Abstract
Objective
The purpose of this study was to describe the relation between various frequently used conformity indices (CIs) and to examine the influence of the target coverage (TC) difference in prescription isodose surface (IDS) on these CI values in dynamic conformal arc (DCA) plans.
Method
73 plans for simple-shaped brain metastases that were previously characterised for dose distribution with regard to the effect of the target volume (TV) and the depth from the skin surface were reviewed. Three different-definition CI values for each TV were calculated at the 80% IDS, and at D99, D95, D90 and D85, considering the interplanner variability in the TC values for the prescription IDS.
Results
The CI used as the Radiation Therapy Oncology Group criterion showed nearly perfect values at D90. The CI defined in the BrainSCAN (BrainLAB AG, Feldkirchen, Germany) treatment planning system (CIBS) denoted lower (superior) values as the TC of the reference IDS decreased. Nakamura’s CI (NCI) had lower variability but demonstrated lower (superior) values at D95. NCI showed the most stringent (higher) values at an 80% IDS, but the differences between the plans were less distinct with NCI.
Conclusion
The TC difference in IDS chosen for dose prescription or evaluation significantly led to CI value variability in a definition-dependent manner, even when NCI was applied. Definition of the reference IDS at a specific TC value according to clinical situation would reduce the CI value variability to a minimum and would make the CIBS sufficient for the objective metric with a perfect value of 1.
The physical goal of intracranial stereotactic radiosurgery (SRS) is to deliver a prescribed dose of radiation to the target geometry in as conformal a manner as possible, while spilling as little as possible into the surrounding normal tissue. Conformity indices (CIs) have been used to quantitatively evaluate the degree of dose conformity [1-3]. However, there have been various CI definitions and terminologies in the literature [1,2], and the choices of CI and the reference isodose surface (IDS) have been left to the planner’s discretion or preference. The most desirable CI and method for evaluation remain to be elucidated [1].
Dynamic conformal arc (DCA) is a state-of-the-art technique of linear accelerator-based SRS using a micro-multileaf collimator (mMLC), and is characterised by the forward-planning method, which easily generates conformal and homogeneous dose distributions and has the freedom of a non-coplanar beam arrangement [4-6]. In this technique, dose prescription is commonly defined at the specific percentage IDS values (e.g. 80% or 90%) normalised to 100% at the isocentre [7]. However, a dose prescription to the specific percentage IDS does not necessarily guarantee a consistent target coverage (TC) value (Figure 1) because TC may be altered by the target volume (TV), shape or leaf margin [8]. Furthermore, the prevailing method of dose prescription in other treatment modality has been the intended “marginal dose” with a specific percentage IDS and an unspecified TC value (e.g. 50% IDS in gamma-knife radiosurgery). The TC value of the marginal dose for individual plans appears to be susceptible to each planner’s discretion. The proximity of a target to an organ-at-risk (OAR) may also compromise the TC for the intended marginal dose to maintain the OAR dose constraint. These situations raise questions such as whether reporting CI values calculated at the intended marginal dose with unspecified TC is really appropriate, or which CI should be chosen to objectively report the dose conformity of the DCA or other plans.
Figure 1.
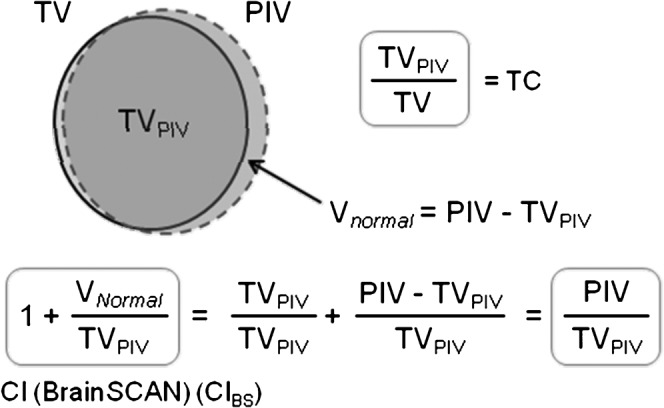
Upper: an example illustrating the definition of volumes in coverage and conformity parameters used in this study, although this is deliberately shown as an imperfect plan in dose conformity. Lower: expression of the conformity index (CI) defined in BrainSCAN (CIBS). PIV, prescription isodose volume; TC, target coverage; TV, target volume; TVPIV, the volume of the target receiving the prescription dose; Vnormal, the volume of the normal tissue receiving the prescription dose.
In this study, we reviewed various reported CIs that were relatively simple in definition, and then clarified the relation between the CI definitions to one another. Next, we chose three different-definition CIs, and examined the influence of the TC difference in IDS chosen for dose prescription or evaluation on these CI values and the inter-CI differences in the DCA plans for simple-shaped brain metastases. Through these analyses, we considered the optimal method for dose conformity evaluation in different clinical situations.
Methods and materials
Study population, treatment system and planning
Seventy-three plans were reviewed from a database of patients with brain metastases who had been treated with the DCA technique between 2005 and 2009 at our institution. The selected lesions were simple in shape, and none were adjacent to any critical structure. The treatment system included the m3 mMLC (BrainLAB AG, Feldkirchen, Germany) [9] as an add-on device on the non-dedicated linear accelerator (Clinac 21EX; Varian, Palo Alto, CA) with 6-MV photon energy (dose rate, 600 MU min–1). BrainSCAN version 5.3 (BrainLAB) was used as the treatment planning system (TPS). To circumvent interplanner variability and to demonstrate baseline conformity, all target definitions and DCA planning were generated using the exact same planning methods, assuming that the prescription dose would be set to 80% IDS [7], although the authors consider that these planning methods and the resulting plans were not necessarily ideal, and that planning parameters such as the arc arrangement, collimator angle and leaf margin should be individually optimised. The characteristics of basic dose distribution of these subjects with regard to the influence of target volume and depth from the skin surface were described previously [8]. Stereotactically localised CT scans were obtained in contiguous 2-mm slices. T1 weighted post-contrast MR images were acquired with 2-mm slices without fiducial markers and were co-registered with the CT scans by using a mutual information-based algorithm implemented in the TPS. Clinical target volume (CTV) was defined as an enhanced lesion on the MR images and was expanded to a planning target volume (PTV; referred to here as TV) with a 2-mm isotropic margin. The PTVs ranged from 0.53 to 19.42 cm3, with a median value of 5.8. The number of arcs per plan used was 3 for a PTV <5 cm3 and 5 for ≥5 cm3, in which the table position was set at 30°, 90° and 300° for 3 arcs, and 10°, 50°, 90°, 310° and 350° for 5 arcs (default setting). The arc length (i.e. the range between the start and stop angles of the gantry) was set at 110° (20–130° or 230–340°). The collimator angle in all arcs was set at 90° in order to secure clearance between the gantry head and the patient. The leaf edge was adapted to the outline of the PTV without leaf margin. All treatment plans were normalised to 100% IDS at the geometric isocentre of the PTV. To circumvent any dose interference resulting from simultaneous treatment of multiple targets, all cases had a single lesion.
Dose conformity evaluation
The dose calculation was based on a pencil-beam algorithm with radiological path length for tissue heterogeneity correction. The grid size of the dose-volume histogram (DVH) calculation was set to 1.0 mm, and an adaptive grid size <1.0 mm was applied to smaller lesions to ensure that there were at least 10 voxels in each dimension inside the PTV. An expanded PTV (ePTV) was created with the addition of an isotropic margin >5 mm to the PTV to directly compute the isodose volumes (IDVs) encompassed by the reference dose for each plan. Various IDVs were calculated from the DVH of the ePTV and were then used to compute the CIs. The IDV calculated by this method was considered more accurate than the sum of the separate IDVs derived from the PTV and the normal tissue, because the whole brain volume was uniformly assumed to be 1500 cm3 for each case in BrainSCAN.
In this study, three different-definition CIs frequently used in the literature were selected: the CI defined in BrainSCAN (CIBS, Figures 1 and 2) [4,6]; the CI used as Radiation Therapy Oncology Group (RTOG) criterion (also referred to as the ratio of prescription IDV/TV; PITV, Figure 2); and the CI proposed by Nakamura et al (Nakamura’s CI; NCI, Figure 2) [11]. These CIs were separately calculated at the specific percentage IDS (80% IDS) and at several PTV coverage values (D99, D95, D90 and D85) for each plan. The D99, D95, D90 and D85 values of the PTV corresponded to the IDS (%) encompassing at least 99%, 95%, 90% and 85% of the PTV, respectively.
Figure 2.
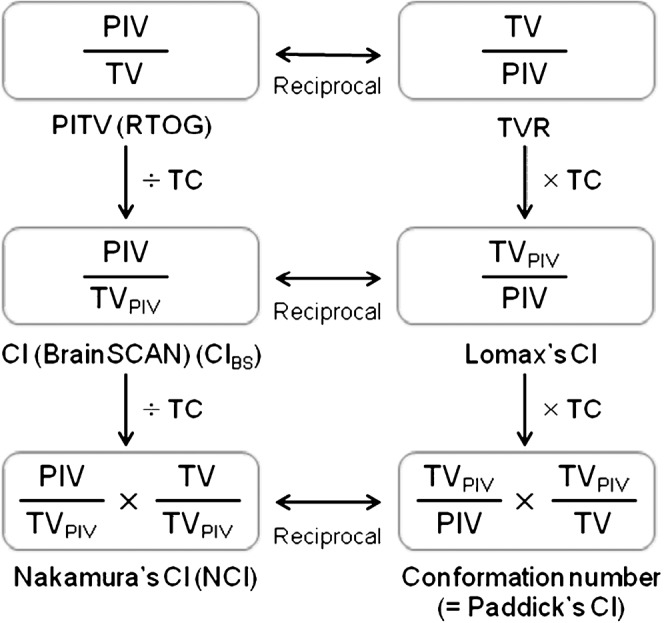
Relation between several representative conformity index (CI) definitions. PIV, prescription isodose volume; PITV, ratio of prescription isodose volume/target volume; RTOG, Radiation Therapy Oncology Group; TVPIV, the volume of the target receiving the prescription dose; TVR, treatment volume ratio; TC, target coverage; TV, target volume.
Statistical analyses
Box-and-whisker plots (BWPs) were used to represent the distribution of variables. In the BWP, the box showed the interquartile range (IQR), the horizontal bar in the box represented the median value, the whiskers denoted the nearest values not beyond 1.5 times the IQR, and the circles beyond the lines indicated the individual outliers. The Wilcoxon signed-rank test was applied to compare paired variables. Spearman’s rank correlation coefficient was applied to evaluate correlations between the variables. The Jonckheere–Terpstra (JT) test was used to assess a trend in the CI values among three groups with ordered CI complexity and four groups with varying PTV coverage. All p-values were calculated with two-sided tests and p-values <0.05 were considered statistically significant. Statistical analyses were performed using PASW Statistics 18 (SPSS Inc., Chicago, IL).
Results
Review of various CI definitions in the literature
Several CI definitions frequently used in the literature were described using three parameters (TV, PIV and TVPIV), as defined in Figure 1, in which the target volume (TV) may be PTV, CTV or gross target volume (GTV), and the reference dose may be the intended marginal dose specified at the specific percentage IDS or the minimum dose to the target (Figure 2).
As shown in Figures 1 and 2, CIBS was regarded as the reciprocal of the CI proposed by Lomax and Scheib (Lomax’s CI; LCI) [12]. The LCI has also been alternatively expressed as the healthy tissue CI [1], the protection factor [13] and the selectivity index [14]. CIBS was regarded as the ratio of PITV/TC [4]. The PITV was also described as the CI in Report 62 of the International Commission on Radiation Units and Measurements (ICRU) [15]. Ma et al [16] termed the PITV as the tissue volume ratio. DiBiase et al [17] defined the TV as the GTV in the PITV. The treatment volume ratio (TVR) defined by Nedzi et al [18] and the radiation CI (RCI) defined by Knöös et al [19] were nearly reciprocal of the PITV [12,13], although the reference dose was defined as the minimum isodose in the target or the 95% isodose according to ICRU 50 guidelines [20]. Although Kwon et al [21] referred to the same index defined by DiBiase et al [17] as the TVR, there should be no misunderstanding that this index is the inverse of the original TVR by Nedzi et al [18]. The conformation number (CN) proposed by van’t Riet et al [22] was defined as the value of LCI×TC [2,12]. Paddick [3] applied the CN to intracranial SRS plans. The NCI was the reciprocal of the CN [5,11] and was also regarded as the value of CIBS/TC or PITV/(TC)2 (Figure 2). Various other denominations or similar definitions related to the six CIs reviewed in this study are summarised in Table 1.
Table 1. Other denominations or similar definitions related to the conformity indices (CIs) described in Figure 2.
| Definition | Other denomination(s) |
| PITV (RTOG) [1,10] | CI defined as ICRU Report 62 [15] |
| Tissue volume ratio by Ma [16] | |
| CI by DiBiase (TV=GTV) [17] | |
| TVR by Kwon (TV=GTV) [21] | |
| TVR (Nedzi) [18] | RCI by Knöös [19] |
| CI (BrainSCAN) [4,6] | |
| Lomax’s CI [12] | Healthy tissue CI [1] |
| Protection factor [13] | |
| Selectivity index [14] | |
| NCI [11] | |
| Conformation number [22] | Paddick’s CI [3] |
NCI, Nakamura’s CI; PITV, ratio of prescription isodose volume/target volume; RCI, radiation CI; RTOG, Radiation Therapy Oncology Group; TVR, treatment volume ratio.
Influence of TC difference in reference IDS on the CI values
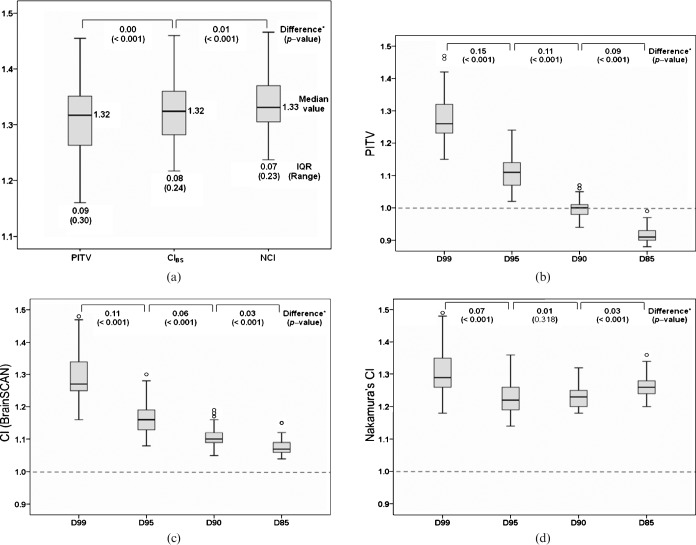
In the present planning method, the TC values for the 80% IDS substantially varied from 93.64% to 99.98%, with a median value of 98.9%, and increased as PTV size increased, as previously reported [8]. The differences between the three CI values calculated at the 80% IDS were statistically significant, although the differences between median values were small (Figure 3a). As the complexity of CI increased from the PITV to the NCI, there was a statistically significant trend in stepwise increase (worsening) of the CI values (JT test, p=0.003), while the range and the IQR significantly decreased.
Figure 3.
Influence of the target coverage difference in reference isodose surface (IDS) on the three representative conformity index (CI) values. Box-and-whisker plots show the three CI values calculated at 80% IDS (a) and each CI value defined at D99, D95, D90, and D85 (b: PITV; c: CIBS; d: Nakamura’s CI, NCI). IQR, interquartile range. *The two-tailed p-values were the results of the Wilcoxon signed-rank test.
The PITV values defined at D99 to D85 significantly decreased as the TC decreased (JT test, p<0.001; Figure 3b). Notably, the PITV at D90 denoted a nearly perfect value. The CIBS values also significantly decreased (improved) as the TC decreased (JT test, p<0.001; Figure 3c), suggesting that CIBS has a tendency to show a false-superior value at lower TC. Although the NCI values also showed a statistically significant stepwise decreasing trend as the TC decreased (JT test, p=0.009; Figure 3d), no significant difference was observed in NCI values between D95 and D90 (p=0.318). Nevertheless, the NCI showed the most superior median value at D95. Although the variation of the CI values relevant to the TC difference of the reference IDS was minimal for the NCI, a significant difference in NCI values was observed between D99 and D95. In all three CI values, stepwise decreases of the range and IQR were observed as TC decreased.
Discussion
Influence of TC difference in IDS chosen for dose prescription or evaluation on various CI values
This study indicated that the variability of CI values related to the TC value in the reference IDS was higher in the order of PITV, CIBS and NCI. The PITV and CIBS values can show a false-superior value when a reference IDS with a lower TC is chosen [1,3], suggesting that the superior PITV or CIBS values based on the reference IDS with unspecified TC do not necessarily guarantee superior conformity. In contrast, the NCI appeared to be mostly robust to the TC difference in the reference IDS, and therefore may be most suitable for the marginal dose with unspecified TC. Nonetheless, considering the difference in values between D99 and D95, the TC difference in the reference IDS still significantly affects interpretation of the NCI values. Any NCI calculated at D95 showed a superior value to that at D99. Although Hazard et al [5] recommended at least D95 for the TC of the standardised prescription IDS (sIDS) value for CI evaluation, the significant variability of the CI values related to the TC difference in chosen IDS still exists even when the sIDS value is adopted. As dose prescription to the specific percentage IDS does not always guarantee a specific TC in the DCA plans [8], objective evaluation of dose conformity at a specific percentage IDS appears to be difficult despite the use of either CI. In such cases, representation of the CI along with the corresponding TC value is necessary for appropriate interpretation of the values [12].
The six CI definitions reviewed in this study were regarded as linked to one another through the TC or the relation of the reciprocal (Figure 2). If the reference dose is defined as the IDS indicating a specific TC, each CI can be directly correlated with others through the TC or the corresponding reciprocal relationship. Therefore, this method allows us to appreciate each CI value more appropriately regardless of which one is chosen. The perfect values for PITV calculated at the D99, D95 and D90 values are 0.99, 0.95 and 0.9, respectively, whereas those for CIBS are regarded as “1” in all cases since CIBS corresponds to the value of PITV/TC (Figure 2). The NCI showed the most stringent values when calculated at the specific percentage IDS, but the interplan differences were less distinct with NCI (Figure 3a). Therefore, CIBS appears to sufficiently represent dose conformity for the reference IDS with a specific TC.
Taken together, each CI has a distinctive tendency for the susceptibility to the TC difference in IDS chosen for dose prescription. When a certain CI is used to report conformity, the definition of the CI should be stipulated and used along with the TC [12].
Consideration of the objective method for conformity evaluation based on different clinical situations
Apart from the intended marginal dose, the definition of CI for the reference IDS indicating specific TC according to individual clinical situations would enable us to fairly compare plan qualities even across different treatment modalities and regardless of the intended marginal dose. Using this method, either CIBS or PITV appears to be sufficient for optimal CI representation.
Three different clinical situations can be considered. Firstly, PTV is equivalent to CTV or GTV without a PTV margin. Secondly, PTV is generated from CTV with a considerable PTV margin. Thirdly, PTV abuts or is located close to OAR and requires considerable dose constraint.
For the first situation, the reference dose should be the minimum dose (Dmin) for the target as described in Report 62 of the ICRU [15] or by Nedzi et al [18]. In such cases, D99 is considered a suitable alternative to Dmin, because the latter is derived from DVH for CTV or GTV and can occur in single voxels of clinically insignificant volume [23]. For the second situation, D95 may be adequate for the reference dose rather than D99 in light of the considerable PTV margin, especially one that is >1 mm. For the third situation, the intentional partial coverage of a part of the PTV boundary confronted by an OAR may be required to ensure sufficient dose constraint [24]. In these cases, the use of D90 may be suitable for CI evaluation. If these methods are used, various CI values with different definitions can be appropriately interpreted by considering the relationship described in Figure 2, and the dose conformity can be objectively evaluated even across plans derived from different treatment modalities regardless of the intended marginal dose. Nevertheless, the intended marginal dose is still important for planners, and for such cases NCI or other CI representation, along with specified TC, is recommended.
Conclusions
The six different CI definitions reviewed in the present study are linked to one another through the TC factor and the relation of the reciprocal. The TC difference in IDS chosen for dose prescription or evaluation significantly influenced the CI values in a definition-dependent manner, even when NCI is applied. The use of reference IDS that indicates a specified TC can minimise the CI value variability, and this objective method of evaluation according to clinical situation would enable us to fairly compare plan quality among rival plans or across different treatment modalities, regardless of the intended marginal dose.
References
- 1.Feuvret L, Noël G, Mazeron JJ, Bey P. Conformity index: a review. Int J Radiat Oncol Biol Phys 2006;64:333–42 [DOI] [PubMed] [Google Scholar]
- 2.Surber G, Hamm K, Kleinert G. Significance of different conformity indices for evaluation of radiosurgery treatment plans for vestibular schwannomas. J Neurosurg 2004;101:334–40 [PubMed] [Google Scholar]
- 3.Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. J Neurosurg 2000;93:219–22 [DOI] [PubMed] [Google Scholar]
- 4.Jin JY, Yin FF, Ryu S, Ajlouni M, Kim JH. Dosimetric study using different leaf-width MLCs for treatment planning of dynamic conformal arcs and intensity-modulated radiosurgery. Med Phys 2005;32:405–11 [DOI] [PubMed] [Google Scholar]
- 5.Hazard LJ, Wang B, Skidmore TB, et al. Conformity of LINAC-based stereotactic radiosurgery using dynamic conformal arcs and micro-multileaf collimator. Int J Radiat Oncol Biol Phys 2009;73:562–70 [DOI] [PubMed] [Google Scholar]
- 6.Wiggenraad RG, Petoukhova AL, Versluis L, van Santvoort JP. Stereotactic radiotherapy of intracranial tumors: a comparison of intensity-modulated radiotherapy and dynamic conformal arc. Int J Radiat Oncol Biol Phys 2009;74:1018–26 [DOI] [PubMed] [Google Scholar]
- 7.Molenaar R, Wiggenraad R, Verbeek-de Kanter A, Walchenbach R, Vecht C. Relationship between volume, dose and local control in stereotactic radiosurgery of brain metastasis. Br J Neurosurg 2009;23:170–8 [DOI] [PubMed] [Google Scholar]
- 8.Ohtakara K, Hayashi S, Hoshi H. Characterisation of dose distribution in linear accelerator-based intracranial stereotactic radiosurgery with the dynamic conformal arc technique: consideration of the optimal method for dose prescription and evaluation. Br J Radiol 2012;85:69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosgrove VP, Jahn U, Pfaender M, Bauer S, Budach V, Wurm RE. Commissioning of a micro multi-leaf collimator and planning system for stereotactic radiosurgery. Radiother Oncol 1999;50:325–36 [DOI] [PubMed] [Google Scholar]
- 10.Shaw E, Kline R, Gillin M, et al. Radiation Therapy Oncology Group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys 1993;27:1231–9 [DOI] [PubMed] [Google Scholar]
- 11.Nakamura JL, Verhey LJ, Smith V, et al. Dose conformity of gamma knife radiosurgery and risk factors for complications. Int J Radiat Oncol Biol Phys 2001;51:1313–19 [DOI] [PubMed] [Google Scholar]
- 12.Lomax NJ, Scheib SG. Quantifying the degree of conformity in radiosurgery treatment planning. Int J Radiat Oncol Biol Phys 2003;55:1409–19 [DOI] [PubMed] [Google Scholar]
- 13.Musat E, Roelofs E, Bar-Deroma R, et al. Dummy run and conformity indices in the ongoing EORTC low-grade glioma trial 22033-26033: first evaluation of quality of radiotherapy planning. Radiother Oncol 2010;95:218–24 [DOI] [PubMed] [Google Scholar]
- 14.Yomo S, Tamura M, Carron R, Porcheron D, Régis J. A quantitative comparison of radiosurgical treatment parameters in vestibular schwannomas: the Leksell Gamma Knife Perfexion versus Model 4C. Acta Neurochir (Wien) 2010;152:47–55 [DOI] [PubMed] [Google Scholar]
- 15.International Commission on Radiation Units and Measurements Prescribing, recording and reporting photon beam therapy. Report 62 (supplement to ICRU Report 50), Bethesda, MD: ICRU; 1999 [Google Scholar]
- 16.Ma L, Xia P, Verhey LJ, Boyer AL. A dosimetric comparison of fan-beam intensity modulated radiotherapy with gamma knife stereotactic radiosurgery for treating intermediate intracranial lesions. Int J Radiat Oncol Biol Phys 1999;45:1325–30 [DOI] [PubMed] [Google Scholar]
- 17.DiBiase SJ, Kwok Y, Yovino S, et al. Factors predicting local tumor control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas. Int J Radiat Oncol Biol Phys 2004;60:1515–19 [DOI] [PubMed] [Google Scholar]
- 18.Nedzi LA, Kooy HM, Alexander E, 3rd, Svensson GK, Loeffler JS. Dynamic field shaping for stereotactic radiosurgery: a modeling study. Int J Radiat Oncol Biol Phys 1993;25:859–69 [DOI] [PubMed] [Google Scholar]
- 19.Knöös T, Kristensen I, Nilsson P. Volumetric and dosimetric evaluation of radiation treatment plans: radiation conformity index. Int J Radiat Oncol Biol Phys 1998;42:1169–76 [DOI] [PubMed] [Google Scholar]
- 20.International Commission on Radiation Units and Measurements Prescribing, recording and reporting photon beam therapy. Report 50, Bethesda, MD: ICRU; 1993 [Google Scholar]
- 21.Kwon AK, Dibiase SJ, Wang B, Hughes SL, Milcarek B, Zhu Y. Hypofractionated stereotactic radiotherapy for the treatment of brain metastases. Cancer 2009;115:890–8 [DOI] [PubMed] [Google Scholar]
- 22.van’t Riet A, Mak AC, Moerland MA, Elders LH, van derZee W. A conformation number to quantify the degree of conformality in brachytherapy and external beam irradiation: application to the prostate. Int J Radiat Oncol Biol Phys 1997;37:731–6 [DOI] [PubMed] [Google Scholar]
- 23.IMRT Documentation Working Group, Holmes T, Das R, Low D, et al. American Society of Radiation Oncology recommendations for documenting intensity-modulated radiation therapy treatments. Int J Radiat Oncol Biol Phys 2009;74:1311–18 [DOI] [PubMed] [Google Scholar]
- 24.Rowe JG, Walton L, Vaughan P, Malik I, Radatz M, Kemeny A. Radiosurgical planning of meningiomas: compromises with conformity. Stereotact Funct Neurosurg 2004;82:169–74 [DOI] [PubMed] [Google Scholar]