre-procedural axial cone-beam CT (CBCT) image shows a 9-mm nodular lesion (arrow) in right middle lobe below the radio-opaque marker on the skin area. Note the measurement of lesion depth from the skin on a workstation. (c) Post-procedural axial CBCT image shows large ground-glass opacity surrounding the nodule, which is suggestive of perilesional haemorrhage (arrowheads).
We determined whether the final diagnosis for each nodule was malignant, benign or indeterminate on its histopathological results and follow-up images. Malignant nodules were defined as malignant features on TFNAB or additional examinations including sputum cytology, bronchoscopic biopsy, transbronchial lung biopsy and surgical resection, and a subsequent clinical and radiological course consistent with malignancy. Benign nodules were defined as a definite benign feature on biopsy or surgical resection and a decrease in size on the follow-up image or no interval change after a follow-up period of 12 months or longer. Indeterminate nodules were defined as non-specific benign features on TFNAB and no additional follow-up images. In the present study, there were no indeterminate nodules. The histopathological specimens were evaluated by a pathologist with 9 years' experience.
Statistical analysis was performed with SPSS 12.0 (SPSS Inc., Chicago, IL). Continuous variables such as patient age, lesion size and lesion depth were expressed as mean±standard diviation (SD) (range), and categorical variables such as patient gender, patient position, presence or absence of emphysema, lesion visibility and post-procedural complications (pneumothorax and haemorrhage) were expressed as frequencies or percentages. Correlation between quantitative parameters (age, lesion size and lesion depth) and the incidence of procedure-related complications was analysed with the Student's t-test. Differences in the incidence of procedure-related complications between patient gender, patient position and presence or absence of emphysema were analysed with the χ2 test. Overall diagnostic accuracy, sensitivity, specificity, positive predictive value and negative predictive value were calculated for the diagnosis of malignancy. We considered a p-value <0.05 to be statistically significant.
Results
In 94 TFNAB procedures, the lesion size was 37±23 mm (range, 8–120 mm). The lesion depth from the pleura along the planned needle path was 16±15.6 mm (range, 0–68 mm). Of all 94 procedures, 23 lesions were abutted to pleura, and 4 patients had emphysema along the needle path. 12 lesions were not seen at projection radiograph, and 64 procedures were done in prone position.
Of 94 procedures, adequate histopathological results were obtained in 90 (95.7%) and inadequate results in 4 procedures. At the final diagnosis, 58 (61.7%) lesions were malignant and 36 (38.3%) were benign. All malignant lesions were proved by initial TFNAB (n=54), surgical resection (n=1), repeated TFNAB (n=1) and a subsequent clinical and radiological course consistent with malignancy (n=2). Of 36 benign lesions, 18 lesions were proved to be specifically benign, such as tuberculosis (n=9), aspergillosis (n=1), hamartoma (n=1), pneumonia (n=7) by TFNAB, and 17 lesions showed non-specific benign features and a decrease in size on follow-up image or no interval change after a follow-up period. One lesion with a non-specific benign feature was proven by positive culture results (tuberculosis) of bronchioloalveolar lavage fluid. The sensitivity, specificity and positive and negative predictive values for a diagnosis of malignancy at TFNAB were 93.1%, 100%, 100% and 90%, respectively. Overall diagnostic accuracy rate was 97.9%.
Of 94 procedures, pneumothorax developed in 24 (25.5%) procedures, of which 23 were detected at immediate CBCT scans and 13 were seen at follow-up chest radiographs (Table 1; Figure 2). There was one case of delayed pneumothorax (1/94, 1.1%), which was only detected at a follow-up chest radiograph 1 h after they procedure and was treated with a chest tube insertion. No other patients had symptomatic pneumothorax that was treated with a chest tube insertion. None of the patient and lesion parameters showed significant impact on the frequency of the pneumothorax in our study (Table 2).
Table 1. Comparison of detection rates of complications at projection radiograph, CBCT, and follow-up chest radiograph after TFNAB using a C-arm CBCT system.
| Imaging method | Pneumothorax (n=24) | Haemorrhage (n=43) |
| Projection radiograph | 1 (4.3%) | 16 (37.2%) |
| CBCT | 23 (95.8%) | 43 (100%) |
| Chest radiograph | 13 (56.5%) | 18 (41.9%) |
CBCT, cone-beam CT; TFNAB, transthoracic fine-needle aspiration biopsy.
Table 2. Comparison of variables and pneumothorax after TFNAB using a C-arm CBCT system.
| Quantitative parameters | With pneumothorax (n=23, 24.5%) | Without pneumothorax (n=71, 75.5%) | p-value |
| Sex (% male) | 65.2 | 74.6 | 0.380 |
| Age (years) | 60.3±11.8 | 61.1±12.4 | 0.791 |
| Lesion size (mm) | 39.1±26.4 | 36.4±22.2 | 0.615 |
| Lesion depth (mm) | 13.7±14.3 | 16.2±16 | 0.502 |
| Abut to pleura (%) | 43.5 | 38.0 | 0.642 |
| Presence of emphysema (%) | 13 | 11.3 | 1.000 |
| Patient position (% Prone) | 69.6 | 67.6 | 0.861 |
| Visibility (%) | 91.3 | 85.9 | 0.724 |
CBCT, cone-beam CT; TFNAB, transthoracic fine-needle aspiration biopsy.
Overall pulmonary haemorrhage occurred in 43 (45.7%) of 94 procedures, which included perilesional haemorrhage (n=4), needle tract haemorrhage (n=29) and both (n=10) (Figures 3 and 4). Of 43 haemorrhages detected at CBCT, 18 were seen at follow-up chest radiographs. No patients required intervention or transfusion to treat pulmonary haemorrhage. Table 3 shows the comparison of variables and haemorrhage after TFNAB using a C-arm CBCT system. The incidence of overall haemorrhage (either perilesional or tract haemorrhage) was higher in patients with smaller lesions, longer pleural distance and pleural abutted lesions (p<0.05). Differences in visibility at projection radiograph were statistically significant between patients with or without perilesional haemorrhage (p<0.05).
Table 3. Comparison of variables and haemorrhage after TFNAB using a C-arm CBCT system.
| Quantitative parameters | With overall haemorrhage (n=43, 45.7%) | Without overall haemorrhage (n=51, 54.3%) | p-value | With perilesional haemorrhage (n=14, 14.9%) | Without perilesional haemorrhage (n=80, 85.1%) | p-value | With needle tract haemorrhage (n=39, 41.5%) | Without needle tract hemorrhage (n=55, 58.5%) | p-value |
| Sex (% male) | 72.1 | 72.5 | 0.961 | 71.4 | 82.9 | 1.000 | 71.8 | 72.7 | 0.921 |
| Age (years) | 60.2±12.1 | 61.6±12.3 | 0.586 | 63±7.7 | 60±12.7 | 0.470 | 60±12.5 | 61.6±12 | 0.533 |
| Lesion size (mm) | 26.6±15.8 | 45.9±24.8 | <0.001 | 23.7±12.3 | 39.4±23.9 | 0.015 | 27.4±16 | 43.9±25 | <0.001 |
| Lesion depth (mm) | 25.6±15.2 | 7.2±9.9 | <0.001 | 30.6±19.6 | 12.9±13 | 0.001 | 25.6±13.6 | 8.5±12.8 | <0.001 |
| Abut to pleura (%) | 4.9 | 68.6 | <0.001 | 7.1 | 45 | 0.007 | 2.6 | 65.5 | <0.001 |
| Presence of emphysema (%) | 7 | 15.7 | 0.191 | 14.3 | 11.3 | 0.666 | 5.1 | 11.3 | 0.666 |
| Patient position (% prone) | 67.4 | 68.6 | 0.902 | 57.1 | 70 | 0.363 | 64.1 | 70.9 | 0.485 |
| Visibility (%) | 81.4 | 92.2 | 0.119 | 57.1 | 92.5 | 0.002 | 84.6 | 89.1 | 0.546 |
CBCT, cone-beam CT; TFNAB, transthoracic fine-needle aspiration biopsy.
Discussion
Flat-panel-based C-arm CBCT systems have been used as imaging guidance modality for variable vascular and nonvascular interventions [8,9]. The CBCT system provides real-time fluoroscopy and 3D CT imaging in one unit and could improve efficiency for guidance and monitoring of TFNAB [8]. With respect to image quality, CBCT provides higher spatial resolution, but has lower contrast resolution and smaller field of view than conventional CT [8,10]. However, these issues are not problematic because intervention is the main purpose of examinations in most CBCT applications, whereas diagnosis is not [8].
Transthoracic fine-needle aspiration biopsy of lung mass has been performed under conventional fluoroscopy, conventional CT or CT fluoroscopy guidance [1-6]. The diagnostic accuracy of TFNAB has been reported to range from 74% to 98% [1-6]. In a recent CBCT study by Jin et al [7], the diagnostic accuracy was 98.4%. In our study, the diagnostic accuracy was 97.9%, which was similar to that of previous TFNAB studies. We believe that CBCT guidance produces accurate and reliable TFNAB results.
In previous TFNAB studies, subsequent CT scans after insertion of an indwelling biopsy needle were obtained to confirm the needle trajectory [2,3,5,6]. Accurate planned data achieved from multiplanar 3D reconstruction CBCT images allow accurate fluoroscopy guidance and continuous monitoring of the needle advancement. In the present study, we achieved comparable diagnostic accuracy of TFNAB without additional scans to visualise the needle advancement, which was enabled by accurate localisation of skin entry and by a vertical approach to the target using a CBCT system. Furthermore, additional radiation exposure to the patients and physician could be avoided.
The incidence of pneumothorax after TFNAB using conventional fluoroscopy, conventional CT or CT fluoroscopy guidance ranged from 5% to 62% [1-6]. In our CBCT study, it was 24.5%, which was similar to the incidence of pneumothorax (25.4%) in the previous CBCT study by Jin et al [7]. Many risk factors of pneumothorax have been reported in the literature, which include patient characteristics (gender, age and pulmonary function), lesion characteristics (size, depth, lobar distribution, surrounded by aerated lung, abutted to pleura and presence of emphysema) and procedure-related factors (patient position, needle size, needle angle and number of pleural passage) [1,3,5,12]. To our knowledge, the visibility of the lesion on fluoroscopy had not been evaluated previously, and showed no statistical significance in the occurrence of pneumothorax in the present study.
In a comparative study between immediate CT scans and follow-up chest radiography after TFNAB, Noh et al reported that the overall incidence of delayed pneumothorax was 2.6% [13]. In our study, the incidence of delayed pneumothorax was 1.1% (1/94 procedures). It occurred in one patient with adenocarcinoma, who underwent lung biopsy across the left major fissure. We think that the transfissural passage of the needle might be one of the possible causes of delayed pneumothorax in our case.
The incidence of parenchymal haemorrhage after TFNAB was 6% on chest radiograph, and ranged from 9.9 to 25.4% on CT in previous studies [1,3,5,6]. In our study, the incidence of parenchymal haemorrhage (perilesional and needle tract haemorrhage) was 45.7% (43/94 procedures). We think that it was higher than that of parenchymal haemorrhage in a previous study because perilesional and needle tract haemorrhage was included and the detection rate of haemorrhage at CBCT (100%) was relatively higher than at fluoroscopy (37%) and at plain radiograph (42%). These complications did not appear to be clinically significant because none of the patients required treatment.
Small lesion size or long lesion depth has been known to increase the risk of bleeding during TFNAB [5,14]. In our study, parenchymal haemorrhage showed higher frequency in small lesions (p<0.01) and a long distance from the pleura (p<0.01). In a small lesion, the needle tip involves a portion of aerated lung and causes large haemorrhage, decreasing the tamponading effect compared with a large lesion [5]. When taking a biopsy from a deep tumour, the needle will cross more lung parenchyma, including more pulmonary vessels, which increase in size with distance to pleura, which in turn increases the risk of a large haemorrhage [5].
There were some limitations to our study. First, 25 of 119 procedures were excluded owing to inadequate data collection, which could affect diagnostic yield and the complication rate. Second, direct dose calculation was not included in our study. However, it is still difficult to compare conventional CT with a CBCT system because conventional dose metric—the CT dose index (CTDI)—is not applicable to CBCT [7,8]. Therefore, a method of measurement should be developed in the future.
In conclusion, transthoracic fine-needle aspiration biopsy using a C-arm CBCT system can detect immediate complications without transferring the patient to the CT suite. This C-arm CBCT system is feasible for imaging guidance of lung lesion and early detection of the procedure-related complications. We believe that a C-arm CBCT system will be an alternative method of imaging-guided TFNAB in patients with lung lesions.
References
- 1.Kurban LA, Gomersall L, Weir J, Wade P. Fluoroscopy-guided percutaneous lung biopsy: a valuable alternative to computed tomography. Acta Radiol 2008;49:876–82 [DOI] [PubMed] [Google Scholar]
- 2.Li H, Boiselle PM, Shepard JO, Trotman-Dickenson B, McLoud TC. Diagnostic accuracy and safety of CT-guided percutaneous needle aspiration biopsy of the lung: comparison of small and large pulmonary nodules. AJR Am J Roentgenol 1996;167:105–9 [DOI] [PubMed] [Google Scholar]
- 3.Heyer CM, Reichelt S, Peters SA, Walther JW, Muller KM, Nicolas V. Computed tomography-navigated transthoracic core biopsy of pulmonary lesions: which factors affect diagnostic yield and complication rates? Acad Radiol 2008;15:1017–26 [DOI] [PubMed] [Google Scholar]
- 4.Goldberg SN, Keogan MT, Raptopoulos V. Percutaneous CT-guided biopsy: improved confirmation of sampling site and needle positioning using a multistep technique at CT fluoroscopy. J Comput Assist Tomogr 2000;24:264–6 [DOI] [PubMed] [Google Scholar]
- 5.Heck SL, Blom P, Berstad A. Accuracy and complications in computed tomography fluoroscopy-guided needle biopsies of lung masses. Eur Radiol 2006;16:1387–92 [DOI] [PubMed] [Google Scholar]
- 6.Yamagami T, Iida S, Kato T, Tanaka O, Nishimura T. Combining fine-needle aspiration and core biopsy under CT fluoroscopy guidance: a better way to treat patients with lung nodules? AJR Am J Roentgenol 2003;180:811–15 [DOI] [PubMed] [Google Scholar]
- 7.Jin KN, Park CM, Goo JM, Lee HJ, Lee Y, Kim JI, et al. Initial experience of percutaneous transthoracic needle biopsy of lung nodules using C-arm cone-beam CT systems. Eur Radiol 2010;20:2108–15 [DOI] [PubMed] [Google Scholar]
- 8.Kalender WA, Kyriakou Y. Flat-detector computed tomography (FD-CT). Eur Radiol 2007;17:2767–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace MJ, Kuo MD, Glaiberman C, Binkert CA, Orth RC, Soulez G. Three-dimensional C-arm cone-beam CT: applications in the interventional suite. J Vasc Interv Radiol 2008;19:799–813 [DOI] [PubMed] [Google Scholar]
- 10.Orth RC, Wallace MJ, Kuo MD. C-arm cone-beam CT: general principles and technical considerations for use in interventional radiology. J Vasc Interv Radiol 2008;19:814–20 [DOI] [PubMed] [Google Scholar]
- 11.Ibukuro K, Tanaka R, Takeguchi T, Fukuda H, Abe S, Tobe K. Air embolism and needle track implantation complicating CT-guided percutaneous thoracic biopsy: single-institution experience. AJR Am J Roentgenol 2009;193:W430–6 [DOI] [PubMed] [Google Scholar]
- 12.Ko JP, Shepard JO, Drucker EA, Aquino SL, Sharma A, Sabloff B, et al. Factors influencing pneumothorax rate at lung biopsy: are dwell time and angle of pleural puncture contributing factors? Radiology 2001;218:491–6 [DOI] [PubMed] [Google Scholar]
- 13.Noh TJ, Lee CH, Kang YA, Kwon SY, Yoon HI, Kim TJ, et al. Chest computed tomography (CT) immediately after CT-guided transthoracic needle aspiration biopsy as a predictor of overt pneumothorax. Korean J Intern Med 2009;24:343–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeow KM, See LC, Lui KW, Lin MC, Tsao TC, Ng KF, et al. Risk factors for pneumothorax and bleeding after CT-guided percutaneous coaxial cutting needle biopsy of lung lesions. J Vasc Interv Radiol 2001;12:1305–12 [DOI] [PubMed] [Google Scholar]