Abstract
Introduction
The current standard for treating operable early stage non-small cell lung cancer is surgical resection and for inoperable cases it is external beam radiotherapy. Lung functions are adversely affected with both the above treatments. CyberKnife treatment limits radiation damage by tracking targets moving with each breath. The effect of CyberKnife treatment on pulmonary function tests has not been well documented.
Methods
Lung cancer patients who underwent CyberKnife treatment and had pre- and post-treatment pulmonary function tests were included. Paired t-tests were conducted. We also conducted subgroup analysis.
Results
Thirty-seven patients were included. Median age was 73 years. No statistical difference between mean pre- and post-CyberKnife pulmonary function tests was found.
Discussion
We observed that CyberKnife better preserves lung function status compared to current standards of care. It has shown to have very minimal side effects.
Keywords: non-small cell lung cancer, radiation pneumonitis, radiotherapy, pulmonary function tests
Introduction
More cancer patients die of lung cancer than of any other cancer.1,2 Early detection and treatment has shown overall improved outcomes in patients with lung cancer.3,4 The current standard of care for early stage non-small cell lung cancer (NSCLC) is invasive local control through surgical resection typically by lobectomy.3 Significant decrease in pulmonary function after lobectomy has been noted in earlier studies.5,6 Win et al found that NSCLC patients who underwent lobectomy “suffered a significant reduction of pulmonary reserve” and lost a great deal of lung function and exercise capacity.6 Functional lung status was measured using pulmonary function tests (PFT) in these studies.
For patients with stage 1 NSCLC who are inoperable or do not want to undergo surgery, external beam radiotherapy is the next best treatment as per the current standards. However, conventional radiotherapy has a high local failure rate of 6.4%–70% and has an estimated five-year overall survival rate of 21% ± 8%.7 Symptomatic radiation pneumonitis is present in 5% to 30% of patients receiving radiotherapy for thoracic malignancies. In addition, 50% to 90% of patients experience declines in pulmonary function test measurements.8–13 The decrease in lung function in both surgical and radiotherapy therapy patients is very significant considering that most lung cancer patients already have poor lung function as most of them also have a history of smoking. Reducing the morbidity of treatment could possibly improve the quality of life in the survivors. There have been significant advances in treatment technology in recent years that will reduce damage to healthy lung tissue during NSCLC treatment by surgery or radiotherapy. One such treatment is CyberKnife.
CyberKnife is a noninvasive, highly specific radiological method of treating cancer tissue with relative preservation of the surrounding healthy tissues. It has been approved and used to treat early stage NSCLC with excellent results. CyberKnife has the unique ability to track dynamic targets that move with breathing. Thus it limits radiation exposure and damage to normal tissue.14 Despite its great promise in treatment of lung cancer, no previous studies have specifically documented CyberKnife’s effect on pulmonary function studies. This study aims to confirm that in treatment of lung cancer, especially early stage, CyberKnife preserves lung function better than the current standard of care. The objective is to compare pre- and post-PFTs of patients treated with CyberKnife for lung cancer and ascertain how much lung function is preserved after the treatment.
Methods and materials
This is an Internal Review Board-approved, retrospective, observational cohort study of patients diagnosed with lung cancer to determine if there are differences in lung function before and after radiosurgery using CyberKnife. Pulmonary function tests were performed on the group before CyberKnife treatment and repeated 3–4 months after treatment.
We included patients diagnosed with primary or metastatic lung cancer who opted for CyberKnife treatment and had pre- and post-treatment pulmonary function tests. We excluded patients who did not fulfill the above criteria.
Abstracted data was entered into a Microsoft Access database and transferred to SPSS statistical software (v 20.0; IBM Corporation, Armonk, NY). Analysis included generation of descriptive statistics to adequately describe the sample. We also conducted paired t-tests to determine differences in pulmonary function data pre- and postoperatively (at 3 to 4 months). In addition, we conducted subgroup analysis based on gender, location, and stage of tumor. We compared eleven parameters of pulmonary function tests including: forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), FEV1/FVC ratio, forced expiratory flow 25%–75%, forced inspiratory vital capacity, slow vital capacity, inspiratory capacity, expiratory reserve volume, diffusing capacity of the lung for carbon monoxide, diffusion capacity corrected for alveolar volume, and alveolar volume.
Results
Thirty-seven patients were included in the study. The median age of the group was 73 years (Table 1). Approximately 49% of the subjects were female, while 51% were males. Three patients were given 5000 cGy in five divided fractions and 34 patients received 6000 cGy in five divided fractions. All the patients had smoking history. Stage 1 cancer was documented in 22 patients, stage 2 in two patients, stage 3 in five patients and stage 4 cancer in eight patients. The location of the tumors is mentioned in Table 1.
Table 1.
Patient characteristics
| Age (median) (years) | 73 |
| Male-n (%) | 19 (51) |
| Smoking-n (%) | 37 (100) |
| Location | |
| Left upper lobe-n (%) | 13 (35) |
| Left lower lobe-n (%) | 4 (11) |
| Right upper lobe-n (%) | 7 (20) |
| Right medial lobe-n (%) | 1 (3) |
| Right lower lobe-n (%) | 10 (30) |
| Right hilar lymph nodal region-n (%) | 2 (5) |
| Stage | |
| Stage I-n (%) | 22 (59) |
| Stage II-n (%) | 2 (5) |
| Stage III-n (%) | 5 (14) |
| Stage IV-n (%) | 8 (22) |
| Dose of radiation received | |
| 5000 cGy-n (%) | 3 (8) |
| 6000 cGy-n (%) | 34 (92) |
Paired t-tests indicated that there was no statistical difference between pre-CyberKnife and post-CyberKnife treatment in terms of mean PFTs for the overall group (Table 2) and for subgroups based on gender and tumor location.
Table 2.
Comparison of pulmonary function tests pre- and post-CyberKnife treatment
| Pulmonary function test results Pre-CyberKnife vs 3–4 months post-CyberKnife | ||||
|---|---|---|---|---|
|
| ||||
| PFT parameter (percentage of predicated) | Mean | n (number of patients) | Standard deviation | P-value |
| FVC – pre | 76.2 | 37 | 21.6 | 0.346 |
| FVC – post | 74.2 | 37 | 19.6 | |
| FEV1 – pre | 56.5 | 37 | 22.1 | 0.781 |
| FEV1 – post | 56.2 | 37 | 21.9 | |
| FEV1/FVC – pre | 74.6 | 37 | 17.9 | 0.905 |
| FEV1/FVC – post | 74.8 | 37 | 18.0 | |
| FEF – pre | 29.4 | 37 | 21.7 | 0.478 |
| FEF – post | 31.0 | 37 | 20.9 | |
| FIVC – pre | 2.1 | 33 | 0.7 | 0.189 |
| FIVC – post | 2.2 | 33 | 0.8 | |
| SVC – pre | 75.1 | 35 | 17.8 | 0.383 |
| SVC – post | 76.9 | 35 | 17.7 | |
| IC – pre | 74.9 | 32 | 17.8 | 0.267 |
| IC – post | 72.1 | 32 | 20.9 | |
| ERV – pre | 106.9 | 32 | 146.8 | 0.668 |
| ERV – post | 112.7 | 32 | 92.0 | |
| DLCO – pre | 56.1 | 33 | 24.4 | 0.672 |
| DLCO – post | 54.8 | 33 | 19.8 | |
| DLVA – pre | 79.6 | 32 | 30.5 | 0.287 |
| DLVA – post | 90.8 | 32 | 53.5 | |
| VA – pre | 71.1 | 32 | 19.1 | 0.299 |
| VA – post | 67.0 | 32 | 21.4 | |
Abbreviations: PFT, pulmonary function test; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; FEF, forced expiratory flow; FIVC, forced inspiratory vital capacity; SVC, slow vital capacity; IC, inspiratory capacity; ERV, expiratory reserve volume; DLCO, diffusing capacity of the lung for carbon monoxide; DLVA, diffusion capacity corrected for alveolar volume; VA, alveolar volume.
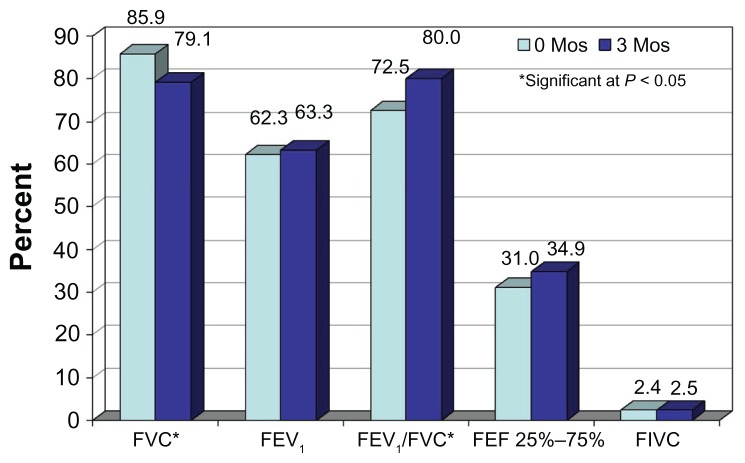
Analysis of subgroups based on stage indicate that stage 4 PFTs were significantly decreased for FVC, but significantly increased for the FEV1/FVC ratio, at 3 months compared to zero months (Figure 1).
Figure 1.
Comparison of mean pulmonary function tests before and 3–4 months after CyberKnife treatment in a stage 4 patient.
Abbreviations: Mos, months; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; FEF, forced expiratory flow; FIVC, forced inspiratory vital capacity.
Discussion
CyberKnife treatment has been shown to be very effective for stage 1 NSCLC in multiple studies published in last few years.15–19 In our study, we observed that CyberKnife is successful in preserving lung function status as was measured by pulmonary function tests. This observation supports the emerging role of CyberKnife in lung cancer management.
Our results are in line with the conclusions of Stephans et al, who studied Novalis treatment (another stereotactic body radiation therapy) and found no significant differences in PFT pre- and posttreatment.20 In Novalis treatment usually an abdominal compression devise is used to limit patients’ respiration, increasing patient discomfort. CyberKnife treatment does not need any method to limit breathing because of its tumor-tracking ability. Collins et al reported similar results for FVC and FEV1.19 They also reported other side effects of CyberKnife treatment being pneumothorax and radiation pneumonitis. They did show a decrease in diffusing capacity of the lung for carbon monoxide at 6 months in the treated patients.19 We did not see any such decrease at 3 months. We will continue to collect long-term follow-up data on our patients to determine if any changes in lung function occur longitudinally.
Most of the patients in the study were referred to a radiation oncology department after they were considered not eligible for surgery or the patient opted for radiation treatment. CyberKnife was offered to these patients because of decreased duration of treatment, convenience to the patient, and possibility of decreased damage to normal lung tissue.
The small sample size, nonrandomization, and retrospective nature of our study are certain limitations of our study and interpretation of the results should be made with caution. Prospective randomized trials comparing CyberKnife to conventional radiotherapy, other types of stereotactic body radiation therapies, and surgical treatments could further clarify the role of CyberKnife treatment.
Conclusion
In our study, we observed that CyberKnife was successful in preserving lung functions at 3–4 months, unlike the current standards of care, and also has a better side-effect profile. Compared to other methods of stereotactic body radiotherapy, it is more comfortable, as it does not need any methods or devices to restrict the patient. The CyberKnife has proved to be a safe and effective treatment. Our study is limited by a small sample size and the results should be interpreted with caution. More research is needed in this field.
Acknowledgments
Rishi Agarwal wishes to thank Dr Babu Paidipaty, a pulmonologist who helped in the understanding of PFTs and was instrumental in manuscript writing. RA also wants to thank Dr Ernie Balcueva who helped in manuscript writing, and Carol Wahl, an administrator at Seton Cancer Institute who was instrumental in data collection.
Footnotes
Authors’ contributions
YHK is the principal author and designed the study. RA is the first author and contributed in data collection, literature search, and manuscript writing. AP, KL, SB, and SV contributed in study design and data collection. PS contributed in data collection and manuscript writing. JC contributed in study design and data analysis.
Authors’ information
YHK is a radiation oncologist at Seton Cancer Institute in Saginaw, Michigan. RA and PS are third year internal medicine residents at Synergy Medical Education Alliance/MSUCHM. AP, KL, SB, and SV are medical students at Michigan State University. JC is a research manager at Synergy Medical Education Alliance/MSUCHM.
Disclosure
Dr Kim is a radiation oncologist at Seton Cancer Institute and uses CyberKnife for his patients. The authors report no conflicts of interest in this work.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Goldstraw P, Crowley J, Chansky K, et al. for the International Association for the Study of Lung Cancer International Staging Committee; Participating Institutions. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 4.Asamura H, Goya T, Koshiishi Y, et al. for Japanese Joint Committee of Lung Cancer Registry. A Japanese Lung Cancer Registry study: prognosis of 13,010 resected lung cancers. J Thorac Oncol. 2008;3(1):46–52. doi: 10.1097/JTO.0b013e31815e8577. [DOI] [PubMed] [Google Scholar]
- 5.Wang JS, Abboud RT, Wang LM. Effect of lung resection on exercise capacity and on carbon monoxide diffusing capacity during exercise. Chest. 2006;129(4):863–872. doi: 10.1378/chest.129.4.863. [DOI] [PubMed] [Google Scholar]
- 6.Win T, Groves AM, Ritchie AJ, Wells FC, Cafferty F, Laroche CM. The effect of lung resection on pulmonary function and exercise capacity in lung cancer patients. Respir Care. 2007;52(6):720–726. [PubMed] [Google Scholar]
- 7.Qiao X, Tullgren O, Lax I, Sirzén F, Lewensohn R. The role of radiotherapy in treatment of stage I non-small cell lung cancer. Lung Cancer. 2003;41(1):1–11. doi: 10.1016/s0169-5002(03)00152-1. [DOI] [PubMed] [Google Scholar]
- 8.Marks LB, Fan M, Clough R, et al. Radiation-induced pulmonary injury: symptomatic versus subclinical endpoints. Int J Radiat Biol. 2000;76(4):469–475. doi: 10.1080/095530000138466. [DOI] [PubMed] [Google Scholar]
- 9.Allen AM, Czerminska M, Jänne PA, et al. Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int J Radiat Oncol Biol Phys. 2006;65(3):640–645. doi: 10.1016/j.ijrobp.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Kahán Z, Csenki M, Varga Z, et al. The risk of early and late lung sequelae after conformal radiotherapy in breast cancer patients. Int J Radiat Oncol Biol Phys. 2007;68(3):673–681. doi: 10.1016/j.ijrobp.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Kong FM, Hayman JA, Griffith KA, et al. Final toxicity results of a radiation-dose escalation study in patients with non–small-cell lung cancer (NSCLC): Predictors for radiation pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys. 2006;65(4):1075–1086. doi: 10.1016/j.ijrobp.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 12.Yom SS, Liao Z, Liu HH, et al. Initial evaluation of treatment-related pneumonitis in advanced-stage non–small-cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68(1):94–102. doi: 10.1016/j.ijrobp.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 13.Ma J, Zhang J, Zhou S, et al. Association between RT-induced changes in lung tissue density and global lung function. Int J Radiat Oncol Biol Phys. 2009;74(3):781–789. doi: 10.1016/j.ijrobp.2008.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibbs IC, Loo BW., Jr CyberKnife stereotactic ablative radiotherapy for lung tumors. Technol Cancer Res Treat. 2010;9(6):589–596. doi: 10.1177/153303461000900607. [DOI] [PubMed] [Google Scholar]
- 15.Brown WT, Wu X, Fayad F, et al. CyberKnife radiosurgery for stage I lung cancer: results at 36 months. Clin Lung Cancer. 2007;8(8):488–492. doi: 10.3816/CLC.2007.n.033. [DOI] [PubMed] [Google Scholar]
- 16.Vahdat S, Oermann EK, Collins SP, et al. CyberKnife radiosurgery for inoperable stage IA non-small cell lung cancer: 18F-fluorodeoxyglucose positron emission tomography/computed tomography serial tumor response assessment. J Hematol Oncol. 2010;3:6. doi: 10.1186/1756-8722-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van der Voort van Zyp NC, Prévost JB, Hoogeman MS, et al. Stereotactic radiotherapy with real-time tumor tracking for non-small cell lung cancer: clinical outcome. Radiother Oncol. 2009;91(3):296–300. doi: 10.1016/j.radonc.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Collins BT, Erickson K, Reichner CA, et al. Radical stereotactic radiosurgery with real-time tumor motion tracking in the treatment of small peripheral lung tumors. Radiat Oncol. 2007;2:39. doi: 10.1186/1748-717X-2-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins BT, Vahdat S, Erickson K, et al. Radical CyberKnife radiosurgery with tumor tracking: an effective treatment for inoperable small peripheral stage I non-small cell lung cancer. J Hematol Oncol. 2009;2:1. doi: 10.1186/1756-8722-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stephans KL, Djemil T, Reddy CA, et al. Comprehensive analysis of pulmonary function Test (PFT) changes after stereotactic body radiotherapy (SBRT) for stage I lung cancer in medically inoperable patients. J Thorac Oncol. 2009;4(7):838–844. doi: 10.1097/JTO.0b013e3181a99ff6. [DOI] [PubMed] [Google Scholar]