Abstract
Objective
LIGHT (TNFSF 14) belongs to the tumor necrosis factor superfamily and is expressed by activated T cells as well as various types of antigen presenting cells. LIGHT binds to its cellular receptors TR2 and LTßR and has a co-stimulatory role in T cell activation. Here, we compared the relative expression of LIGHT in different immune cells and the biological activity of immune cell-derived LIGHT on endothelial cells.
Methods and Results
Surface expression of LIGHT and mRNA production by PBMC and isolated T cells (CD4+ or CD8+) significantly increased after stimulation with PMA (Phorbolester-12-Myristat-13-Acetat) + ionomycin. No LIGHT expression on PMA stimulated monocytes or monocytic-like THP-1 cells could be detected; differentiation of monocytes and THP-1 cells into macrophages, however, resulted in up-regulation of LIGHT. Supernatants of stimulated T cells contained higher concentrations of soluble LIGHT than macrophage supernatants normalized to cell numbers; release of soluble LIGHT was found to be dependent on metalloproteinase activity. Size determination of released soluble LIGHT by size exclusion chromatography revealed a molecular mass of ~60 kDa, suggesting a trimeric form. Released soluble LIGHT induced expression of proinflammatory antigens ICAM-1, tissue factor and IL-8 in human endothelial cells and caused apoptosis of IFN-γ pretreated endothelial cells. Soluble LIGHT was detected at low levels in sera of healthy controls and was significantly enhanced in sera of patients with chronic hepatitis C and rheumatoid arthritis (24.93 ± 9.41 vs.129.53 ± 49.14 and 172.13 ± 77.64; p < 0.0005).
Conclusion
These findings suggest that among immune cells activated T lymphocytes are the main source of soluble LIGHT with released amounts of soluble LIGHT markedly higher compared to platelets. Immune cell-derived membrane-bound and soluble trimeric LIGHT is biologically active, inducing proinflammatory changes in endothelial cells. Enhanced plasma levels of soluble LIGHT in patients with chronic infections suggest a role of LIGHT in systemic inflammatory activation.
Keywords: LIGHT, endothelial cells, inflammation
Introduction
The tumor necrosis factor related cytokines provide essential communication pathways that help orchestrate inflammatory and immune responses. They play an integral role in regulation of innate and adaptive immunity [1,2]. LIGHT belongs to the tumor necrosis superfamily and acts as a co-stimulatory molecule for T cells, including the enhancement of T cell proliferation and secretion of IFN-γ. LIGHT exists in a membrane-bound and soluble form. It is a ligand for TR2, LTßR and TR6, all of which are TNF receptor family members. Studies in animal models suggest that LIGHT signaling pathways may be crucial for the development of various autoimmune disorders, at least in part because of their effects on T cells and T-cell homing into inflamed tissues [3,4]. In an experimental mouse model it was shown that soluble LIGHT is involved in the pathogenesis of hepatitis via LIGHT-LTßR interaction [5]. Several studies suggest that LIGHT is involved in atherogenesis via induction of proatherogenetic cytokines and decreasing plaque stability by inducing metalloproteinase activity in macrophages [6].
Recently, Otterdal et al. [7] reported that LIGHT was associated with platelets und released upon activation. Thrombus material obtained at the site of plaque rupture in patients with STEMI (ST segment elevation myocardial infarction) contained platelet-derived LIGHT, suggesting platelets as the origin of LIGHT. In line with these findings we previously showed that the adhesion of platelets to endothelial cells is mediated by platelet-LIGHT [8]. Furthermore, patients with STEMI showed enhanced plasma levels of soluble LIGHT compared to healthy controls [5,10]. Recently, it was shown that concentrations of platelet-derived cytokines are markedly influenced by preclinical conditions and may be released only ex vivo [9]; raising the question if soluble LIGHT in patient sera really originates from platelets or possibly from other cell types, e. g. lymphocytes or macrophages. Similarly, the origin of circulating soluble LIGHT in other human autoimmune or inflammatory disease states (rheumatoid arthritis, infection) has not yet been studied - leaving the relative contribution of different cell types to circulating soluble LIGHT unresolved.
In the present study we analyzed different immune cells for expression and the release of membrane-bound and soluble LIGHT to quantify the different sources of LIGHT. Our findings show that T lymphocytes show high expression levels of membrane-bound and release large amounts of soluble LIGHT while monocytes and THP-1 cells only begin to express LIGHT upon differentiation into macrophages. Release of soluble LIGHT is shown to be matrix metalloproteinase-dependent, soluble LIGHT appears to be shed as a trimeric form which is biologically active.
Finally, enhanced serum levels of soluble LIGHT were detected in patients with chronic inflammatory disorders.
Methods
Cell culture
Human umbilical vein endothelial cells were isolated from umbilical cords by enzymatic digestion as previously described [10] and serially cultured in medium.
PBMC were obtained from healthy adult volunteers by density gradient centrifugation of heparinized venous blood. Mononuclear cells were collected from the interphase after Ficoll separation (Amersham Pharmacia Biotech, Freiburg, Germany). For the isolation of the single subpopulations of PBMCs magnetic Micro-Beads (Miltenyi Biotec, Bergisch Gladbach, Germany) were used according to the manufacturer's instructions. A total of 2.5 × 106 T cells per ml were stimulated with 100 ng/ml PMA (Sigma, Germany) and 1 μg/ml ionomycin (Sigma) for different time of points. Human monocytic cell line THP-1 was cultured in RPMI medium.
THP-1 cells were differentiated by overnight incubation with PMA (100 ng/ml).
PMA-differentiated THP-1 macrophages were stimulated in 12-well plates (2.5 × 106 cells/ml, Nunclon) with TNF-α (10 ng/ml; Sigma, Germany) for 2, 4 and 16 hours.
Monocytes were isolated from PBMC using magnetic beads and cultured in RPMI medium in the presence of cytokine MCSF at a concentration 100 nM for 6 days to generate macrophages. Differentiation of monocytes into macrophages was confirmed by the surface expression of CD80 and CD86. Stimulation of macrophages was performed in 12-well plates (5 × 106 cells/ml) with TNF-α (10 ng/ml) for 2, 4 and 16 hours.
For the generation of the supernatants 2.5 × 106 cells in 1 ml medium of each cell were activated respectively for 24 and 48 hours.
Light CDNA, construction of lightcontaining vector and cell transfection
Total RNA isolated from T cells was used to generate cDNA by RT-PCR (MBI-Fermentas). Total RNA was prepared from T cells using QiaAmp RNA blood mini kit. LIGHT cDNA was amplified by PCR using pfu DNA polymerase (Stratagene). The size of LIGHT cDNA was 731 bp. LIGHT cDNA was then cloned in to plasmid pcDNA 3.1D/V5-His-TOPO (Invitrogen, Germany) to generate pTOPOLIGHT. The integrity of pTOPOLIGHT was confirmed by restriction digestion analysis and sequencing.
Transfection of 293 cells
293 cells were transfected with plasmid pTopoLIGHT using Superfectin reagent (Qiagen) as recommended by the manufacturer.
Gel Exclusion Chromatography
Isolation of soluble LIGHT from culture supernatant of transiently LIGHT-expressing 293 cells or PMA + ionomycin stimulated PBMC and determination of apparent molecular weights of proteins and protein complexes were achieved by size exclusion chromatography on a calibrated Superdex 200 column (1.6 _ 60 cm; Pharmacia), connected to a FPLC system (Pharmacia). Sample volumes were 0.5 ml for analytical and up to 2 ml for preparative purposes. Eluted proteins were collected in fractions of 2.2 ml, concentrations determined by ELISA subjected to SDS-PAGE, and analyzed by silver staining or immunoblotting. (3 fractions of isolated soluble LIGHT from PBMC supernatant were pooled and used for endothelial cell stimulation assays).
Filtration of light containing supernatants
LIGHT containing medium (500 μl) was collected from stimulated PBMC, pipetted into the sample reservoir of Microcon 30 kDa, 50 kDa and 100 kDa cutoff centrifugal filter device (Millipore Corp., Bedford, Massachusetts, USA) and centrifuged according to the manufacturer's instructions. For the calculation of percent filtrate and retentate recovery a corresponding formula provided by the manufacturer was used.
Western blot analysis
Western blotting was performed according to standard procedures. For western blotting of the supernatants of activated PBMCs, Microcon centrifugal filters YM50 were used to concentrate soluble LIGHT and then suspended in SDS sample buffer for SDS-PAGE (12-15%). The separated proteins were transferred electrophoretically to nitrocellulose membranes, and immunodetection was carried out using specific antibodies against LIGHT. Peroxidase-conjugated donkey anti-rabbit were used, and detection was carried out with an enhanced chemiluminescence (ECL) reagent (Pierce). Chemiluninescence was quantified using a FluorS-MultiImager (BioRad, Munich, Germany).
Flow cytometry
Expression of LIGHT on THP-1 cells, CD4+, CD8+ and CD14+ was measured by indirect immunofluorescence flow cytometric analysis.
Cells were processed following standard procedures as described before [11]. A total of 5 × 105 cells were stimulated with PMA (Phorbolester-12-Myristat-13 Acetat; 100 ng/ml) + ionomycin (1 μg/ml) or TNF-α and stained with anti-LIGHT or control antibodies for 60 minutes at 4°C in the dark. All analyses were performed on flow cytometer (Becton Dickinson, Heidelberg, Germany) using CellQuest software FAC-Scan (BD Biosciences, Heidelberg, Germany).
Real Time-Pcr Analysis of mrna expression
The mRNA expression of ICAM-1, IL-8, ß-Actin and LIGHT was evaluated by Real Time PCR with Light-Cycler (Roche, Germany) according to the manufacturer's instructions.
A long (NM_003807) and short (NM_172014) form of LIGHT RNA has been reported earlier. To distinguish both types of RNA, PCR primers which spans the region missing in the shorter form was designed. By using RT-PCR, the presence of longer or a shorter RNA form was detected by amplicon having product sizes of 194 bps and 84 bps respectively.
Determination of tissue factor activity in huvec by one-stage clotting assay
One-stage clotting assays were performed as previously described [12]. Human endothelial cells (106/well) were incubated with isolated soluble LIGHT for 6 hours. TF activity was calculated by comparing the measured clotting time with a standard curve made with known amounts of TF. The measured amount of TF was expressed as picograms of TF/106 cells ± S.D.
ELISA Assays
Plasma levels of LIGHT in patients with hepatitis C and rheumatoid arthritis and LIGHT expression in the supernatants of stimulated cells was performed by ELISA according to the manufacturer's instructions ((R&D Systems, Wiesbaden, Germany)
Apoptosis assay
Endothelial cells (10 × 105 cells / well) were cultured with endothelial cell medium and preincubated with IFN-γ (250 U/ml) for 24 hours and then stimulated with cycloheximide 2.5 μg/ml (ICN Biomedicals) in combination with TNF-α (10 ng/ml) or recombinant LIGHT (100 ng/ml).
After stimulation for 24 hours the cells were harvested and washed in PBS following fixation in cold 70% ethanol. After fixation for at least 30 min at 4°C, cells were washed twice with PBS and stained with 50 μg/ml RNase and 50 μg/ml propidium iodide (Sigma) and analyzed by FACS.
Patients
22 patients, 15 females, age range 25 - 69 years, with rheumatoid arthritis defined by American College of Rheumatology criteria [13], were identified in rheumatology clinic with approval from the local research ethics committee.
10 patients (6 female; age range 18 - 50 years) with chronic hepatitis C infection (defined by positive serum antibodies to HCV by means of a second or third generation HCV enzyme linked immunosorbent assay and detectable serum HCV RNA) were recruited to the study. Plasma samples from all individuals were collected and stored at -80°C until analysis.
16 healthy volunteers (10 female; age range 20 -48 years) with no previous diagnosis of RA or other chronic inflammatory diseases served as normal controls.
Participating subjects gave written informed consent. The investigation conforms to the principles outlined in the Declaration of Helsinki.
Data presentation and statistics
Comparisons between group means were performed using Mann-Whitney U-test, Student t-test or ANOVA analysis as appropriate. Data are presented as mean + SD. P < 0.05 was considered as statistically significant.
Results
Expression of light on different activated immune cells
Stimulation of purified CD4+ and CD8+ T cells with PMA (100 ng/ml) + ionomycin (1 μg/ml) induced a marked increase in surface expression of LIGHT. No LIGHT expression could be detected on monocytes and undifferentiated THP-1 cells after stimulation with TNF-α (10 ng/ml). All cells were stimulated for 24 hours and analyzed by flow cytometry (Figure 1).
Figure 1.
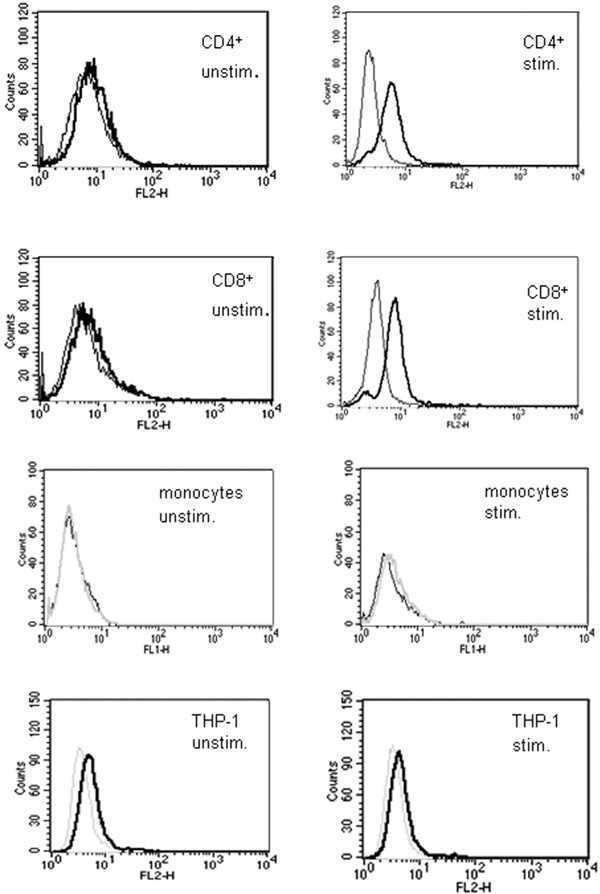
Surface expression of LIGHT on CD4+ and CD8+. Expression of LIGHT on the surface of CD4+, CD8+, CD14+ and THP-1 cells was measured by FACS. Cells were stimulated with PMA and ionomycin for 24 hours and stained with anti-LIGHT monoclonal antibody. LIGHT was not detectable on unstimulated cells, but activation of the cells resulted in appearance of LIGHT on the cell surface of CD4+ and CD8+ T cells, but not on monocytes and THP-1 cells. Thin curves correspond to the negative control and thick black curves correspond to specific staining for LIGHT. These data correspond to one representative experiment of three performed (Figure. 1).
Mrna expression of light in activated immune cells
mRNA LIGHT expression in resting and stimulated T cells (PMA 100 ng/ml + ionomycin 1 μg/ml ), monocytes, macrophages and differentiated THP-1 cells (TNF-α 10 ng/ml) following specific time patterns (2, 4 and 16 hours) was evaluated by RT-PCR. LIGHT levels increased in response to stimulation with a maximum at 2 - 4 hours after activation in T cells, macrophages and differentiated THP-1 cells (Figure 2A-2C). No mRNA expression for LIGHT was seen in undifferentiated monocytes. In all mRNA / PCR experiments only a single band of LIGHT cDNA was detected, even using specifically designed primers to detect a potential differentially spliced mRNA form (see methods).
Figure 2.
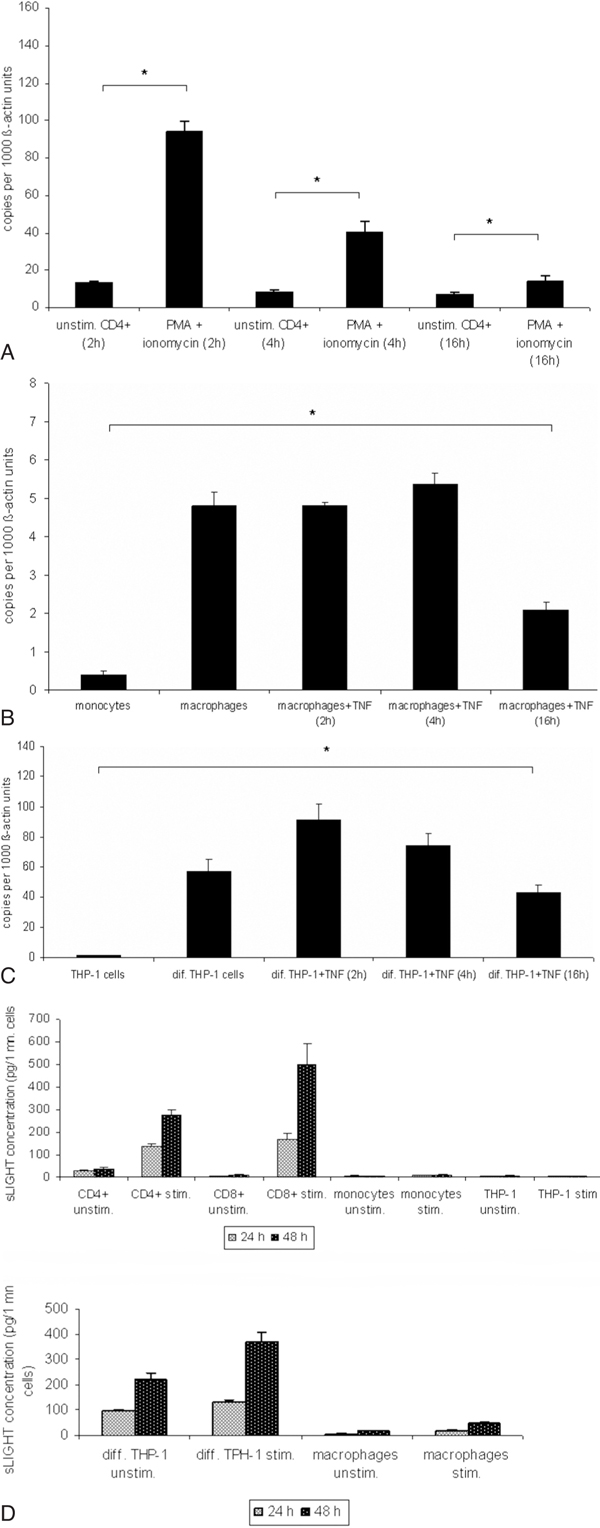
A-2C, mRNA expression of LIGHT in immune cells. T cells were stimulated with PMA and ionomycin for 2, 4 or 16 hours. PMA differentiated THP-1 cells, monocyte-derived macrophages were further stimulated with TNF-α for 2, 4 or 16 hours. Expression of LIGHT was assessed at different time points by RT-PCR. Maximum of LIGHT expression in T cells was observed after 2 hours after stimulation (Figure 2A). *P < 0.05 vs unstimulated cells. In differentiated THP-1 cells and monocyte-derived macrophages maximum LIGHT expression peaked after 4 hours at the mRNA-level (Figure 2B and 2C). *P < 0.05 vs monocytes and THP-1 cells. These data correspond to one representative experiment from three performed.
Release of soluble light by activated immune cells
Secretion of soluble LIGHT into the supernatants of PBMC or stimulated CD4+ and CD8+ (PMA 100 ng/ml + ionomycin 1 μg/ml) monocytes, macrophages, THP-1 cells and differentiated THP-1 (TNF-α 10 ng/ml) cells was analyzed by ELISA. Significant concentrations of soluble LIGHT could be detected in the supernatants of PBMC or CD4+ and CD8+ cells and differentiated THP-1 cells, which increased markedly upon cell activation, but not in monocytes and undifferentiated THP-1 cells. Differentiated THP-1 and T cell supernatants contained similar amounts of soluble LIGHT. Supernatant concentrations of LIGHT were higher in the CD8+ subset than in CD4+ cells (Figure 2D). A low concentration of soluble LIGHT was detected in the supernatants of stimulated monocyte-derived macrophages.
In all cell types the specificity of ELISA results was supported by identical findings after ultrafiltration (30000 rpm, 12 h).
Soluble light is generated through proteolytic cleavage of mlight by matrix metalloproteinase inhibitors
The involvement of metalloproteinases in the regulation of soluble LIGHT was investigated by testing the effect of a broad-spectrum synthetic metalloproteinase inhibitor (GM6001, a hydroxamate inhibitor of MMPs). GM6001 (30 μM) inhibited significantly the release of soluble LIGHT into the supernatant of peripheral blood mononuclear cells and isolated CD4+ T cell subset (Figure 3A and 3B) when added 30 minutes prior and during the stimulation with PMA 100 ng/ml + ionomycin 1 μg/ml.
Figure 3.
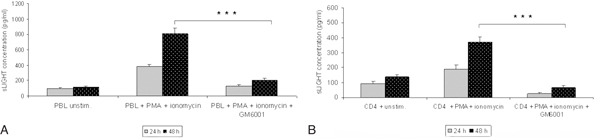
A-3B, Matrix metalloproteinase (GM6001) mediates shedding of soluble LIGHT. 30 minutes prior to activation of PBL and CD4+ subsets with PMA metalloproteinase inhibitor GM6001 (30 μM) was added to the medium. After 24 hours and 48 hours the conditioned medium was analyzed for the presence of soluble LIGHT by ELISA. MMP inhibitor markedly reduced the release of soluble LIGHT in lymphocytes (Figure 3A and 3B). Values represent the mean ± SD of soluble LIGHT analyzed in triplicate. ***P < 0.0005 vs without GM6001 treated cells.
Monomeric size of soluble light
Western blot analysis (reducing conditions) of lymphocyte derived soluble LIGHT revealed a molecular mass of approximately ~23 -25 kDa, similar to the molecular mass of recombinant soluble LIGHT (25 kDa), which represents only the extracellular part (Figure 4).
Figure 4.

Monomeric size of soluble LIGHT. To determine the monomoric size of soluble LIGHT, supernatants of PMA (100 ng/ml + ionomycin 1 μg/ml) activated PBMCs were collected after 48 hours and concentrated with Microcon centrifugal filters YM50. Soluble LIGHT production in supernatant was then assessed by ELISA. Molecular mass of monomeric LIGHT was detected by Western blotting. 50 ng of recombinant sLIGHT were loaded in one lane as a control (Figure 4).
Size determination of soluble light
For initial estimation of the molecular mass of soluble LIGHT filters with a molecular weight cut-off values of > 100 kDa, 50 kDa and 30 kDa were used. Retentate fraction of soluble LIGHT in microcon filters with 30 kDa and 50 kDa molecular weight cut off was about 90%. Spinning of soluble LIGHT through a filter with a 100 kDa MWCO resulted in a ~ 30% retentate fraction, indicating a molecular mass of soluble LIGHT between 50 - 100 kDa.
Size determination of lymphocyte-derived soluble LIGHT by size exclusion chromatography on a calibrated Superdex 200 gel filtration column resulted in a molecular mass of approximately 60 kDa, indicating a trimeric form of soluble LIGHT. The apparent molecular mass of soluble LIGHT was calculated from standard curves as a mean value of three independent measurements (data not shown).
Inflammatory effects of lymphocyte derived light on huvec
To investigate the biological activity of cell-derived soluble LIGHT, endothelial cells were incubated with LIGHT-containing supernatants derived from activated lymphocytes (PMA 100 ng/ml + ionomycin 1 μg/ml). After stimulation of HUVECs for 3 hours a marked up-regulation of ICAM-1 and IL-8 was shown by RTPCR (Figure 5A). Similar results were obtained with soluble LIGHT isolated by size exclusion chromatography (Figure 5B). Pre-incubation of LIGHT-containing supernatants with anti-LIGHT mAb resulted in a partial reduction of these cytokines; with isolated soluble LIGHT antibody preincubation resulted in almost complete inhibition of up-regulation of ICAM-1 and IL-8. Membrane-bound LIGHT of stimulated PBMC was shown to induce up-regulation of ICAM-1 and IL-8 when co-incubating whole-cell PBMC with endothelial cells (Figure 5C). Pre-incubation of PBMC with anti- LIGHT resulted in partial inhibition of ICAM-1 and IL-8 up-regulation, suggesting a substantial contribution of cell-bound LIGHT to this up-regulation. Similar results were obtained when using PMA-stimulated CD4+-T cells (data not shown). Furthermore, induction of tissue factor activity by isolated soluble LIGHT in endothelial cells could be shown by one stage-clotting assay (Figure 5D), which again was inhibitable by antibody pre-incubation.
Figure 5.
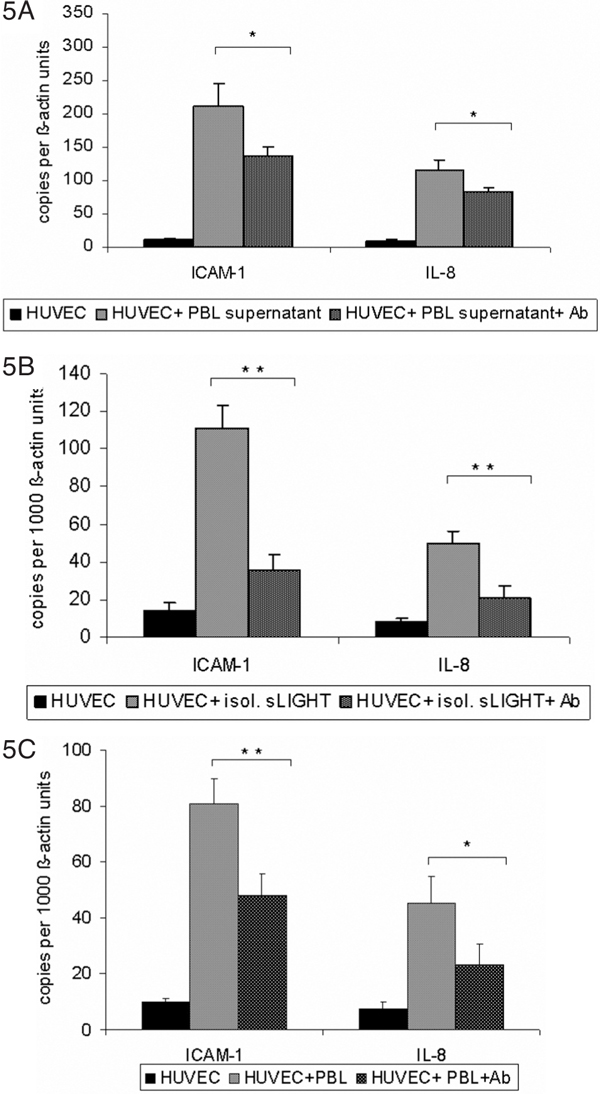
A-C, Induction of ICAM1 and IL8 by lymphocytederived membranebound and soluble LIGHT in human endothelial cells. Human endothelial cells were co-incubated in supernatant containing soluble LIGHT, isolated soluble LIGHT and stimulated (PMA 100 ng/ml + ionomycin 1 μg/ml) and unstimulated whole lymphocytes for 3 hours. Thereafter, HUVECS were harvested and analyzed for expression of the adhesion molecule ICAM-1 and IL-8 by real-time PCR. After stimulation ICAM-1 and IL-8 were significantly increased in all experiments (Figure 5A - 5C). To confirm that the stimulation of the endothelial cells was due to LIGHT, specific anti-LIGHT-antibodies were used. After pre-incubation of whole lymphocytes and LIGHT containing medium with anti-LIGHT antibody, cytokine induction was partially abolished. After pre-treatment of isolated LIGHT containing medium with anti LIGHT, the effects were even completely abolished (Figure 5B). These data correspond to one representative experiment of three performed. *P < 0.05 and **P < 0.005 vs stimulated cells without blocking antibody.
Figure 5D.
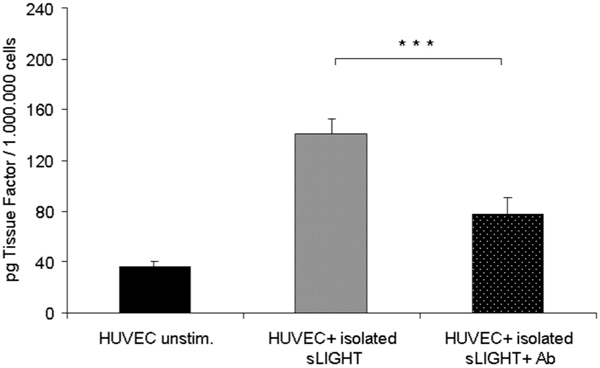
Induction of tissue factor (TF) activity by recombinant LIGHT in human endothelial cells. Endothelial cells were stimulated with isolated soluble LIGHT, harvested after 6 hours and assayed for procoagulant activity as described under "Materials and Methods". LIGHT induced a marked up-regulation of tissue factor in endothelial cells. TF activity of each sample was determined based on comparison with a standard curve established with known amounts of recombinant TF. The data represent the mean of three independent experiments performed (Figure 5D). ***P < 0.0005 vs stimulated cells without bocking antibody.
Light-mediated apoptosis in huvec
There have been many studies addressing the role of LIGHT in apoptosis, especially with tumour cell lines expressing both receptors TR2 and LTßR. LIGHT alone did not induce apoptosis in endothelial cells.
Since previous studies reported that LIGHT, in combination with IFN-β induced cell death through LTßR in several cancer cell lines, endothelial cells were preincubated with IFN-β (25 U/ml) for 24 hours prior to apoptosis induction. Such IFN-β pretreated endothelial cells were fully susceptible to LIGHT-mediated apoptosis (Figure 6). IFN-β alone had no effect on endothelial cells (not shown).
Figure 6.
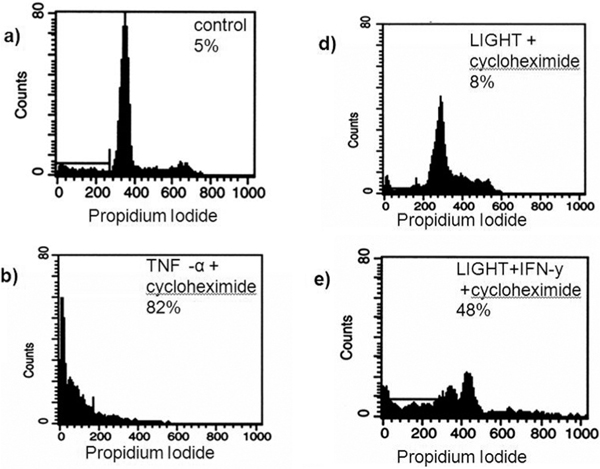
Induction of apoptosis in endothelial cells by recombinant LIGHT. LIGHT induced apoptosis endothelial cells after preincubation with IFN-γ. Endothelial cells were incubated with medium, soluble LIGHT, TNF-α, cycloheximide alone or in combination with TNF-α or LIGHT for 24 hours. Thereafter harvested and analyzed for propidium iodide exclusion by FACS analysis. IFN-γ plus cycloheximide alone did not induce apoptosis (control). LIGHT combined with cycloheximide induced apoptosis in endothelial cells after pre-treatment with IFN-γ (Figure 6). IFN-γ alone had no effect on endothelial cells (not shown). The data represent the mean ± SD of three independent experiments performed.
Increased plasma levels of light in hepatitis c and rheumatoid arthritis patients
Involvement of LIGHT in human atherosclerosis, synovialitis of rheumatoid arthritis or pathogenesis of experimental rodent liver inflammation has been reported. To elucidate the potential systemic relevance of soluble LIGHT in inflammatory diseases like hepatitis C or rheumatoid arthritis, plasma levels of LIGHT of these patients was examined by ELISA (Figure 7). While healthy controls showed low, but distinct serum concentrations of soluble LIGHT (n = 16), patients with hepatitis C (n = 10) and rheumatoid arthritis (n = 22) showed several-fold increased plasma levels of soluble LIGHT. (Hepatitis C: 129.53 ± 49.14 pg/ml, rheumatoid arthritis: 172.13 ± 77.64 pg/ml; both p < 0.0005).
Figure 7.
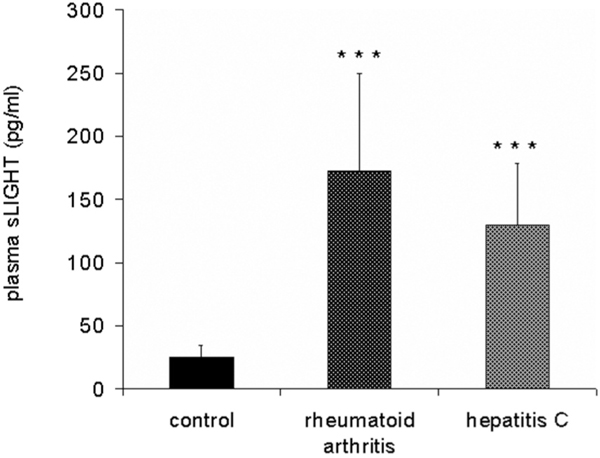
Enhanced plasma levels of soluble LIGHT in patients with hepatitis C and rheumatoid arthritis. Plasma levels of LIGHT was measured by ELISA in patients with hepatitis C (n = 10), rheumatoid arthritis (n = 22) and 16 healthy controls. Soluble LIGHT concentration was normalized to cell numbers (pg/1 million cells). The data represent the mean ± SD of three independent experiments performed (Figure 7). ***P < 0.0005 vs healthy controls.
Discussion
The tumor necrosis factor superfamily member LIGHT plays an important role in regulating the immune response and LIGHT may play a prominent role in predominantly T cell mediated pathologies. Several studies have demonstrated the expression of LIGHT in different cell types and its potential role in inflammatory diseases [15,16]. Recently, we have shown that platelet-associated LIGHT is involved in the adhesion of platelets to endothelial cells under static and high-shear dynamic flow conditions [11]. Furthermore our result showed enhanced plasma levels of LIGHT in patients with acute myocardial infarction. These findings were in agreement with the results of Otterdal et al.10 and strengthen the notion of the potential role of platelet-derived LIGHT in coronary heart disease. Although the expression of LIGHT has been shown in different cell types, including platelets, the relative contribution of various cellular origins to soluble LIGHT as well as the relevance and biological activity of soluble LIGHT secreted by different cells has not been investigated. In this study we compared the quantitative expression of LIGHT in different immune cells and the biological activity of soluble LIGHT upon endothelial cells as an exemplary readout system.
Here, we could show that among the immune cells, CD4+/CD8+ T cells are the main source of soluble LIGHT. Circulating monocytes do not express LIGHT, but differentiation into macrophages resulted in expression of LIGHT, even more so in differentiated THP-1 cells. Secretion of soluble LIGHT was matrix metalloproteinase-dependent and led to inflammatory changes in endothelial cells measured as up-regulation of adhesion molecules (ICAM-1), pro-coagulant factors (TF) and release of chemokines (IL-8). Size determination of soluble LIGHT revealed a molecular mass of approximately 60 kDa, suggesting a trimeric form similar to other TNFSF members. Furthermore, patients with chronic inflammatory diseases such as hepatitis C and rheumatoid arthritis showed enhanced concentrations of soluble LIGHT compared to healthy controls.
Basal cellular expression of LIGHT on mRNA and surface protein levels was significantly increased in peripheral blood mononuclear cells or isolated CD4+/ CD8+ T cells after stimulation. Stimulated monocytes or monocyte-like THP-1 cells lacked expression of LIGHT, but differentiation of THP-1 cells into macrophages resulted in a marked upregulation of LIGHT. Monocyte-derived macrophages showed only a weak expression of LIGHT.
Soluble LIGHT could be detected in large amounts in the supernatants of CD4+/ CD8+ T cells as well as differentiated THP-1 cells, but not in the supernatants of THP-1 cells and monocytes. Ultrafiltration experiments excluded membrane fragments of ruptured / dead cells as the source of soluble LIGHT. In recently published data involvement of platelet-derived LIGHT in acute coronary syndromes was suggested [10,11], but based on recently published data by Ivandic et al. [12] that release of platelet-mediated cytokines are mostly influenced by preanalytical conditions through ex vivo activation, the actual relevance and contribution of platelet-derived LIGHT in atherosclerosis might have been misinterpreted or overestimated. Clinical relevance of other platelet-derived inflammatory cytokines was shown for soluble CD40 ligand [17]; increased concentrations of sCD40L were reported mostly in disorders associated with platelet activation such as acute and stable coronary artery disease [18,19]. However, Ivandic et al.12 recently reported that increased sCD40 L concentrations by platelets were dependent on sample preparation; no disease-related in vivo activation of platelets was observed.
LIGHT has been claimed that it exists in several distinct molecular forms, which are directed to distinct cellular compartments, including the extracullar space, the membrane, and the cytosol [20], however, PCR data in our experiments consistently showed a single band for LIGHT (not shown). Furthermore, our data indicate that similar to soluble CD40 ligand [21] shedding of soluble LIGHT is mediated by metalloproteinase from the cell surface. Metalloproteinase inhibitor reduced significantly the release of soluble LIGHT and no separate mRNA or cDNA form of soluble LIGHT was detectable.
Size determination of immune cell-derived soluble LIGHT by gel exclusion chromatography and filter cartridge centrifugation revealed a molecular mass of approximately 60 kDa and provide first evidence for a trimeric form since western blot analysis shows a monomeric size of 23 - 25 kDa. It can be speculated that similar to other TNFSF members, LIGHT is assembled to and functions as a homotrimer. These results implicate that release of LIGHT, at least partly, is due to the action of metal-dependent enzymes.
Endothelial cells play a critical role in the control of vascular function and participate in inflammatory and immune reactions. Herein we showed that immune cell-derived membrane-bound and soluble LIGHT is a potent inducer of ICAM-1, tissue factor and IL-8. After incubation of endothelial cells with LIGHT containing supernatant or isolated soluble LIGHT inflammatory cytokines were up-regulated. The effects of LIGHT-containing supernatants could however, only partially be blocked by anti-LIGHT antibody. When using LIGHT isolated und purified by gel exclusion chromatography from cell supernatants, LIGHT-mediated activation of endothelial cells could be almost completely blocked by specific antibody, strongly supporting the notion of specific biologic activity of immune cell-mediated soluble LIGHT. While several studies have examined the cytotoxic effects of LIGHT on tumor cell lines in culture and its ability to prevent tumor formation in mice [3,22], induction of apoptosis in endothelial cell has not been addressed yet. Our findings indicate that soluble LIGHT is also able to induce apoptosis in IFN-γ-pretreated endothelial cells. IFN-γ has been reported to be involved in inflammatory responses, e. g. its expression in atherosclerotic plaques [23]. Since, we found that LIGHT-mediated apoptosis occurs only in IFN-γ-pretreated endothelial cells, it is likely that both, LIGHT and IFN-γ, work in concert to enhance inflammatory reactions.
Recently Dahl et al. [24] could show myocardial expression of LIGHT in ischemic and non-ischemic regions of the left ventricle in patients with end-stage heart failure undergoing cardiac transplantation. In this patient collective HVEM (herpesvirus entry mediator) was up-regulated in circulating monocytes, accompanied by increased expression of LIGHT in circulating T cells. Furthermore, LIGHT-mediated IL-6 expression in PBMC and endothelial cells could be shown in patients with chronic heart failure, underscoring a role for LIGHT in the progression of heart failure. In our study we show increased plasma levels of soluble LIGHT in patients with chronic hepatitis C and rheumatoid arthritis as an example for inflammatory diseases where activated T cells play a key role. As our data show that monocytes do not express LIGHT and resident macrophages show only a slight expression while circulating T cells have the highest LIGHT expression, it might be speculated that activated T cells are the main source of circulating LIGHT in inflammatory or infectious conditions. In terms of platelet-derived LIGHT, the quantitative contribution by platelets is difficult to asses because of ex vivo activation.
These findings implicate that soluble LIGHT originates not only from platelets, but largely from immune cell activation during systemic inflammation or infection.
In summary, rather than representing "disposal mechanism" shedding/release of soluble LIGHT represents a relevant biological activation process leading to pro-inflammatory changes e. g. in endothelial cells, where the interaction with T cell activation and platelet function/adhesion may accelerate atherothrombotic pathways. Soluble LIGHT however, does not appear to be exclusively produced by platelets and macrophages as suggested previously, but elevated LIGHT serum concentrations seen in diverse inflammatory or infectious pathologies, most likely are derived from activated immune cells. The diagnostic/ prognostic efficacy of soluble LIGHT determination in various clinical states will have to be determined in more detail in future studies. Nonetheless, diagnostic determinations or therapeutic inhibition of the LIGHT pathway may become useful research developments in various clinical pathologies.
References
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3(9):745–56. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- Tamada K, Shimozaki K, Chapoval AI, Zhai Y, Su J, Chen SF, Hsieh SL, Nagata S, Ni J, Chen L. LIGHT, a TNF-like molecule, costimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T cell response. J Immunol. 2000;64(8):4105–10. doi: 10.4049/jimmunol.164.8.4105. [DOI] [PubMed] [Google Scholar]
- Scholz H, Sandberg W, Damas JK, Smith C, Andreassen AK, Gullestad L, Froland SS, Yndestad A, Aukrust P, Halvorsen B. Enhanced plasma levels of LIGHT in unstable angina: possible pathogenic role in foam cell formation and thrombosis. Circulation. 2005;112:2121–2129. doi: 10.1161/CIRCULATIONAHA.105.544676. [DOI] [PubMed] [Google Scholar]
- Schneider K, Potter KG, Ware CF. Lymphotoxin and LIGHT signaling pathways and target genes. Immunol Rev. 2004;202:49–66. doi: 10.1111/j.0105-2896.2004.00206.x. Review. [DOI] [PubMed] [Google Scholar]
- Anand S, Wang P, Yoshimura K, Choi IH, Hilliard A, Chen YH, Wang CR, Schulick R, Flies AS, Flies DB, Zhu G, Xu Y, Pardoll DM, Chen L, Tamada K. Essential role of TNF family molecule LIGHT as a cytokine in the pathogenesis of hepatitis. J Clin Invest. 2006;116(4):1045–51. doi: 10.1172/JCI27083. Epub 2006 Mar 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WH, Kim SH, Lee Y, Lee BB, Kwon B, Song H, Kwon BS, Park JE. Tumor necrosis factor receptor superfamily 14 is involved in atherogenesis by inducing proinflammatory cytokines and matrix metalloproteinases. Arterioscler Thromb Vasc Biol. 2001;21(12):2004–10. doi: 10.1161/hq1201.098945. [DOI] [PubMed] [Google Scholar]
- Otterdal K, Smith C, Oie E, Pedersen TM, Yndestad A, Stang E, Endresen K, Solum NO, Aukrust P, Damas JK. Platelet-derived LIGHT induces inflammatory responses in endothelial cells and monocytes. Blood. 2006. [DOI] [PubMed]
- Celik S, Langer H, Stellos K, May AE, Shankar V, Kurz K, Katus HA, Gawaz MP, Dengler TJ. Platelet-associated LIGHT (TNFSF14) mediates adhesion of platelets to human vascular endothelium. Thromb Haemost. 2007;98(4):798–805. [PubMed] [Google Scholar]
- Ivandic BT, Spanuth E, Haase D, Lestin HG, Katus HA. Increased plasma concentrations of soluble CD40 ligand in acute coronary syndrome depend on in vitro platelet activation. Clin Chem. 2007;53(7):1231–4. doi: 10.1373/clinchem.2007.085332. Epub 2007 May 10. [DOI] [PubMed] [Google Scholar]
- Pober JS, Bevilacqua MP, Mendrick DL, Lapierre LA, Fiers W, Gimbrone MA Jr. Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986;36:1680–1687. [PubMed] [Google Scholar]
- Kluger MS, Johnson DR, Pober JS. Mechanism of sustained E-selectin expression in cultured human dermal microvascular endothelial cells. J Immunol. 1997;158:887–896. [PubMed] [Google Scholar]
- Nawroth PP, Stern DM, Kisiel W, Bach R. Cellular requirements for tissue factor generation by bovine aortic endothelial cells in culture. Thromb Res. 1985;40(5):677–91. doi: 10.1016/0049-3848(85)90305-6. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Edworth SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheumatism. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu GL, Ruben S, Murphy M, Eisenberg RJ, Cohen GH, Spear PG, Ware CF. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8:21–30. doi: 10.1016/S1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- Anand S, Wang P, Yoshimura K, Choi IH, Hilliard A, Chen YH, Wang CR, Schulick R, Flies AS, Flies DB, Zhu G, Xu Y, Pardoll DM, Chen L, Tamada K. Essential role of TNF family molecule LIGHT as a cytokine in the pathogenesis of hepatitis. J Clin Invest. 2006;116(4):1045–51. doi: 10.1172/JCI27083. Epub 2006 Mar 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WJ, Kang YJ, Koh EM, Ahn KS, Cha HS, Lee WH. LIGHT is involved in the pathogenesis of rheumatoid arthritis by inducing the expression of pro-inflammatory cytokines and MMP-9 in macrophages. Immunology. 2005;114(2):272–9. doi: 10.1111/j.1365-2567.2004.02004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukrust P, Muller F, Ueland T, Berget T, Aaser E, Brunsvig A, Solum NO, Forfang K, Froland SS, Gullestad L. Enhanced levels of soluble and membrane-bound CD40 ligand in patients with unstable angina. Possible reflection of T lymphocyte and platelet involvement in the pathogenesis of acute coronary syndromes. Circulation. 1999;100:614–620. doi: 10.1161/01.cir.100.6.614. [DOI] [PubMed] [Google Scholar]
- Garlichs CD, John S, Schmeisser A, Eskafi S, Stumpf C, Karl M, Goppelt-Struebe M, Schmieder R, Daniel WG. Upregulation of CD40 and CD40 ligand (CD154) in patients with moderate hypercholesterolemia. Circulation. 2001;104(20):2395–400. doi: 10.1161/hc4501.099312. [DOI] [PubMed] [Google Scholar]
- Marx N, Imhof A, Froehlich J, Siam L, Ittner J, Wierse G, Schmidt A, Maerz W, Hombach V, Koenig W. Effect of rosiglitazone treatment on soluble CD40L in patients with type 2 diabetes and coronary artery disease. Circulation. 2003;107(15):1954–7. doi: 10.1161/01.CIR.0000069272.06194.91. Epub 2003 Apr 14. [DOI] [PubMed] [Google Scholar]
- Granger SW, Butrovich KD, Houshmand P, Edwards WR, Ware CF. enomic characterization of LIGHT reveals linkage to an immune response locus on chromosome 19p13.3 and distinct isoforms generated by alternate splicing or proteolysis. J Immunol. 2001;167(9):5122–8. doi: 10.4049/jimmunol.167.9.5122. [DOI] [PubMed] [Google Scholar]
- Contin C, Pitard V, Itai T, Nagata S, Moreau JF, Déchanet-Merville J. Membrane-anchored CD40 is processed by the tumor necrosis factor-alpha-converting enzyme. Implications for CD40 signaling. J Biol Chem. 2003;278(35):32801–9. doi: 10.1074/jbc.M209993200. Epub 2003 Jun 16. [DOI] [PubMed] [Google Scholar]
- Zhai Y, Guo R, Hsu TL, Yu GL, Ni J, Kwon BS, Jiang GW, Lu J, Tan J, Ugustus M, Carter K, Rojas L, Zhu F, Lincoln C, Endress G, Xing L, Wang S, Oh KO, Gentz R, Ruben S, Lippman ME, Hsieh SL, Yang D. LIGHT, a novel ligand for lymphotoxin beta receptor and TR2/HVEM induces apoptosis and suppresses in vivo tumor formation via gene transfer. J Clin Invest. 1998;102(6):1142–51. doi: 10.1172/JCI3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellides G, Tereb DA, Kirkiles-Smith NC, Kim RW, Wilson JH, Schechner JS, Lorber MI, Pober JS. Interferongamma elicits arteriosclerosis in the absence of leukocytes. Nature. 2000;403(6766):207–11. doi: 10.1038/35003221. [DOI] [PubMed] [Google Scholar]
- Dahl CP, Gullestad L, Fevang B, Holm AM, Landrø L, Vinge LE, Fiane AE, Sandberg WJ, Otterdal K, Frøland SS, Damås JK, Halvorsen B, Aukrust P, Oie E, Yndestad A. Increased expression of LIGHT/TNFSF14 and its receptors in experimental and clinical heart failure. Eur J Heart Fail. 2008;10(4):352–9. doi: 10.1016/j.ejheart.2008.02.010. Epub 2008 Mar 18. [DOI] [PubMed] [Google Scholar]


