Abstract
Significance: Regulation of mitochondrial H2O2 homeostasis and its involvement in the regulation of redox-sensitive signaling and transcriptional pathways is the consequence of the concerted activities of the mitochondrial energy- and redox systems. Recent Advances: The energy component of this mitochondrial energy-redox axis entails the formation of reducing equivalents and their flow through the respiratory chain with the consequent electron leak to generate 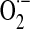 and H2O2. The mitochondrial redox component entails the thiol-based antioxidant system, largely accounted for by glutathione- and thioredoxin-based systems that support the activities of glutathione peroxidases, peroxiredoxins, and methionine sulfoxide reductase. The ultimate reductant for these systems is NADPH: mitochondrial sources of NADPH are the nicotinamide nucleotide transhydrogenase, isocitrate dehydrogenase-2, and malic enzyme. NADPH also supports the glutaredoxin activity that regulates the extent of S-glutathionylation of mitochondrial proteins in response to altered redox status. Critical Issues: The integrated network of these mitochondrial thiols constitute a regulatory device involved in the maintenance of steady-state levels of H2O2, mitochondrial and cellular redox and metabolic homeostasis, as well as the modulation of cytosolic redox-sensitive signaling; disturbances of this regulatory device affects transcription, growth, and ultimately influences cell survival/death. Future Directions: The modulation of key mitochondrial thiol proteins, which participate in redox signaling, maintenance of the bioenergetic machinery, oxidative stress responses, and cell death programming, provides a pivotal direction in developing new therapies towards the prevention and treatment of several diseases. Antioxid. Redox Signal. 17, 1714–1727.
and H2O2. The mitochondrial redox component entails the thiol-based antioxidant system, largely accounted for by glutathione- and thioredoxin-based systems that support the activities of glutathione peroxidases, peroxiredoxins, and methionine sulfoxide reductase. The ultimate reductant for these systems is NADPH: mitochondrial sources of NADPH are the nicotinamide nucleotide transhydrogenase, isocitrate dehydrogenase-2, and malic enzyme. NADPH also supports the glutaredoxin activity that regulates the extent of S-glutathionylation of mitochondrial proteins in response to altered redox status. Critical Issues: The integrated network of these mitochondrial thiols constitute a regulatory device involved in the maintenance of steady-state levels of H2O2, mitochondrial and cellular redox and metabolic homeostasis, as well as the modulation of cytosolic redox-sensitive signaling; disturbances of this regulatory device affects transcription, growth, and ultimately influences cell survival/death. Future Directions: The modulation of key mitochondrial thiol proteins, which participate in redox signaling, maintenance of the bioenergetic machinery, oxidative stress responses, and cell death programming, provides a pivotal direction in developing new therapies towards the prevention and treatment of several diseases. Antioxid. Redox Signal. 17, 1714–1727.
Introduction
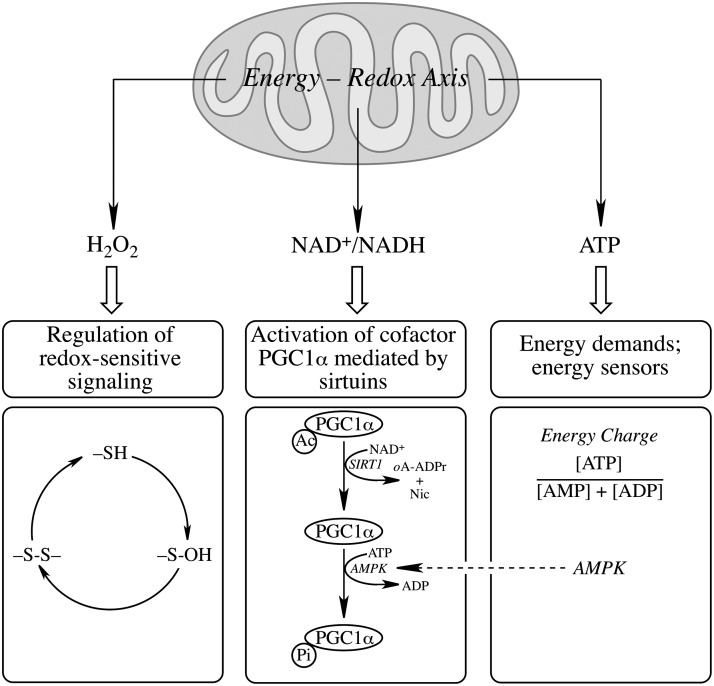
Mitochondria meet the cell's energy demands that support metabolic, osmotic, and mechanical functions; they are sources of H2O2, and play a pivotal role as mediators of the intrinsic apoptotic pathway. Organs that demand significantly larger amounts of energy, such as the central nervous system, are particularly susceptible to an energy crisis and concomitant cell death. Mitochondria integrate distinct cytosolic signaling pathways and (a) generate second messengers, such as H2O2, implicated in the modulation of redox-sensitive signaling pathways, (b) are involved in the regulation of NAD+/NADH homeostasis, influencing the activation of the cofactor PGC1α via sirtuins, and (c) are the cell's generators of ATP that supports the cell's energy demands (Fig. 1). The generation of H2O2 reports the mitochondrial energy charge to cytosol (176) and is implicated in the regulation of the cell's redox status, thus transducing redox signals into a wide variety of responses, such as proliferation, differentiation, and cellular death pathways (119). Cells with high metabolic rate are exposed to large quantities of oxidants, which renders them more vulnerable to oxidative stress-induced cell death (2); thus, high levels of oxidants disrupt redox signaling and mediate detrimental effects inherent in mitochondrial dysfunction in a variety of pathologies including neurodegenerative disorders (13, 14, 162), diabetes (85, 107), cardiovascular disease (159), and aging (112, 126, 177). Hence, oxidants such as H2O2 have a dual function: on the one hand, H2O2 is involved in the fine tuning of signaling and transcription through modulation of redox-sensitive pathways; on the other hand, higher levels of H2O2, as expected with a diminished energy-conservation capacity of mitochondria, are involved in oxidative damage to cell constituents, a well-documented phenomenon under the term oxidative stress.
FIG. 1.
The mitochondrial energy–redox axis and generation of redox- and energy messengers. Mitochondria maintain a fine tuning of NAD+/NADH ratios, generate H2O2 involved in the regulation of redox-sensitive signaling and transcriptional pathways, and ATP to meet the energy demands of the cell. The regulation of redox-sensitive signaling is exemplified with the 2-electron pathway (Equations 4 and 8 in the text). Activation of PGC1α is given as an example of regulation by NAD+/NADH ratios and of interaction with the energy demands/energy sensors panel. There is also interaction between the latter panel and the regulation of redox-sensitive signaling by H2O2, for its generation reports the mitochondrial energy charge to cytosol (176) and is implicated in the regulation of the cell's redox status. oA-ADPr, 2’-o-acetyl-ADP-ribose; Ac, acetyl moiety; Nic, nicotinamide.
Cell death occurs mainly by apoptosis and necrosis, pathways that differ functionally and mechanistically. The critical role of mitochondria in the intrinsic apoptotic pathway is well documented (89, 172, 184) and entails changes in respiratory capacity and mitochondrial membrane potential, as well as increased mitochondrial permeability transition (95). Necrosis, on the other hand, is usually triggered by infection, trauma, or toxins (114), and is associated with major ultrastructural abnormalities of mitochondria (95). Generally, apoptosis may occur with low or moderate, but lethal oxidative stimuli, whereas necrosis would result from severe oxidative challenges that overcome the cellular antioxidant defenses and energy-transducing pathways (100). The intracellular ATP levels constitute a critical signal directing the cells towards either type of cell death (203), because apoptosis requires energy in the form of ATP to assemble the apoptotic machinery (104, 151), which is dissipated during necrosis due to depletion of energy stores and damage of energy-transducing capacity in mitochondria.
Mitochondrial thiols that maintain redox reactions mainly include GSH and thioredoxin-2 (Trx2) and the associated enzymes—glutathione reductase (GR) and thioredoxin reductase (TrxR)—supporting the activities of glutathione peroxidase, glutaredoxin-2 (Grx2), and peroxiredoxin-3 (Prx3) and −5 (Prx5) (34, 70, 148). These mitochondrial thiols have been shown to influence the cellular death pathway (49, 175). Under physiological conditions, the mitochondrial thiol-based antioxidant systems maintain steady-state levels of H2O2 and an adequate cell's redox status, thereby preventing cell death by the pathways mentioned above. Hence, the mitochondrial thiol state is a critical mediator of metabolic-, signaling-, and cell death-related processes. Thiol groups in proteins play an important role in redox signaling by shuffling between oxidized and reduced states (41).
The Mitochondrial GSH Pool and Redox Status
GSH, synthesized by two ATP-dependent steps involving γ glutamylcysteine synthetase and GSH synthase (128), is found in two major pools in cytosol and mitochondria: the latter is the most abundant thiol in mitochondria and acts as a cofactor for glutathione peroxidase, glutathione-S-transferases, and sulfiredoxins (110, 150). Cellular viability and redox status are controlled in part by GSH (45), which plays a dual role by participating in the reduction of peroxides and acting as an nucleophile upon conversion of electrophilic centers to thioether bonds (147). GSH is imported from the cytosol via transporters in the outer and inner mitochondrial membranes (54): dicarboxylate- and 2-oxoglutarate carriers in the inner mitochondrial membrane were identified first in kidney (25, 26, 97, 113) and then in liver (204). The involvement of GSH in redox pathways results in GSSG formation. However, at variance with cytosolic GSSG, mitochondrial GSSG cannot be exported, thus increasing mitochondria susceptibility to protein thiol oxidation and increasing the significance of systems involved in the interconversion between GSH and GSSG to maintain the redox status and provide an environment appropriate for disulfide bond formation during folding of nascent proteins (71).
The mitochondrial GSH:GSSG ratio is greater than 100:1 and is widely used as an indicator of the redox status, calculated by the Nernst equation (Ehc=E0+30 log ([GSSG]/GSH]2) (80). The redox potential of mitochondrial GSH/GSSG couple was calculated as approximately −300 mV and that of the Trx2reduced/Trx2oxidized couple as −340 mV (87). Although these redox couples are maintained independently in nonequilibrium steady state across different subcellular compartments, these values indicate a more reducing environment in mitochondria than in cytosol (−280 mV for Trx1 and −260 to −200 mV for GSH/GSSG) and in endoplasmic reticulum (−185 mV for GSH/GSSG) (58, 82).
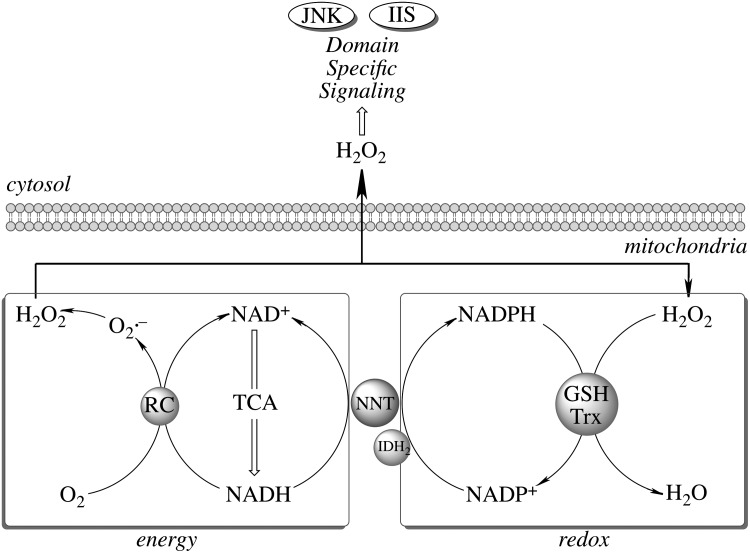
The mitochondrial redox status cannot be viewed independent of its energy-transducing capacity but integrated in a mitochondrial energy–redox axis (Fig. 2). The energy component of this axis is encompassed by the generation of reducing equivalents (NADH and FP2H2) by the tricarboxylic acid cycle (TCA) and their flow through the respiratory chain with concomitant generation of 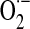 and H2O2. The redox component is the domain of H2O2 removal systems—mainly glutathione peroxidase and Prx3—that use GSH and Trx2 as electron donors. The ultimate reductant of these systems is NADPH (supporting the activities of glutathione reductase and thioredoxin reductase). Hence, the steady-state levels of mitochondrion-generated H2O2 in cytosol (and its involvement in domain-specific signaling) are largely determined by maintenance of the mitochondrial energy-redox axis and are strictly dependent on the mitochondrial GSH pool and associated enzymes (161). Mitochondrial NADPH is mainly formed through three pathways: NADP+-dependent isocitrate dehydrogenase (IDH2), malic enzyme, and nicotinamide nucleotide transhydrogenase (NNT). Of these pathways, 50% of the mitochondrial NADPH pool is uncoupler sensitive, thus suggesting that the NNT-catalyzed reduction of NADP+ accounts for more than 50% of the mitochondrial NADPH pool (154). NNT—a nuclear encoded mitochondrial 110 kDa protein located on the inner mitochondrial membrane (64)—catalyzes the reversible reduction of NADP+ to NADPH and the conversion of NADH to NAD+ (Equation 1).
and H2O2. The redox component is the domain of H2O2 removal systems—mainly glutathione peroxidase and Prx3—that use GSH and Trx2 as electron donors. The ultimate reductant of these systems is NADPH (supporting the activities of glutathione reductase and thioredoxin reductase). Hence, the steady-state levels of mitochondrion-generated H2O2 in cytosol (and its involvement in domain-specific signaling) are largely determined by maintenance of the mitochondrial energy-redox axis and are strictly dependent on the mitochondrial GSH pool and associated enzymes (161). Mitochondrial NADPH is mainly formed through three pathways: NADP+-dependent isocitrate dehydrogenase (IDH2), malic enzyme, and nicotinamide nucleotide transhydrogenase (NNT). Of these pathways, 50% of the mitochondrial NADPH pool is uncoupler sensitive, thus suggesting that the NNT-catalyzed reduction of NADP+ accounts for more than 50% of the mitochondrial NADPH pool (154). NNT—a nuclear encoded mitochondrial 110 kDa protein located on the inner mitochondrial membrane (64)—catalyzes the reversible reduction of NADP+ to NADPH and the conversion of NADH to NAD+ (Equation 1).
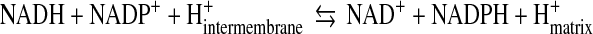 |
[Eq. 1] |
FIG. 2.
Role of the mitochondrial energy–redox axis in maintenance of cellular H2O2 levels. Reducing equivalents from the tricarboxylic acid cycle flow through the respiratory chain (RC); electron leak accounts for 2%–3% of O2 consumed in the form of 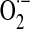 and H2O2. Reduction of H2O2 is supported by thiol-based systems, for which the ultimate reductant is NADPH. Sources of mitochondrial NADPH: nicotinamide nucleotide transhydrogenase (NNT), isocitrate dehydrogenase-2 (IDH2), and malic enzyme. Domain-specific signaling entailing regulation of redox-sensitive JNK- and insulin/IGF1 signaling (IIS) pathways,
and H2O2. Reduction of H2O2 is supported by thiol-based systems, for which the ultimate reductant is NADPH. Sources of mitochondrial NADPH: nicotinamide nucleotide transhydrogenase (NNT), isocitrate dehydrogenase-2 (IDH2), and malic enzyme. Domain-specific signaling entailing regulation of redox-sensitive JNK- and insulin/IGF1 signaling (IIS) pathways,
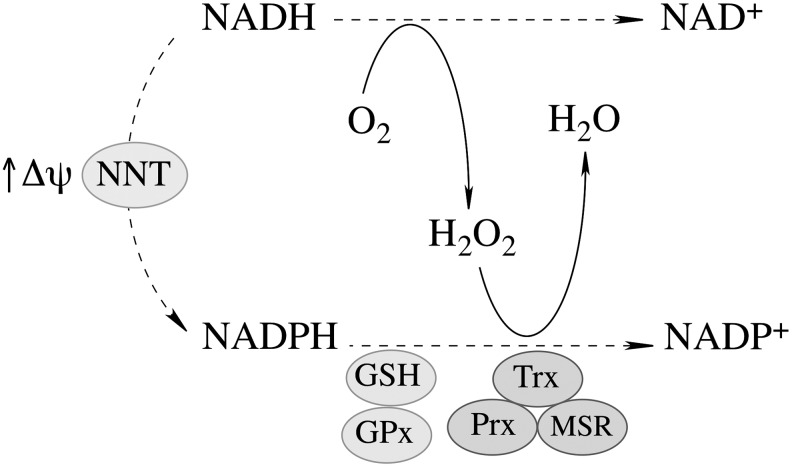
The proton gradient across the mitochondrial inner membrane strongly stimulates the forward reaction (Fig. 3), [i.e., the generation of NADPH and the subsequent H2O2 reduction (197)]. NNT plays an important role in regulating cellular redox homeostasis, energy metabolism, and apoptotic pathways (196). Knockdown of NNT in PC12 cells results in an altered redox status encompassed by decreased cellular NADPH levels and GSH/GSSG ratios and increased H2O2 levels, as well as an impaired mitochondrial energy-transducing capacity. The activation of redox-sensitive signaling (JNK) by H2O2 after NNT suppression induces mitochondrion-dependent intrinsic apoptosis and results in decreased cell viability (196). The oxidized cellular redox state and decline in bioenergetics, as a consequence of NNT knockdown, cannot be viewed as independent events, but rather as interdependent relationships coordinated by the mitochondrial energy–redox axis. Disruption of electron flux from fuel substrates to redox components due to NNT suppression induces not only mitochondrial dysfunction but also cellular disorders or cell death through redox-sensitive signaling.
FIG. 3.
NNT-supported mitochondrial thiol-based antioxidant system. At high Δψ, the forward reaction catalyzed by NNT is favored (see Equation 1 in the text), thus supporting the generation of NADPH, the ultimate reductant for the GSH and thioredoxin-based mitochondrial antioxidant systems. GSH, via glutathione reductase, supports the glutathione peroxidase (GPx) system, whereas thioredoxin (Trx), via thioredoxin reductase, is a requirement for peroxiredoxin- and methionine sulfoxide reductase activities.
Mitochondrial Protein S-Glutathionylation
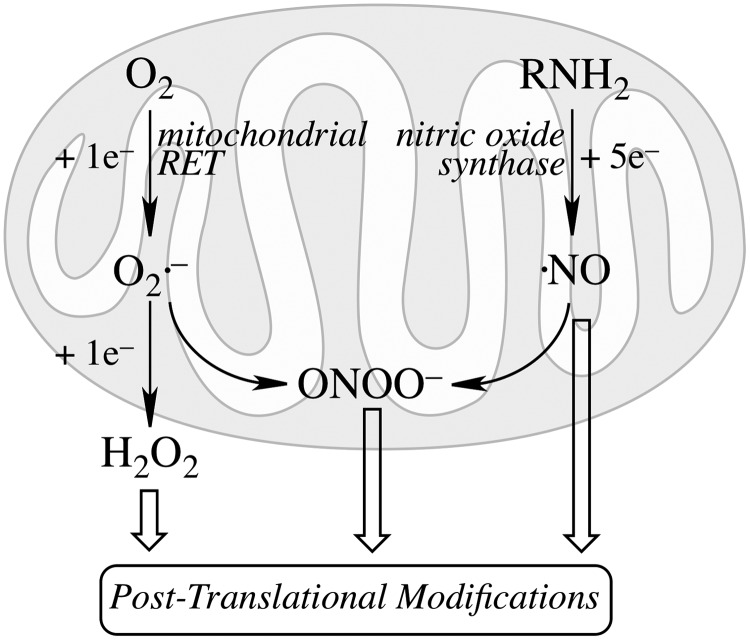
Mitochondrial generation of oxidants and free radicals is associated with reversible and irreversible modifications of target proteins (Fig. 4), mainly involving S-nitrosylation and S-glutathionylation of redox-sensitive cysteinyl residues (Fig. 5) and nitration of tyrosyl residues. The increased GSSG formation and the lack of export of GSSG from mitochondria renders these organelles more susceptible to oxidative conditions and S-glutathionylation reactions through thiol-disulfide exchange (Equation 2) that may be associated with impairment or protection of protein function. Protein mixed disulfides are also formed upon the reaction of GSH with S-nitrosylated proteins by a S-thiolation mechanism (Equation 3) and sulfenic acid intermediates (Equation 4). Protein mixed disulfides can be formed by one- or two-electron pathways: the former yields a protein thiyl radical (Equation 5) that upon conjugation with a thiol (e.g., GSH) forms a protein disulfide anion radical (Equation 6); the disulfide anion radical reduces O2 to 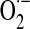 with formation of a protein disulfide (Equation 7). The 2-electron pathway yields a sulfenic acid derivative (Equation 8; e.g., upon reaction of the thiol with H2O2), which is converted into a protein disulfide upon nucleophilic addition by a thiol (Equation 4; e.g., GSH) (see also Fig.1).
with formation of a protein disulfide (Equation 7). The 2-electron pathway yields a sulfenic acid derivative (Equation 8; e.g., upon reaction of the thiol with H2O2), which is converted into a protein disulfide upon nucleophilic addition by a thiol (Equation 4; e.g., GSH) (see also Fig.1).
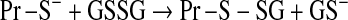 |
[Equation 2] |
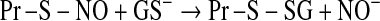 |
[Equation 3] |
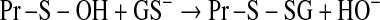 |
[Equation 4] |
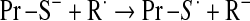 |
[Equation 5] |
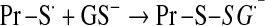 |
[Equation 6] |
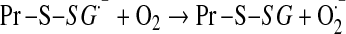 |
[Equation 7] |
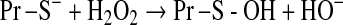 |
[Equation 8] |
FIG. 4.
Mitochondrial generation of oxidants and their involvement in protein post-translational modifications. Univalent reduction of O2, largely by reverse electron transfer (RET) in the respiratory chain, generates 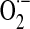 , which stoichiometrically disproportionates to H2O2. The 5-electron oxidation of the guanidine group of arginine by nitric oxide synthase (nNOS or eNOS) generates nitric oxide (.NO). The reaction of .NO (from NOS activity) with
, which stoichiometrically disproportionates to H2O2. The 5-electron oxidation of the guanidine group of arginine by nitric oxide synthase (nNOS or eNOS) generates nitric oxide (.NO). The reaction of .NO (from NOS activity) with 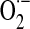 (from mitochondrial RET) generates the oxidant peroxynitrite (ONOO−). These species are involved by different mechanisms in post-translational modifications of proteins.
(from mitochondrial RET) generates the oxidant peroxynitrite (ONOO−). These species are involved by different mechanisms in post-translational modifications of proteins.
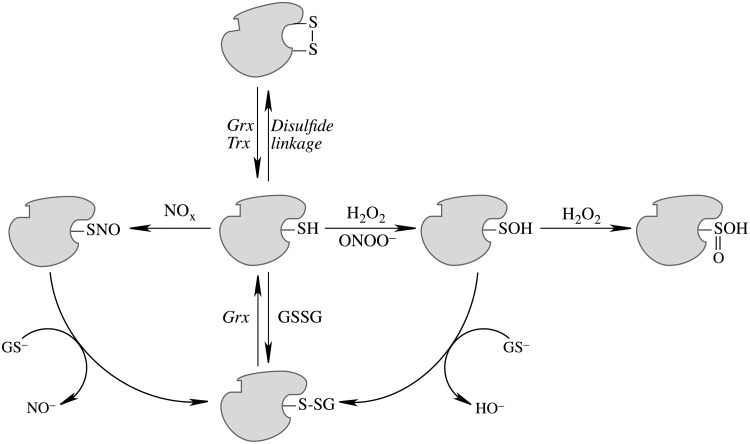
FIG. 5.
Post-translational modifications entailing cysteinyl moieties of proteins. Disulfide linkage is reversed by glutaredoxins and thioredoxins. Oxidation to sulfenic acid by H2O2 and ONOO−; hyperoxidation of Pr-S-OH to sulfinic acid (Pr-SOOH) by H2O2; GSSG-mediated protein glutathionylation, reversed by glutaredoxins. The S-glutathionylated protein can also be formed by S-thiolation of Pr-S-NO and nucleophilic attack of GS– on Pr-S-OH. NOx, a species such as NO+ or GSNO or N2O3.
Regardless of the molecular mechanisms, S-glutathionylation is one of the most important protein post-translational modifications and is viewed as a regulatory device for proteins involved in energy metabolism, redox signaling, and apoptosis (40, 41, 63, 92, 116). In mitochondria, aconitase (57), α-ketoglutarate dehydrogenase (133), isocitrate dehydrogenase (90), succinyl-CoA transferase (51), and aldehyde dehydrogenase (185) can be inhibited upon glutathionylation. Electron transport chain complexes I (170), II (24), and V (51,186) are also sensitive to glutathionylation. S-glutathionylation of succinyl-CoA transferase and ATP synthase (F1 complex, α-subunit) in brain mitochondria resulted in a decrease of activity and a substantially low reduction potential (−171 mV); supplementation of mitochondria with respiratory substrates to complex I or complex II increased NADH and NADPH levels, restored GSH levels through reduction of GSSG and deglutathionylation of mitochondrial proteins, and resulted in a more reducing mitochondrial environment (−291 mV) (51). Excessive protein glutathionylation upon treatment with diamide at high concentrations resulted in bioenergetics failure and cell death; however, low diamide concentrations lead to an apparently adaptive response [i.e., increased glycolytic flux and cell viability remained unchanged (63)]. Treatment of mitochondria from human dopaminergic neuroblastoma cells with neuromelanin increased GSH and free thiol levels by releasing GSH from glutathionylated mitochondrial complex I, thereby exposing critical thiols to detrimental oxidation and subsequent mitochondrial permeability transition and apoptosis (125). These results support the notion that reversible formation of mixed disulfides could serve as a mechanism that protects critical sulfhydryls in mitochondria from further oxidation (e.g., protein sulfinic and sulfonic acids) (62, 145).
The deglutathionylation of protein mixed disulfides is the domain of Grx (116) by a monothiol mechanism (67). The oxidized form of Grx is reduced by GSH, regenerated from GSSG by NADPH-supported glutathione reductase (GR). Cytosolic Grx1 is involved in multiple cellular processes (30, 68). Mitochondrial Grx2 (52) is about 1.5–3-fold more efficient than cytosolic Grx1 in protein de-glutathionylation (102) and is strongly implicated in mitochondrial redox control. Oxidized Grx1 is exclusively reduced by GSH, whereas oxidized Grx2 can also be a substrate for TrxR (79), which enables Grx2 catalysis in a wide range of GSH/GSSG values and conditions of oxidative stress (15).
The role of Grx2 function in the maintenance of the mitochondrial redox status gains further significance when considering that Grx2 knockdown led to increased sensitivity to cell death (103), whereas overexpression of Grx2 decreased the susceptibility of cells to oxidants and inhibited cytochrome c release and caspase activation (48); moreover, inhibition of Grx1 by cadmium did not sensitize to oxidative damage (103). The protein level of Grx2 is less than 1/20 of that of Grx1: this emphasizes the regulatory role of Grx2 upon specific mitochondrial protein targets rather than an antioxidant itself. The cytoprotective role of Grx2 may be related to the activation of Akt signaling and involves the redox-sensitive transcription factor NF-κB and anti-apoptotic Bcl-2 (123). Human Grx2 has been characterized as an iron–sulfur center-containing component of the thioredoxin family that may serve as a redox sensor that controls the activation of Grx2 during conditions of oxidative stress (68); this expands the interaction between oxidants, mitochondrial redox status, and protein glutathionylation. Grx1 activity in the mitochondrial intermembrane space (47, 137) is involved in the regulation of complex I and VDAC activity (88), mitochondrial membrane potential, and apoptosis, and implicated in neurodegenerative diseases (155).
Mitochondrial Thiols, H2O2, and Domain-Specific Signaling
Imbalanced H2O2 regulation can shift the cell from a reduced state to an oxidized state and further induce apoptosis and/or necrosis (6). Moreover, mitochondria provide a setting for relatively high 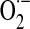 levels (compared with cytosol) and its reaction with .NO (mostly diffusing from cytosol, while the existence of mitochondrial NOS is still debatable (127, 142, 180)) generates ONOO− at diffusion-controlled rates, an oxidant that may be associated with the initial steps of cell death pathways (136). Thus, maintenance of mitochondrial H2O2 homeostasis is critical for regulation of domain-specific redox-sensitive signaling pathways (171). This concept requires careful consideration of spatial regulation of H2O2 signals (178), its generation at specific cellular locations (82), occurrence of H2O2 gradients across distinct cellular compartments (5) and their regulated transfer by aquaporin (117), and the modulation of Prx activity close to the site of H2O2 generation (191).
levels (compared with cytosol) and its reaction with .NO (mostly diffusing from cytosol, while the existence of mitochondrial NOS is still debatable (127, 142, 180)) generates ONOO− at diffusion-controlled rates, an oxidant that may be associated with the initial steps of cell death pathways (136). Thus, maintenance of mitochondrial H2O2 homeostasis is critical for regulation of domain-specific redox-sensitive signaling pathways (171). This concept requires careful consideration of spatial regulation of H2O2 signals (178), its generation at specific cellular locations (82), occurrence of H2O2 gradients across distinct cellular compartments (5) and their regulated transfer by aquaporin (117), and the modulation of Prx activity close to the site of H2O2 generation (191).
Maintenance of mitochondrial H2O2 homeostasis is the domain of glutathione peroxidase and Prx: the latter are a family of thiol peroxidases involved in peroxide reduction. Mitochondrial Prx3 and Prx5 are involved in the enzymatic degradation of H2O2, organic hydroperoxides, and ONOO− (46, 139). Prx3 belongs to the typical 2-cysteine class of Prx (38) and is the target of up to 90% of H2O2 generated in the mitochondrial matrix with a high reaction rate (2×107 M−1·s−1) especially at low levels of H2O2 (36, 38, 148). Accordingly, overexpression of Prx-3 reduces H2O2 production and lipid peroxidation and protects cells from different inducers of apoptosis such as hypoxia, TNFα, cadmium, and oxidant-generating drugs (19, 21, 131, 188). Prx3 is overexpressed in human breast cancers (129) and, hence, it prevents apoptosis induced either by radiation therapy or cisplatin (31). Conversely, Prx3 knockdown leads to increased mitochondrial oxidant production and protein carbonyl content, altered mitochondrial morphology, and renders cells susceptible to apoptosis (19, 44, 60, 101, 120). Prx3 levels are found significantly lower in brains of Alzheimer's disease patients (91) and deficiency in Prx3 is also associated with amyotrophic lateral sclerosis (ALS), Parkinson's disease, and Down syndrome (94, 192), thus emphasizing the significance of mitochondrial Prx in neurodegenerative disorders. Mitochondrial Prx5, a 17 kDa atypical 2-Cys Prx (157), is less effective than Prx3 in reducing H2O2 but has a higher reactivity towards ONOO− (173). Overexpression of the Prx5 inhibits H2O2 accumulation, TNFα-induced JNK activation, H2O2-induced DNA damage, and p53-induced apoptosis (11, 12, 205), whereas Prx5-deficient cells show higher levels of protein and DNA oxidative damage and are more susceptible to apoptosis (44, 96, 146).
Regulation of mitochondrial Prx activity is performed at the level of gene expression and by its oxidation (73, 194). A disrupted mitochondrial redox status activates transcription of Prx3 by FOXO3a, nuclear factor erythroid 2-related factor (Nrf2), and PGC1α, and adaptively strengthens antioxidant defenses (7, 28, 134). Post-translationally, Prx3 oxidation is found as an early event during receptor-mediated apoptosis, which leads to increased mitochondrial H2O2 levels and further affects assembly of the apoptotic machinery (37).
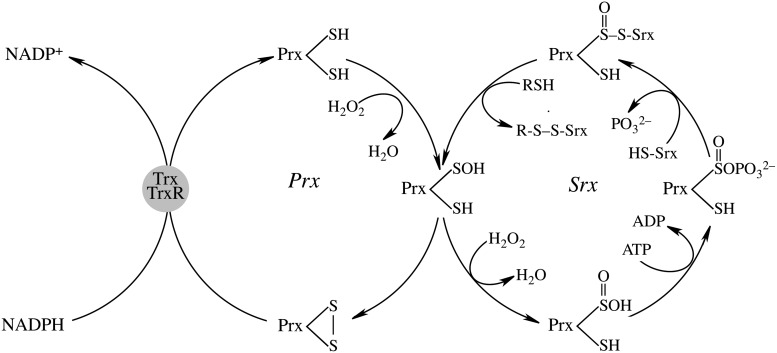
Prxs are considered important regulators of the cellular H2O2 steady-state levels: for the typical 2-Cys Prx (Prx1-3), the peroxidatic cysteine, CysP-SH, at the redox-sensitive N-terminal, is oxidized by H2O2 to CysP-SOH, followed by reaction with a nearby resolving cysteine (CysR-SH) at the N-terminal of the other subunit to form an intermolecular disulfide and release H2O. This disulfide is then reduced by Trxs. At high concentrations of H2O2, oxidation of the intermediate Prx-Cys-SOH to Prx-Cys-SO2H (sulfinic acid form) results in loss of peroxidase activity (35). Sulfiredoxin (Srx) can reduce the sulfinic acid form of Prx back to Prx-Cys-SOH (189). Mammalian Srx translocates to mitochondria under oxidative conditions to reduce over-oxidized mitochondrial Prx3 (130, 190) in an ATP-driven reaction followed by binding of Srx to 2-Cys Prx enzymes and release of the γ-phosphate from ATP to the sulfinic moiety and reduction of the resulting sulfinic phosphoryl ester by either mitochondrial GSH or thioredoxin (20, 74, 83, 149, 153) (Fig. 6).
FIG. 6.
The mitochondrial peroxiredoxin–sulfiredoxin system in the recovery of hyperoxidized peroxiredoxin. Mitochondrial peroxiredoxin-3 is a typical 2 cysteine peroxiredoxin. The scheme shows only modifications of one Cys-SP of peroxiredoxin. This mechanism is based on References 20, 74, 83, 149, and 153.
Expression of Srx is primarily regulated by the Nrf2 (10, 138, 164) along with other phase II enzymes: Trx, Prx, GPx, and MnSOD. In addition to Nrf2, the expression of Srx is also regulated by AP-1 in pancreatic β-cells (53) and in rat neurons (183). Hence, Srx expression is induced in various oxidative and nitrosative stress conditions and is seen as an adaptive and protective mechanism to avoid sustained excessive oxidant production due to hyperoxidation of Prx and its inactivation (1, 9, 10, 164). Currently, the major role of Srx is to catalyze the reversible sulfinic modification of 2-Cys Prxs but not the sulfinic acid form of other over-oxidized proteins such as GAPDH and DJ-1 (149). The biological significance of this reversible hyperoxidation of Prx enzymes is still unclear: it has been proposed that inactivation of Prx by over-oxidation results in higher levels of H2O2, which may be engaged in regulation of distinct signaling pathways (193); albeit attractive, this proposal needs to be viewed in light of the spatial considerations for H2O2 signaling (82, 178). Srx−/− mice show normal viability but an increased mortality during endotoxic shock; this may suggest a protective role of Srx through regulation of Prx function and cellular H2O2 levels (141).
The reducing power for Prx is transmitted through thiols of the Trx system: NADPH→TrxR→Trx→Prx (65, 199). Trx is highly efficient in redox reactions via thiol-disulfide exchanges (108, 118, 143), thus impacting cellular functions such as antioxidant defenses and redox control of transcription and signal transduction (8, 66). Trx is also involved in the reduction of methionine sulfoxides via methionine sulfoxide reductases (MSR). Mitochondrial Trx2 is found at its highest levels in metabolically active tissues (18, 167) and its oxidation after exposure to peroxides and diamide is an early event in oxidative stress; overexpression of Trx2 increases mitochondrial membrane potential, inhibits cytochrome c release from mitochondria (42), and protects the cells against TNFα-, diamide-, and tert-butylhydroperoxide-induced oxidation, cytotoxicity, and cell death (22, 23, 59). Trx-2-deficient cells show accumulation of intracellular oxidants, cytochrome c release, and activation of the intrinsic apoptotic pathway (169) and Trx2−/− mice show increased apoptosis in early embryos leading to embryonic lethality (132) that coincides with mitochondria maturation. This strengthens the association of mitochondrial metabolic function and oxidant regulation (i.e., the mitochondrial energy–redox axis). This is also supported by studies showing that Trx2+/− mice show reduced ATP production and electron-transport chain complexes activities (140). Knocking down TrxR leads to Trx2 oxidation and this sensitizes cells to oxidant-induced cell death (152). The mitochondrial generation of oxidants and the reducing power of TrxR2 determine the redox status of Trx2, which can be viewed as a marker of mitochondrial dysfunction and oxidant-induced cell death (81, 82, 87, 124).
Trx2 may be involved in the regulation of apoptosis through its interaction with Apoptosis Signal-regulating Kinase 1 (ASK1) (200). Upon pro-inflammatory cytokine (TNFα) or oxidative stress (H2O2) stimulation, mitochondrion-localized ASK1 disassociates from Trx2 and mediates a JNK-independent caspase-mediated apoptotic pathway (200). Overexpression of Trx2 inhibits ASK1-induced apoptosis, while knockdown of Trx2 increases TNFα/ASK1-induced cytochrome c release (156, 200). The mitochondrial permeability transition (MPT) plays significant roles in activation of apoptosis and necrosis (72, 144, 174, 198): Trx2 protects isolated mitochondria from MPT induced by peroxide or Ca++ (61) and Trx2-deficient cells show lower mitochondrial membrane potential (182). Whether Trx2 regulates the MPT by either interacting with MPT pore or modulating the mitochondrial oxidant levels is still unclear (61). Mitochondrial GSH also plays an important role in maintaining mitochondrial inner membrane permeability (111). The association of Trx2 with cytochrome c both in vivo and in vitro could provide a mechanism for the inhibitory role of Trx2 in apoptotic signaling (169). Taken together, the interactions involving Trx2, ASK1, cytochrome c, and MPT seem to be critical in the regulation of mitochondria-mediated apoptotic pathway.
Mitochondrial GSH in Cell Viability and Function
The properties and roles of mitochondrial GSH were first studied during the mid-1960s to mid-1970s (77, 78) and then advanced in the 1980s (115), showing the role of mitochondrial GSH in maintaining cell viability. Depletion of mitochondrial GSH decreased the cellular viability (158), whereas increasing mitochondrial GSH protected against oxidative and nitrosative stress (121). Increased mitochondrial GSH oxidation and decreased GSH/GSSG ratios as a function of age were accompanied by oxidative damage of the mtDNA (43, 165, 166, 181).
In the central nervous system, mitochondrial GSH depletion in astrocytes led to cell death via necrosis rather than apoptosis and decreases in mitochondrial GSH below 50% resulted in neuronal degeneration (69, 122). Expectedly, loss of mitochondrial GSH in neurons was accompanied by increase in oxidant levels, collapse of mitochondrial membrane potential, and cell death (195). The chemoprotectant 3H-1,2-dithiole-3-thione protected against oxidative and electrophilic neurotoxicity in neuroblastoma cells and primary neurons due to its ability to increase mitochondrial GSH (76). Interestingly, dopamine at nontoxic concentrations strongly increased mitochondrial GSH and afforded a greater protection against cytotoxicity (75). GSH was substantially decreased in cerebral cortex and striatum mitochondria in a model of brain focal ischemia, in which the loss in mitochondrial GSH did not correlate with minimal total GSH losses in the tissue (4). In this model, bilateral injections of GSH monoethylester—prior to induction of unilateral focal ischemia—increased mitochondrial GSH in the striatum of ischemic and nonischemic hemispheres, albeit with no reduction of infarct volume. This could be potentially used to study the effects of modulating brain mitochondrial glutathione in a range of brain disorders and warrants further research (3). The above studies establish the importance of the role of mitochondrial GSH in maintaining brain function.
In liver, TNF-α increased the susceptibility of hepatocytes after mitochondrial GSH depletion and restoration of mitochondrial GSH levels had protective effects against TNF-α (32). Decreased intracellular GSH levels markedly enhance the cytotoxicity of alkylating agents; however, it shifts the mode of cell death to necrosis rather than apoptosis. This study poses an important question as to whether raising GSH levels enables the switch from necrosis to apoptosis, thus viewing apoptosis as a more desirable cell death pathway that circumvents the destructive inflammatory response associated with necrosis (49).
Mitochondria have also been shown to undergo morphological and functional changes in chronic experimental models of alcoholism in which ethanol is oxidized to acetaldehyde in liver (168). In chronic models of alcoholism, there is a distinct mitochondrial damage characterized by abnormalities like its swelling, disruption, disorganization of the normal cristae organization, all of which finally translates into a lower energy-transducing capacity (i.e., ATP levels) (17, 39, 168). These effects stem partly from a low mitochondrial GSH pool, as a consequence of dysfunctional GSH transport into the mitochondria, which weakens binding of cytochrome c to cardiolipin in the inner mitochondrial membrane and affects membrane permeabilization (84, 110, 135). Decreased mitochondrial GSH is also linked to disrupted Ca++ homeostasis via disturbances in the pyridine nucleotide pool mainly caused by decreased mitochondrial GSH (99, 106).
The transport of GSH into mitochondria was found to be closely associated with the apoptotic machinery due to the interaction of GSH with the BH3 groove of Bcl-2; pro-apoptotic Bax and BH3-only proteins suppressed GSH transport into the mitochondria upon inhibition of GSH-Bcl-2 binding (206). Bcl-2 binding to GSH enhanced its affinity for the 2-oxoglutarate carrier on the inner mitochondrial membrane (187).
Conclusions and Perspectives
The interlaced networks of mitochondrial thiols constitute a regulatory device to maintain mitochondrial redox status and modulate cytosolic redox signaling in normal and stress conditions. Disturbances in this regulatory device can affect transcription, growth, and ultimately influences cell survival/death. Modification of sulhydryl groups on signal proteins by oxidants and their control exerted by thiol-containing molecules such as glutathione, Grx, Trx, and Prx, forms the core of redox signaling. Each of them plays a distinct role in the overall process. GSH/GSSG determines the mitochondrial redox status due to its high molecular concentration and can be seen as a “redox buffer”; Prx3 acts more in H2O2 removal and therefore affects the H2O2 signal pathway as a “redox sensor”; Trx2 acts more as a “redox transmitter” to transfer the reducing equivalents from NADPH to other thiol-molecules such as Prx3. The primary role of Grx2 in mitochondria is to control protein glutathionylation/deglutathionylation and thereby regulate functions of important mitochondrial enzymes in response to change in mitochondrial redox status. Mitochondrial thiols thereby form an intricate network that constitutes complex crosstalk involved in oxidants detoxification and maintenance of cellular and mitochondrial redox homeostasis, as well as the modulation of cytosolic redox-sensitive signaling and cell death. Emerging evidence suggests that the mitochondrial thiol/disulfide systems are critical for the progression of several pathologies (Table 1). Thus, the modulation of key mitochondrial thiol proteins, which participate in oxidative stress responses, redox signaling, maintenance of the bioenergetic machinery, and cell death programming, provides a pivotal direction in developing new therapies towards the prevention and treatment of these diseases.
Table 1.
Mitochondrial Thiols and Related Enzymatic Systems in Disease
| Thiols involved | References | |
|---|---|---|
| Neurodegeneration | ||
| Models of Alzheimer's disease | Prx3, GSH | 16, 55, 91 |
| Models of Parkinson's disease | Grx1*, Grx2, GSH, Protein-SSG, Prx3, Prx5 | 27, 44, 55, 60, 94, 98, 125, 155 |
| Excitotoxicity | Prx3 | 60 |
| Down syndrome | Prx3 | 91, 94 |
| Liver diseases | ||
| Alcohol-induced hepatoxicity | Srx | 9 |
| Alcoholic steatohepatitis | GSH | 33, 105, 201, 202 |
| Nonalcoholic steatohepatitis | GSH | 109 |
| Chronic alcoholism | GSH | 50 |
| Acetaminophen toxicity | GSH | 179, 201 |
| Liver cirrhosis | GSH | 93 |
| Cardiovascular diseases | ||
| Ischemic preconditioning | Protein-SSG, Protein-SNO | 24, 62 |
| Dilated cardiomyopathy | TrxR2 | 160 |
| Myocardial infarction | Prx3 | 7 |
| Vascular muscle function | Protein-SSG | 63 |
| Diabetes | ||
| Glucose tolerance | Prx3 | 21 |
| Diabetic retinopathy | GSH | 86 |
| Cancer | ||
| Hepatocellular carcinomas | TrxR2, Prx3 | 29 |
| Human breast cancer | Prx3 | 129 |
| Colon cancer cell protection | Prx3 | 131 |
| Neoplastic transformation | Prx3 | 188 |
| Aging | GSH, Prx5 | 43, 146, 165, 181 |
| Stroke - Focal cerebral ischemia | GSH | 3, 163 |
The table summarizes the involvement of selective mitochondrial thiols in different disease models addressed in this review. *Glutaredoxin-1 in the inter-membrane space.
Abbreviations Used
- ASK1
apoptosis signal-regulating kinase-1
- FP2H2
reduced flavoprotein in succinate dehydrogenase
- GPx
glutathione peroxidase
- GR
glutathione reductase
- Grx
glutaredoxin
- IDH2
isocitrate dehydrogenase 2
- IIS
insulin/IGF1 signaling
- JNK
c-Jun N-terminal kinase
- LDH
lactate dehydrogenase
- MPT
mitochondrial permeability transition
- MSR
methionine sulfoxide reductases
- NNT
nicotinamide nucleotide transhydrogenase
- Nrf2
nuclear factor erythroid 2-related factor 2
- PGC1α
peroxisome proliferator-activated receptor γ coactivator 1α
- Prx
peroxiredoxin
- Srx
sulfiredoxin
- TCA
tricarboxylic acid
- TNFα
tumor necrosis factor α
- Trx
thioredoxin
- TrxR
thioredoxin reductase
Acknowledgments
This study is supported by National Institutes of Health Grants R01AG016718 and P01AG026572 (to Roberta Díaz Brinton; Project 1 to EC) and Grant 17RT-0171.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Abbas K. Breton J. Planson AG. Bouton C. Bignon J. Seguin C. Riquier S. Toledano MB. Drapier JC. Nitric oxide activates an Nrf2/sulfiredoxin antioxidant pathway in macrophages. Free Radic Biol Med. 2011;51:107–114. doi: 10.1016/j.freeradbiomed.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 2.Andersen JK. Oxidative stress in neurodegeneration: Cause or consequence? Nat Med. 2004;10:S18–25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 3.Anderson MF. Nilsson M. Sims NR. Glutathione monoethylester prevents mitochondrial glutathione depletion during focal cerebral ischemia. Neurochem Int. 2004;44:153–159. doi: 10.1016/s0197-0186(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 4.Anderson MF. Sims NR. The effects of focal ischemia and reperfusion on the glutathione content of mitochondria from rat brain subregions. J. Neurochem. 2002;81:541–549. doi: 10.1046/j.1471-4159.2002.00836.x. [DOI] [PubMed] [Google Scholar]
- 5.Antunes F. Cadenas E. Estimation of H2O2 gradients across biomembranes. FEBS Lett. 2000;475:121–126. doi: 10.1016/s0014-5793(00)01638-0. [DOI] [PubMed] [Google Scholar]
- 6.Antunes F. Cadenas E. Cellular titration of apoptosis with steady state concentrations of H2O2: Submicromolar levels of H2O2 induce apoptosis through Fenton chemistry independent of the cellular thiol state. Free Radic Biol Med. 2001;30:1008–1018. doi: 10.1016/s0891-5849(01)00493-2. [DOI] [PubMed] [Google Scholar]
- 7.Araki M. Nanri H. Ejima K. Murasato Y. Fujiwara T. Nakashima Y. Ikeda M. Antioxidant function of the mitochondrial protein SP-22 in the cardiovascular system. J Biol Chem. 1999;274:2271–2278. doi: 10.1074/jbc.274.4.2271. [DOI] [PubMed] [Google Scholar]
- 8.Aslund F. Beckwith J. Bridge over troubled waters: sensing stress by disulfide bond formation. Cell. 1999;96:751–3. doi: 10.1016/s0092-8674(00)80584-x. [DOI] [PubMed] [Google Scholar]
- 9.Bae SH. Sung SH. Cho EJ. Lee SK. Lee HE. Woo HA. Yu DY. Kil IS. Rhee SG. Concerted action of sulfiredoxin and peroxiredoxin I protects against alcohol-induced oxidative injury in mouse liver. Hepatology. 2011;53:945–953. doi: 10.1002/hep.24104. [DOI] [PubMed] [Google Scholar]
- 10.Bae SH. Woo HA. Sung SH. Lee HE. Lee SK. Kil IS. Rhee SG. Induction of sulfiredoxin via an Nrf2-dependent pathway and hyperoxidation of peroxiredoxin III in the lungs of mice exposed to hyperoxia. Antioxid Redox Signal. 2009;11:937–948. doi: 10.1089/ars.2008.2325. [DOI] [PubMed] [Google Scholar]
- 11.Banmeyer I. Marchand C. Clippe A. Knoops B. Human mitochondrial peroxiredoxin 5 protects from mitochondrial DNA damages induced by hydrogen peroxide. FEBS Lett. 2005;579:2327–2333. doi: 10.1016/j.febslet.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 12.Banmeyer I. Marchand C. Verhaeghe C. Vucic B. Rees JF. Knoops B. Overexpression of human peroxiredoxin 5 in subcellular compartments of Chinese hamster ovary cells: Effects on cytotoxicity and DNA damage caused by peroxides. Free Radic Biol Med. 2004;36:65–077. doi: 10.1016/j.freeradbiomed.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Barnham KJ. Masters CL. Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 14.Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol. 1995;38:357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- 15.Beer SM. Taylor ER. Brown SE. Dahm CC. Costa NJ. Runswick MJ. Murphy MP. Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: Implications for mitochondrial redox regulation and antioxidant defense. J Biol Chem. 2004;279:47939–47951. doi: 10.1074/jbc.M408011200. [DOI] [PubMed] [Google Scholar]
- 16.Boyd-Kimball D. Sultana R. Abdul HM. Butterfield DA. Gamma-glutamylcysteine ethyl ester-induced up-regulation of glutathione protects neurons against Abeta(1–42)-mediated oxidative stress and neurotoxicity: Implications for Alzheimer's disease. J Neurosci Res. 2005;79:700–706. doi: 10.1002/jnr.20394. [DOI] [PubMed] [Google Scholar]
- 17.Bruguera M. Bertran A. Bombi JA. Rodes J. Giant mitochondria in hepatocytes: A diagnostic hint for alcoholic liver disease. Gastroenterology. 1977;73:1383–1387. [PubMed] [Google Scholar]
- 18.Chae HZ. Kim HJ. Kang SW. Rhee SG. Characterization of three isoforms of mammalian peroxiredoxin that reduce peroxides in the presence of thioredoxin. Diabetes Res Clin Pract. 1999;45:101–112. doi: 10.1016/s0168-8227(99)00037-6. [DOI] [PubMed] [Google Scholar]
- 19.Chang TS. Cho CS. Park S. Yu S. Kang SW. Rhee SG. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. J Biol Chem. 2004;279:41975–41984. doi: 10.1074/jbc.M407707200. [DOI] [PubMed] [Google Scholar]
- 20.Chang TS. Jeong W. Woo HA. Lee SM. Park S. Rhee SG. Characterization of mammalian sulfiredoxin and its reactivation of hyperoxidized peroxiredoxin through reduction of cysteine sulfinic acid in the active site to cysteine. J Biol Chem. 2004;279:50994–51001. doi: 10.1074/jbc.M409482200. [DOI] [PubMed] [Google Scholar]
- 21.Chen L. Na R. Gu M. Salmon AB. Liu Y. Liang H. Qi W. Van Remmen H. Richardson A. Ran Q. Reduction of mitochondrial H2O2 by overexpressing peroxiredoxin 3 improves glucose tolerance in mice. Aging Cell. 2008;7:866–878. doi: 10.1111/j.1474-9726.2008.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y. Cai J. Jones DP. Mitochondrial thioredoxin in regulation of oxidant-induced cell death. FEBS Lett. 2006;580:6596–6602. doi: 10.1016/j.febslet.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y. Cai J. Murphy TJ. Jones DP. Overexpressed human mitochondrial thioredoxin confers resistance to oxidant-induced apoptosis in human osteosarcoma cells. J Biol Chem. 2002;277:33242–33248. doi: 10.1074/jbc.M202026200. [DOI] [PubMed] [Google Scholar]
- 24.Chen YR. Chen CL. Pfeiffer DR. Zweier JL. Mitochondrial complex II in the post-ischemic heart: Oxidative injury and the role of protein S-glutathionylation. J Biol Chem. 2007;282:32640–32654. doi: 10.1074/jbc.M702294200. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z. Lash LH. Evidence for mitochondrial uptake of glutathione by dicarboxylate and 2-oxoglutarate carriers. J Pharmacol Exp Ther. 1998;285:608–618. [PubMed] [Google Scholar]
- 26.Chen Z. Putt DA. Lash LH. Enrichment and functional reconstitution of glutathione transport activity from rabbit kidney mitochondria: Further evidence for the role of the dicarboxylate and 2-oxoglutarate carriers in mitochondrial glutathione transport. Arch Biochem Biophys. 2000;373:193–202. doi: 10.1006/abbi.1999.1527. [DOI] [PubMed] [Google Scholar]
- 27.Chinta SJ. Andersen JK. Reversible inhibition of mitochondrial complex I activity following chronic dopaminergic glutathione depletion in vitro: Implications for Parkinson's disease. Free Radic Biol Med. 2006;41:1442–1448. doi: 10.1016/j.freeradbiomed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Chiribau CB. Cheng L. Cucoranu IC. Yu YS. Clempus RE. Sorescu D. FOXO3A regulates peroxiredoxin III expression in human cardiac fibroblasts. J Biol Chem. 2008;283:8211–8217. doi: 10.1074/jbc.M710610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi JH. Kim TN. Kim S. Baek SH. Kim JH. Lee SR. Kim JR. Overexpression of mitochondrial thioredoxin reductase and peroxiredoxin III in hepatocellular carcinomas. Anticancer Res. 2002;22:3331–3335. [PubMed] [Google Scholar]
- 30.Chrestensen CA. Starke DW. Mieyal JJ. Acute cadmium exposure inactivates thioltransferase (glutaredoxin), inhibits intracellular reduction of protein-glutathionyl-mixed disulfides, and initiates apoptosis. J Biol Chem. 2000;275:26556–26565. doi: 10.1074/jbc.M004097200. [DOI] [PubMed] [Google Scholar]
- 31.Chung YM. Yoo YD. Park JK. Kim YT. Kim HJ. Increased expression of peroxiredoxin II confers resistance to cisplatin. Anticancer Res. 2001;21:1129–1133. [PubMed] [Google Scholar]
- 32.Colell A. Garcia-Ruiz C. Miranda M. Ardite E. Mari M. Morales A. Corrales F. Kaplowitz N. Fernandez-Checa JC. Selective glutathione depletion of mitochondria by ethanol sensitizes hepatocytes to tumor necrosis factor. Gastroenterology. 1998;115:1541–1551. doi: 10.1016/s0016-5085(98)70034-4. [DOI] [PubMed] [Google Scholar]
- 33.Coll O. Colell A. Garcia-Ruiz C. Kaplowitz N. Fernandez-Checa JC. Sensitivity of the 2-oxoglutarate carrier to alcohol intake contributes to mitochondrial glutathione depletion. Hepatology. 2003;38:692–702. doi: 10.1053/jhep.2003.50351. [DOI] [PubMed] [Google Scholar]
- 34.Costa NJ. Dahm CC. Hurrell F. Taylor ER. Murphy MP. Interactions of mitochondrial thiols with nitric oxide. Antioxid Redox Signal. 2003;5:291–305. doi: 10.1089/152308603322110878. [DOI] [PubMed] [Google Scholar]
- 35.Cox AG. Pearson AG. Pullar JM. Jonsson TJ. Lowther WT. Winterbourn CC. Hampton MB. Mitochondrial peroxiredoxin 3 is more resilient to hyperoxidation than cytoplasmic peroxiredoxins. Biochem J. 2009;421:51–58. doi: 10.1042/BJ20090242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cox AG. Peskin AV. Paton LN. Winterbourn CC. Hampton MB. Redox potential and peroxide reactivity of human peroxiredoxin 3. Biochemistry. 2009;48:6495–6501. doi: 10.1021/bi900558g. [DOI] [PubMed] [Google Scholar]
- 37.Cox AG. Pullar JM. Hughes G. Ledgerwood EC. Hampton MB. Oxidation of mitochondrial peroxiredoxin 3 during the initiation of receptor-mediated apoptosis. Free Radic Biol Med. 2008;44:1001–1009. doi: 10.1016/j.freeradbiomed.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 38.Cox AG. Winterbourn CC. Hampton MB. Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem J. 2010;425:313–325. doi: 10.1042/BJ20091541. [DOI] [PubMed] [Google Scholar]
- 39.Cunningham CC. Bailey SM. Ethanol consumption and liver mitochondria function. Neurosignals. 2001;10:271–282. doi: 10.1159/000046892. [DOI] [PubMed] [Google Scholar]
- 40.Dalle-Donne I. Colombo G. Gagliano N. Colombo R. Giustarini D. Rossi R. Milzani A. S-glutathiolation in life and death decisions of the cell. Free Radic Res. 2011;45:3–15. doi: 10.3109/10715762.2010.515217. [DOI] [PubMed] [Google Scholar]
- 41.Dalle-Donne I. Milzani A. Gagliano N. Colombo R. Giustarini D. Rossi R. Molecular mechanisms and potential clinical significance of S-glutathionylation. Antioxid. Redox Signal. 2008;10:445–473. doi: 10.1089/ars.2007.1716. [DOI] [PubMed] [Google Scholar]
- 42.Damdimopoulos AE. Miranda-Vizuete A. Pelto-Huikko M. Gustafsson JA. Spyrou G. Human mitochondrial thioredoxin. Involvement in mitochondrial membrane potential and cell death. J Biol Chem. 2002;277:33249–33257. doi: 10.1074/jbc.M203036200. [DOI] [PubMed] [Google Scholar]
- 43.de la Asuncion J. Millan A. Pla R. Bruseghini L. Esteras A. Pallardo F. Sastre J. Vina J. Mitochondrial glutathione oxidation correlates with age-associated oxidative damage to mitochondrial DNA. FASEB J. 1996;10:333–338. doi: 10.1096/fasebj.10.2.8641567. [DOI] [PubMed] [Google Scholar]
- 44.De Simoni S. Goemaere J. Knoops B. Silencing of peroxiredoxin 3 and peroxiredoxin 5 reveals the role of mitochondrial peroxiredoxins in the protection of human neuroblastoma SH-SY5Y cells toward MPP+ Neurosci Lett. 2008;433:219–224. doi: 10.1016/j.neulet.2007.12.068. [DOI] [PubMed] [Google Scholar]
- 45.Deneke SM. Fanburg BL. Regulation of cellular glutathione. Am J Physiol. 1989;257:L163–L173. doi: 10.1152/ajplung.1989.257.4.L163. [DOI] [PubMed] [Google Scholar]
- 46.Dubuisson M. Vander Stricht D. Clippe A. Etienne F. Nauser T. Kissner R. Koppenol WH. Rees JF. Knoops B. Human peroxiredoxin 5 is a peroxynitrite reductase. FEBS Lett. 2004;571:161–165. doi: 10.1016/j.febslet.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 47.Ehrhart J. Gluck M. Mieyal J. Zeevalk GD. Functional glutaredoxin (thioltransferase) activity in rat brain and liver mitochondria. Parkinsonism Relat Disord. 2002;8:395–400. doi: 10.1016/s1353-8020(02)00020-2. [DOI] [PubMed] [Google Scholar]
- 48.Enoksson M. Fernandes AP. Prast S. Lillig CH. Holmgren A. Orrenius S. Overexpression of glutaredoxin 2 attenuates apoptosis by preventing cytochrome c release. Biochem Biophys Res Commun. 2005;327:774–779. doi: 10.1016/j.bbrc.2004.12.067. [DOI] [PubMed] [Google Scholar]
- 49.Fernandes RS. Cotter TG. Apoptosis or necrosis: Intracellular levels of glutathione influence mode of cell death. Biochem Pharmacol. 1994;48:675–681. doi: 10.1016/0006-2952(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 50.Fernandez-Checa JC. Garcia-Ruiz C. Ookhtens M. Kaplowitz N. Impaired uptake of glutathione by hepatic mitochondria from chronic ethanol-fed rats. Tracer kinetic studies in vitro and in vivo and susceptibility to oxidant stress. J Clin Invest. 1991;87:397–405. doi: 10.1172/JCI115010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia J. Han D. Sancheti H. Yap LP. Kaplowitz N. Cadenas E. Regulation of mitochondrial glutathione redox status and protein glutathionylation by respiratory substrates. J Biol Chem. 2010;285:39646–39654. doi: 10.1074/jbc.M110.164160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gladyshev VN. Liu A. Novoselov SV. Krysan K. Sun QA. Kryukov VM. Kryukov GV. Lou MF. Identification and characterization of a new mammalian glutaredoxin (thioltransferase), Grx2. J Biol Chem. 2001;276:30374–30380. doi: 10.1074/jbc.M100020200. [DOI] [PubMed] [Google Scholar]
- 53.Glauser DA. Brun T. Gauthier BR. Schlegel W. Transcriptional response of pancreatic beta cells to metabolic stimulation: Large scale identification of immediate-early and secondary response genes. BMC Mol Biol. 2007;8:54. doi: 10.1186/1471-2199-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Griffith OW. Meister A. Origin and turnover of mitochondrial glutathione. Proc Natl Acad Sci USA. 1985;82:4668–4672. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gu M. Owen AD. Toffa SE. Cooper JM. Dexter DT. Jenner P. Marsden CD. Schapira AH. Mitochondrial function, GSH and iron in neurodegeneration and Lewy body diseases. J Neurol Sci. 1998;158:24–29. doi: 10.1016/s0022-510x(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 56. This reference has been deleted.
- 57.Han D. Canali R. Garcia J. Aguilera R. Gallaher TK. Cadenas E. Sites and mechanisms of aconitase inactivation by peroxynitrite: Modulation by citrate and glutathione. Biochemistry. 2005;44:11986–11996. doi: 10.1021/bi0509393. [DOI] [PubMed] [Google Scholar]
- 58.Hansen JM. Go YM. Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol Toxicol. 2006;46:215–234. doi: 10.1146/annurev.pharmtox.46.120604.141122. [DOI] [PubMed] [Google Scholar]
- 59.Hansen JM. Zhang H. Jones DP. Mitochondrial thioredoxin-2 has a key role in determining tumor necrosis factor-alpha-induced reactive oxygen species generation, NF-kappaB activation, and apoptosis. Toxicol Sci. 2006;91:643–650. doi: 10.1093/toxsci/kfj175. [DOI] [PubMed] [Google Scholar]
- 60.Hattori F. Murayama N. Noshita T. Oikawa S. Mitochondrial peroxiredoxin-3 protects hippocampal neurons from excitotoxic injury in vivo. J Neurochem. 2003;86:860–868. doi: 10.1046/j.1471-4159.2003.01918.x. [DOI] [PubMed] [Google Scholar]
- 61.He M. Cai J. Go YM. Johnson JM. Martin WD. Hansen JM. Jones DP. Identification of thioredoxin-2 as a regulator of the mitochondrial permeability transition. Toxicol Sci. 2008;105:44–50. doi: 10.1093/toxsci/kfn116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hill BG. Darley-Usmar VM. S-nitrosation and thiol switching in the mitochondrion: A new paradigm for cardioprotection in ischaemic preconditioning. Biochem J. 2008;412:e11–13. doi: 10.1042/BJ20080716. [DOI] [PubMed] [Google Scholar]
- 63.Hill BG. Higdon AN. Dranka BP. Darley-Usmar VM. Regulation of vascular smooth muscle cell bioenergetic function by protein glutathiolation. Biochim Biophys Acta. 2010;1797:285–295. doi: 10.1016/j.bbabio.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoek JB. Rydström J. Physiological roles of nicotinamide nucleotide transhydrogenase. Biochem J. 1988;254:1–10. doi: 10.1042/bj2540001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hofmann B. Hecht HJ. Flohe L. Peroxiredoxins. Biol Chem. 2002;383:347–364. doi: 10.1515/BC.2002.040. [DOI] [PubMed] [Google Scholar]
- 66.Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989;264:13963–13966. [PubMed] [Google Scholar]
- 67.Holmgren A. Aslund F. Glutaredoxin. Methods Enzymol. 1995;252:283–292. doi: 10.1016/0076-6879(95)52031-7. [DOI] [PubMed] [Google Scholar]
- 68.Holmgren A. Johansson C. Berndt C. Lonn ME. Hudemann C. Lillig CH. Thiol redox control via thioredoxin and glutaredoxin systems. Biochem Soc Trans. 2005;33:1375–1377. doi: 10.1042/BST0331375. [DOI] [PubMed] [Google Scholar]
- 69.Huang J. Philbert MA. Cellular responses of cultured cerebellar astrocytes to ethacrynic acid-induced perturbation of subcellular glutathione homeostasis. Brain Res. 1996;711:184–192. doi: 10.1016/0006-8993(95)01376-8. [DOI] [PubMed] [Google Scholar]
- 70.Hurd TR. Costa NJ. Dahm CC. Beer SM. Brown SE. Filipovska A. Murphy MP. Glutathionylation of mitochondrial proteins. Antioxid Redox Signal. 2005;7:999–1010. doi: 10.1089/ars.2005.7.999. [DOI] [PubMed] [Google Scholar]
- 71.Hwang C. Sinskey A. Lodish H. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257:1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- 72.Imberti R. Nieminen AL. Herman B. Lemasters JJ. Mitochondrial and glycolytic dysfunction in lethal injury to hepatocytes by t-butylhydroperoxide: Protection by fructose, cyclosporin A and trifluoperazine. J Pharmacol Exp Ther. 1993;265:392–400. [PubMed] [Google Scholar]
- 73.Immenschuh S. Baumgart-Vogt E. Peroxiredoxins, oxidative stress, and cell proliferation. Antioxid Redox Signal. 2005;7:768–777. doi: 10.1089/ars.2005.7.768. [DOI] [PubMed] [Google Scholar]
- 74.Jeong W. Park SJ. Chang TS. Lee DY. Rhee SG. Molecular mechanism of the reduction of cysteine sulfinic acid of peroxiredoxin to cysteine by mammalian sulfiredoxin. J Biol Chem. 2006;281:14400–14407. doi: 10.1074/jbc.M511082200. [DOI] [PubMed] [Google Scholar]
- 75.Jia Z. Zhu H. Misra B. Li Y. Misra H. Dopamine as a potent inducer of cellular glutathione and NAD(P)H:quinone oxidoreductase 1 in PC12 neuronal cells: A potential adaptive mechanism for dopaminergic neuroprotection. Neurochem Res. 2008;33:2197–2205. doi: 10.1007/s11064-008-9670-4. [DOI] [PubMed] [Google Scholar]
- 76.Jia Z. Zhu H. Misra HP. Li Y. Potent induction of total cellular GSH and NQO1 as well as mitochondrial GSH by 3H-1,2-dithiole-3-thione in SH-SY5Y neuroblastoma cells and primary human neurons: Protection against neurocytotoxicity elicited by dopamine, 6-hydroxydopamine, 4-hydroxy-2-nonenal, or hydrogen peroxide. Brain Res. 2008;1197:159–169. doi: 10.1016/j.brainres.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jocelyn PC. Some properties of mitochondrial glutathione. Biochim Biophys Acta. 1975;396:427–436. doi: 10.1016/0005-2728(75)90148-6. [DOI] [PubMed] [Google Scholar]
- 78.Jocelyn PC. Dickson J. Glutathione and the mitochondrial reduction of hydroperoxides. Biochim Biophys Acta. 1980;590:1–12. doi: 10.1016/0005-2728(80)90141-3. [DOI] [PubMed] [Google Scholar]
- 79.Johansson C. Lillig CH. Holmgren A. Human mitochondrial glutaredoxin reduces S-glutathionylated proteins with high affinity accepting electrons from either glutathione or thioredoxin reductase. J Biol Chem. 2004;279:7537–7543. doi: 10.1074/jbc.M312719200. [DOI] [PubMed] [Google Scholar]
- 80.Jones DP. Redox potential of GSH/GSSG couple: Assay and biological significance. In: Helmut S, editor; Lester P, editor. Methods Enzymol. Academic Press; 2002. pp. 93–112. [DOI] [PubMed] [Google Scholar]
- 81.Jones DP. Disruption of mitochondrial redox circuitry in oxidative stress. Chem Biol Interact. 2006;163:38–53. doi: 10.1016/j.cbi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 82.Jones DP. Redox sensing: Orthogonal control in cell cycle and apoptosis signalling. J Intern Med. 2010;268:432–448. doi: 10.1111/j.1365-2796.2010.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jonsson TJ. Murray MS. Johnson LC. Poole LB. Lowther WT. Structural basis for the retroreduction of inactivated peroxiredoxins by human sulfiredoxin. Biochemistry. 2005;44:8634–8642. doi: 10.1021/bi050131i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kagan VE. Tyurin VA. Jiang J. Tyurina YY. Ritov VB. Amoscato AA. Osipov AN. Belikova NA. Kapralov AA. Kini V. Vlasova II. Zhao Q. Zou M. Di P. Svistunenko DA. Kurnikov IV. Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 85.Kaneto H. Kawamori D. Matsuoka TA. Kajimoto Y. Yamasaki Y. Oxidative stress and pancreatic beta-cell dysfunction. Am J Ther. 2005;12:529–533. doi: 10.1097/01.mjt.0000178773.31525.c2. [DOI] [PubMed] [Google Scholar]
- 86.Kanwar M. Chan PS. Kern TS. Kowluru RA. Oxidative damage in the retinal mitochondria of diabetic mice: Possible protection by superoxide dismutase. Invest Ophthalmol Vis Sci. 2007;48:3805–3811. doi: 10.1167/iovs.06-1280. [DOI] [PubMed] [Google Scholar]
- 87.Kemp M. Go YM. Jones DP. Nonequilibrium thermodynamics of thiol/disulfide redox systems: A perspective on redox systems biology. Free Radic Biol Med. 2008;44:921–937. doi: 10.1016/j.freeradbiomed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kenchappa RS. Ravindranath V. Glutaredoxin is essential for maintenance of brain mitochondrial complex I: Studies with MPTP. FASEB J. 2003;17:717–719. doi: 10.1096/fj.02-0771fje. [DOI] [PubMed] [Google Scholar]
- 89.Kerr JFR. Winterford CM. Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 90.Kil IS. Park JW. Regulation of mitochondrial NADP+-dependent isocitrate dehydrogenase activity by glutathionylation. J Biol Chem. 2005;280:10846–10854. doi: 10.1074/jbc.M411306200. [DOI] [PubMed] [Google Scholar]
- 91.Kim SH. Fountoulakis M. Cairns N. Lubec G. Protein levels of human peroxiredoxin subtypes in brains of patients with Alzheimer's disease and Down syndrome. J Neural Transm Suppl. 2001;61:223–235. doi: 10.1007/978-3-7091-6262-0_18. [DOI] [PubMed] [Google Scholar]
- 92.Klatt P. Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem. 2000;267:4928–4944. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- 93.Krahenbuhl S. Talos C. Lauterburg BH. Reichen J. Reduced antioxidative capacity in liver mitochondria from bile duct ligated rats. Hepatology. 1995;22:607–612. doi: 10.1002/hep.1840220234. [DOI] [PubMed] [Google Scholar]
- 94.Krapfenbauer K. Engidawork E. Cairns N. Fountoulakis M. Lubec G. Aberrant expression of peroxiredoxin subtypes in neurodegenerative disorders. Brain Res. 2003;967:152–160. doi: 10.1016/s0006-8993(02)04243-9. [DOI] [PubMed] [Google Scholar]
- 95.Kroemer G. Dallaporta B. Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 96.Kropotov A. Gogvadze V. Shupliakov O. Tomilin N. Serikov VB. Tomilin NV. Zhivotovsky B. Peroxiredoxin V is essential for protection against apoptosis in human lung carcinoma cells. Exp Cell Res. 2006;312:2806–2815. doi: 10.1016/j.yexcr.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 97.Lash LH. Mitochondrial glutathione transport: Physiological, pathological and toxicological implications. Chem Biol Interact. 2006;163:54–67. doi: 10.1016/j.cbi.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee DW. Kaur D. Chinta SJ. Rajagopalan S. Andersen JK. A disruption in iron-sulfur center biogenesis via inhibition of mitochondrial dithiol glutaredoxin 2 may contribute to mitochondrial and cellular iron dysregulation in mammalian glutathione-depleted dopaminergic cells: Implications for Parkinson's disease. Antioxid Redox Signal. 2009;11:2083–2094. doi: 10.1089/ars.2009.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lehninger AL. Vercesi A. Bababunmi EA. Regulation of Ca2+ release from mitochondria by the oxidation-reduction state of pyridine nucleotides. Proc Natl Acad Sci USA. 1978;75:1690–1694. doi: 10.1073/pnas.75.4.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leist M. Single B. Castoldi AF. Kuhnle S. Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: A switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li L. Shoji W. Takano H. Nishimura N. Aoki Y. Takahashi R. Goto S. Kaifu T. Takai T. Obinata M. Increased susceptibility of MER5 (peroxiredoxin III) knockout mice to LPS-induced oxidative stress. Biochem Biophys Res Commun. 2007;355:715–721. doi: 10.1016/j.bbrc.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 102.Lillig CH. Holmgren A. Thioredoxin and related molecules—From biology to health and disease. Antioxid Redox Signal. 2007;9:25–47. doi: 10.1089/ars.2007.9.25. [DOI] [PubMed] [Google Scholar]
- 103.Lillig CH. Lonn ME. Enoksson M. Fernandes AP. Holmgren A. Short interfering RNA-mediated silencing of glutaredoxin 2 increases the sensitivity of HeLa cells toward doxorubicin and phenylarsine oxide. Proc Natl Acad Sci USA. 2004;101:13227–13232. doi: 10.1073/pnas.0401896101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu X. Kim CN. Yang J. Jemmerson R. Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 105.Lluis JM. Colell A. Garcia-Ruiz C. Kaplowitz N. Fernandez-Checa JC. Acetaldehyde impairs mitochondrial glutathione transport in HepG2 cells through endoplasmic reticulum stress. Gastroenterology. 2003;124:708–724. doi: 10.1053/gast.2003.50089. [DOI] [PubMed] [Google Scholar]
- 106.Lötscher HR. Winterhalter KH. Carafoli E. Richter C. Hydroperoxide-induced loss of pyridine nucleotides and release of calcium from rat liver mitochondria. J Biol Chem. 1980;255:9325–9330. [PubMed] [Google Scholar]
- 107.Lowell BB. Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 108.Maher P. Redox control of neural function: Background, mechanisms, and significance. Antioxid Redox Signal. 2006;8:1941–1970. doi: 10.1089/ars.2006.8.1941. [DOI] [PubMed] [Google Scholar]
- 109.Mari M. Caballero F. Colell A. Morales A. Caballeria J. Fernandez A. Enrich C. Fernandez-Checa JC. Garcia-Ruiz C. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 2006;4:185–198. doi: 10.1016/j.cmet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 110.Marí M. Morales A. Colell A. García-Ruiz C. Fernández-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal. 2009;11:2685–2700. doi: 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Masini A. Ceccarelli D. Trenti T. Gallesi D. Muscatello U. Mitochondrial inner membrane permeability changes induced by octadecadienoic acid hydroperoxide. Role of mitochondrial GSH pool. Biochim Biophys Acta. 1992;1101:84–89. doi: 10.1016/0167-4838(92)90471-o. [DOI] [PubMed] [Google Scholar]
- 112.Mattson MP. Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McKernan TB. Woods EB. Lash LH. Uptake of glutathione by renal cortical mitochondria. Arch Biochem Biophys. 1991;288:653–663. doi: 10.1016/0003-9861(91)90248-h. [DOI] [PubMed] [Google Scholar]
- 114.Mehendale H. Roth R. Gandolfi A. Klaunig J. Lemasters J. Curtis L. Novel mechanisms in chemically induced hepatotoxicity. FASEB J. 1994;8:1285–1295. doi: 10.1096/fasebj.8.15.8001741. [DOI] [PubMed] [Google Scholar]
- 115.Meredith MJ. Reed DJ. Depletion in vitro of mitochondrial glutathione in rat hepatocytes and enhancement of lipid peroxidation by adriamycin and 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU). Biochem. Pharmacol. 1983;32:1383–1388. doi: 10.1016/0006-2952(83)90451-3. [DOI] [PubMed] [Google Scholar]
- 116.Allen EMG. Mieyal JJ. Protein-thiol oxidation and cell death: Regulatory role of glutaredoxins. Antioxid Redox Signal. 2012;17:1748–1763. doi: 10.1089/ars.2012.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miller EW. Dickinson BC. Chang CJ. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc Natl Acad Sci USA. 2010;107:15681–15686. doi: 10.1073/pnas.1005776107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Miranda-Vizuete A. Damdimopoulos AE. Spyrou G. The mitochondrial thioredoxin system. Antioxid Redox Signal. 2000;2:801–810. doi: 10.1089/ars.2000.2.4-801. [DOI] [PubMed] [Google Scholar]
- 119.Moran LK. Gutteridge JM. Quinlan GJ. Thiols in cellular redox signalling and control. Curr Med Chem. 2001;8:763–772. doi: 10.2174/0929867013372904. [DOI] [PubMed] [Google Scholar]
- 120.Mukhopadhyay SS. Leung KS. Hicks MJ. Hastings PJ. Youssoufian H. Plon SE. Defective mitochondrial peroxiredoxin-3 results in sensitivity to oxidative stress in Fanconi anemia. J Cell Biol. 2006;175:225–235. doi: 10.1083/jcb.200607061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Muyderman H. Nilsson M. Sims NR. Highly selective and prolonged depletion of mitochondrial glutathione in astrocytes markedly increases sensitivity to peroxynitrite. J Neurosci. 2004;24:8019–8028. doi: 10.1523/JNEUROSCI.1103-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Muyderman H. Wadey AL. Nilsson M. Sims NR. Mitochondrial glutathione protects against cell death induced by oxidative and nitrative stress in astrocytes. J Neurochem. 2007;102:1369–1382. doi: 10.1111/j.1471-4159.2007.04641.x. [DOI] [PubMed] [Google Scholar]
- 123.Nagy N. Malik G. Tosaki A. Ho YS. Maulik N. Das DK. Overexpression of glutaredoxin-2 reduces myocardial cell death by preventing both apoptosis and necrosis. J Mol Cell Cardiol. 2008;44:252–60. doi: 10.1016/j.yjmcc.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 124.Nalvarte I. Damdimopoulos AE. Spyrou G. Human mitochondrial thioredoxin reductase reduces cytochrome c and confers resistance to complex III inhibition. Free Radic Biol Med. 2004;36:1270–1278. doi: 10.1016/j.freeradbiomed.2004.02.072. [DOI] [PubMed] [Google Scholar]
- 125.Naoi M. Maruyama W. Yi H. Yamaoka Y. Shamoto-Nagai M. Akao Y. Gerlach M. Tanaka M. Riederer P. Neuromelanin selectively induces apoptosis in dopaminergic SH-SY5Y cells by deglutathionylation in mitochondria: Involvement of the protein and melanin component. J Neurochem. 2008;105:2489–2500. doi: 10.1111/j.1471-4159.2008.05329.x. [DOI] [PubMed] [Google Scholar]
- 126.Navarro A. Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007;292:C670–686. doi: 10.1152/ajpcell.00213.2006. [DOI] [PubMed] [Google Scholar]
- 127.Navarro A. Boveris A. Mitochondrial nitric oxide synthase, mitochondrial brain dysfunction in aging, and mitochondria-targeted antioxidants. Adv Drug Deliv Rev. 2008;60:1534–1544. doi: 10.1016/j.addr.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 128.Noctor G. Arisi A-CM. Jouanin L. Kunert KJ. Rennenberg H. Foyer CH. Glutathione: Biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot. 1998;49:623–647. [Google Scholar]
- 129.Noh DY. Ahn SJ. Lee RA. Kim SW. Park IA. Chae HZ. Overexpression of peroxiredoxin in human breast cancer. Anticancer Res. 2001;21:2085–2090. [PubMed] [Google Scholar]
- 130.Noh YH. Baek JY. Jeong W. Rhee SG. Chang TS. Sulfiredoxin translocation into mitochondria plays a crucial role in reducing hyperoxidized peroxiredoxin III. J Biol Chem. 2009;284:8470–8477. doi: 10.1074/jbc.M808981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nonn L. Berggren M. Powis G. Increased expression of mitochondrial peroxiredoxin-3 (thioredoxin peroxidase-2) protects cancer cells against hypoxia and drug-induced hydrogen peroxide-dependent apoptosis. Mol Cancer Res. 2003;1:682–689. [PubMed] [Google Scholar]
- 132.Nonn L. Williams RR. Erickson RP. Powis G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol Cell Biol. 2003;23:916–922. doi: 10.1128/MCB.23.3.916-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nulton-Persson AC. Starke DW. Mieyal JJ. Szweda LI. Reversible inactivation of alpha-ketoglutarate dehydrogenase in response to alterations in the mitochondrial glutathione status. Biochemistry. 2003;42:4235–4242. doi: 10.1021/bi027370f. [DOI] [PubMed] [Google Scholar]
- 134.Olmos Y. Valle I. Borniquel S. Tierrez A. Soria E. Lamas S. Monsalve M. Mutual dependence of Foxo3a and PGC-1alpha in the induction of oxidative stress genes. J Biol Chem. 2009;284:14476–14484. doi: 10.1074/jbc.M807397200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ott M. Robertson JD. Gogvadze V. Zhivotovsky B. Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci USA. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pacher P. Beckman JS. Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pai HV. Starke DW. Lesnefsky EJ. Hoppel CL. Mieyal JJ. What is the functional significance of the unique location of glutaredoxin 1 (GRx1) in the intermembrane space of mitochondria? Antioxid Redox Signal. 2007;9:2027–2033. doi: 10.1089/ars.2007.1642. [DOI] [PubMed] [Google Scholar]
- 138.Papadia S. Soriano FX. Leveille F. Martel MA. Dakin KA. Hansen HH. Kaindl A. Sifringer M. Fowler J. Stefovska V. McKenzie G. Craigon M. Corriveau R. Ghazal P. Horsburgh K. Yankner BA. Wyllie DJ. Ikonomidou C. Hardingham GE. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11:476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Peng Y. Yang PH. Guo Y. Ng SS. Liu J. Fung PC. Tay D. Ge J. He ML. Kung HF. Lin MC. Catalase and peroxiredoxin 5 protect Xenopus embryos against alcohol-induced ocular anomalies. Invest Ophthalmol Vis Sci. 2004;45:23–29. doi: 10.1167/iovs.03-0550. [DOI] [PubMed] [Google Scholar]
- 140.Perez VI. Lew CM. Cortez LA. Webb CR. Rodriguez M. Liu Y. Qi W. Li Y. Chaudhuri A. Van Remmen H. Richardson A. Ikeno Y. Thioredoxin 2 haploinsufficiency in mice results in impaired mitochondrial function and increased oxidative stress. Free Radic Biol Med. 2008;44:882–892. doi: 10.1016/j.freeradbiomed.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 141.Planson AG. Palais G. Abbas K. Gerard M. Couvelard L. Delaunay A. Baulande S. Drapier JC. Toledano MB. Sulfiredoxin protects mice from lipopolysaccharide-induced endotoxic shock. Antioxid Redox Signal. 2011;14:2071–2080. doi: 10.1089/ars.2010.3552. [DOI] [PubMed] [Google Scholar]
- 142.Poderoso JJ. The formation of peroxynitrite in the applied physiology of mitochondrial nitric oxide. Arch Biochem Biophys. 2009;484:214–220. doi: 10.1016/j.abb.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 143.Powis G. Montfort WR. Properties and biological activities of thioredoxins. Annu Rev Biophys Biomol Struct. 2001;30:421–455. doi: 10.1146/annurev.biophys.30.1.421. [DOI] [PubMed] [Google Scholar]
- 144.Precht TA. Phelps RA. Linseman DA. Butts BD. Le SS. Laessig TA. Bouchard RJ. Heidenreich KA. The permeability transition pore triggers Bax translocation to mitochondria during neuronal apoptosis. Cell Death Differ. 2005;12:255–265. doi: 10.1038/sj.cdd.4401552. [DOI] [PubMed] [Google Scholar]
- 145.Queiroga CS. Almeida AS. Martel C. Brenner C. Alves PM. Vieira HL. Glutathionylation of adenine nucleotide translocase induced by carbon monoxide prevents mitochondrial membrane permeabilization and apoptosis. J Biol Chem. 2010;285:17077–17088. doi: 10.1074/jbc.M109.065052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Radyuk SN. Michalak K. Klichko VI. Benes J. Rebrin I. Sohal RS. Orr WC. Peroxiredoxin 5 confers protection against oxidative stress and apoptosis and also promotes longevity in Drosophila. Biochem J. 2009;419:437–445. doi: 10.1042/BJ20082003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Reed DJ. Glutathione: Toxicological implications. Annu Rev Pharmacol Toxicol. 1990;30:603–631. doi: 10.1146/annurev.pa.30.040190.003131. [DOI] [PubMed] [Google Scholar]
- 148.Rhee SG. Chae HZ. Kim K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 149.Rhee SG. Jeong W. Chang TS. Woo HA. Sulfiredoxin, the cysteine sulfinic acid reductase specific to 2-Cys peroxiredoxin: Its discovery, mechanism of action, and biological significance. Kidney Int Suppl. 2007;106:S3–8. doi: 10.1038/sj.ki.5002380. [DOI] [PubMed] [Google Scholar]
- 150.Rhee SG. Woo HA. Multiple functions of peroxiredoxins: Peroxidases, sensors and regulators of the intracellular messenger HO, and protein chaperones. Antioxid Redox Signal. 2011;15:781–794. doi: 10.1089/ars.2010.3393. [DOI] [PubMed] [Google Scholar]
- 151.Richter C. Schweizer M. Cossarizza A. Franceschi C. Control of apoptosis by the cellular ATP level. FEBS Lett. 1996;378:107–110. doi: 10.1016/0014-5793(95)01431-4. [DOI] [PubMed] [Google Scholar]
- 152.Rohrbach S. Gruenler S. Teschner M. Holtz J. The thioredoxin system in aging muscle: Key role of mitochondrial thioredoxin reductase in the protective effects of caloric restriction? Am J Physiol Regul Integr Comp Physiol. 2006;291:R927–935. doi: 10.1152/ajpregu.00890.2005. [DOI] [PubMed] [Google Scholar]
- 153.Roussel X. Boukhenouna S. Rahuel-Clermont S. Branlant G. The rate-limiting step of sulfiredoxin is associated with the transfer of the gamma-phosphate of ATP to the sulfinic acid of overoxidized typical 2-Cys peroxiredoxins. FEBS Lett. 2011;585:574–578. doi: 10.1016/j.febslet.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 154.Rydstrom J. Mitochondrial NADPH, transhydrogenase, and disease. Biochim Biophys Acta. 2006;1757:721–726. doi: 10.1016/j.bbabio.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 155.Saeed U. Durgadoss L. Valli RK. Joshi DC. Joshi PG. Ravindranath V. Knockdown of cytosolic glutaredoxin 1 leads to loss of mitochondrial membrane potential: Implication in neurodegenerative diseases. PLoS One. 2008;3:e2459. doi: 10.1371/journal.pone.0002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Saxena G. Chen J. Shalev A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J Biol Chem. 2010;285:3997–4005. doi: 10.1074/jbc.M109.034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Seo MS. Kang SW. Kim K. Baines IC. Lee TH. Rhee SG. Identification of a new type of mammalian peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate. J Biol Chem. 2000;275:20346–20354. doi: 10.1074/jbc.M001943200. [DOI] [PubMed] [Google Scholar]
- 158.Shan X. Jones DP. Hashmi M. Anders MW. Selective depletion of mitochondrial glutathione concentrations by (R,S)-3-hydroxy-4-pentenoate potentiates oxidative cell death. Chem Res Toxicol. 1993;6:75–81. doi: 10.1021/tx00031a012. [DOI] [PubMed] [Google Scholar]
- 159.Sheeran FL. Rydstrom J. Shakhparonov MI. Pestov NB. Pepe S. Diminished NADPH transhydrogenase activity and mitochondrial redox regulation in human failing myocardium. Biochim Biophys Acta. 2010;1797:1138–1148. doi: 10.1016/j.bbabio.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 160.Sibbing D. Pfeufer A. Perisic T. Mannes AM. Fritz-Wolf K. Unwin S. Sinner MF. Gieger C. Gloeckner CJ. Wichmann HE. Kremmer E. Schafer Z. Walch A. Hinterseer M. Nabauer M. Kaab S. Kastrati A. Schomig A. Meitinger T. Bornkamm GW. Conrad M. von Beckerath N. Mutations in the mitochondrial thioredoxin reductase gene TXNRD2 cause dilated cardiomyopathy. Eur Heart J. 2011;32:1121–1133. doi: 10.1093/eurheartj/ehq507. [DOI] [PubMed] [Google Scholar]
- 161.Sies H. Moss K. A role of mitochondrial glutathione peroxidase in modulating mitochondrial oxidations in liver. Eur J Biochem. 1978;84:377–383. doi: 10.1111/j.1432-1033.1978.tb12178.x. [DOI] [PubMed] [Google Scholar]
- 162.Simonian NA. Coyle JT. Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 1996;36:83–106. doi: 10.1146/annurev.pa.36.040196.000503. [DOI] [PubMed] [Google Scholar]
- 163.Sims NR. Anderson MF. Mitochondrial contributions to tissue damage in stroke. Neurochem Int. 2002;40:511–526. doi: 10.1016/s0197-0186(01)00122-x. [DOI] [PubMed] [Google Scholar]
- 164.Singh A. Ling G. Suhasini AN. Zhang P. Yamamoto M. Navas-Acien A. Cosgrove G. Tuder RM. Kensler TW. Watson WH. Biswal S. Nrf2-dependent sulfiredoxin-1 expression protects against cigarette smoke-induced oxidative stress in lungs. Free Radic Biol Med. 2009;46:376–386. doi: 10.1016/j.freeradbiomed.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sohal RS. Arnold L. Orr WC. Effect of age on superoxide dismutase, catalase, glutathione reductase, inorganic peroxides, TBA-reactive material, GSH/GSSG, NADPH/NADP+ and NADH/NAD+ in Drosophila melanogaster. Mech Ageing Dev. 1990;56:223–235. doi: 10.1016/0047-6374(90)90084-s. [DOI] [PubMed] [Google Scholar]
- 166.Sohal RS. Dubey A. Mitochondrial oxidative damage, hydrogen peroxide release, and aging. Free Radic Biol Med. 1994;16:621–626. doi: 10.1016/0891-5849(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 167.Spyrou G. Enmark E. Miranda-Vizuete A. Gustafsson J. Cloning and expression of a novel mammalian thioredoxin. J Biol Chem. 1997;272:2936–2941. doi: 10.1074/jbc.272.5.2936. [DOI] [PubMed] [Google Scholar]
- 168.Svoboda DJ. Manning RT. Chronic alcoholism with fatty metamorphosis of the liver. Mitochondrial alterations in hepatic cells. Am J Pathol. 1964;44:645–662. [PMC free article] [PubMed] [Google Scholar]
- 169.Tanaka T. Hosoi F. Yamaguchi-Iwai Y. Nakamura H. Masutani H. Ueda S. Nishiyama A. Takeda S. Wada H. Spyrou G. Yodoi J. Thioredoxin-2 (TRX-2) is an essential gene regulating mitochondria-dependent apoptosis. EMBO J. 2002;21:1695–1703. doi: 10.1093/emboj/21.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Taylor ER. Hurrell F. Shannon RJ. Lin TK. Hirst J. Murphy MP. Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J Biol Chem. 2003;278:19603–19610. doi: 10.1074/jbc.M209359200. [DOI] [PubMed] [Google Scholar]
- 171.Terada LS. Specificity in reactive oxidant signaling: Think globally, act locally. J Cell Biol. 2006;174:615–623. doi: 10.1083/jcb.200605036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Thompson C. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 173.Trujillo M. Clippe A. Manta B. Ferrer-Sueta G. Smeets A. Declercq JP. Knoops B. Radi R. Pre-steady state kinetic characterization of human peroxiredoxin 5: Taking advantage of Trp84 fluorescence increase upon oxidation. Arch Biochem Biophys. 2007;467:95–106. doi: 10.1016/j.abb.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 174.Tsujimoto Y. Nakagawa T. Shimizu S. Mitochondrial membrane permeability transition and cell death. Biochim Biophys Acta. 2006;1757:1297–1300. doi: 10.1016/j.bbabio.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 175.Ueda S. Masutani H. Nakamura H. Tanaka T. Ueno M. Yodoi J. Redox control of cell death. Antioxid Redox Signal. 2002;4:405–414. doi: 10.1089/15230860260196209. [DOI] [PubMed] [Google Scholar]
- 176.Valdez LB. Zaobornyj T. Boveris A. Mitochondrial metabolic states and membrane potential modulate mtNOS activity. Biochim Biophys Acta. 2006;1757:166–172. doi: 10.1016/j.bbabio.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 177.Van Remmen H. Jones DP. Current thoughts on the role of mitochondria and free radicals in the biology of aging. J Gerontol A Biol Sci Med Sci. 2009;64:171–174. doi: 10.1093/gerona/gln058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Veal E. Day A. Hydrogen peroxide as a signaling molecule. Antioxid Redox Signal. 2011;15:147–151. doi: 10.1089/ars.2011.3968. [DOI] [PubMed] [Google Scholar]
- 179.Vendemiale G. Grattagliano I. Altomare E. Turturro N. Guerrieri F. Effect of acetaminophen administration on hepatic glutathione compartmentation and mitochondrial energy metabolism in the rat. Biochem Pharmacol. 1996;52:1147–1154. doi: 10.1016/0006-2952(96)00414-5. [DOI] [PubMed] [Google Scholar]
- 180.Venkatakrishnan P. Nakayasu ES. Almeida IC. Miller RT. Absence of nitric-oxide synthase in sequentially purified rat liver mitochondria. J Biol Chem. 2009;284:19843–19855. doi: 10.1074/jbc.M109.003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Vina J. Sastre J. Anton V. Bruseghini L. Esteras A. Asensi M. Effect of aging on glutathione metabolism. Protection by antioxidants. EXS. 1992;62:136–144. doi: 10.1007/978-3-0348-7460-1_14. [DOI] [PubMed] [Google Scholar]
- 182.Wang D. Masutani H. Oka S. Tanaka T. Yamaguchi-Iwai Y. Nakamura H. Yodoi J. Control of mitochondrial outer membrane permeabilization and Bcl-xL levels by thioredoxin 2 in DT40 cells. J Biol Chem. 2006;281:7384–7391. doi: 10.1074/jbc.M509876200. [DOI] [PubMed] [Google Scholar]
- 183.Wei Q. Jiang H. Matthews CP. Colburn NH. Sulfiredoxin is an AP-1 target gene that is required for transformation and shows elevated expression in human skin malignancies. Proc Natl Acad Sci USA. 2008;105:19738–19743. doi: 10.1073/pnas.0810676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Weil M. Jacobson MD. Coles HS. Davies TJ. Gardner RL. Raff KD. Raff MC. Constitutive expression of the machinery for programmed cell death. J Cell Biol. 1996;133:1053–1059. doi: 10.1083/jcb.133.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wenzel P. Hink U. Oelze M. Schuppan S. Schaeuble K. Schildknecht S. Ho KK. Weiner H. Bachschmid M. Munzel T. Daiber A. Role of reduced lipoic acid in the redox regulation of mitochondrial aldehyde dehydrogenase (ALDH-2) activity. Implications for mitochondrial oxidative stress and nitrate tolerance. J Biol Chem. 2007;282:792–799. doi: 10.1074/jbc.M606477200. [DOI] [PubMed] [Google Scholar]
- 186.West MB. Hill BG. Xuan YT. Bhatnagar A. Protein glutathiolation by nitric oxide: An intracellular mechanism regulating redox protein modification. FASEB J. 2006;20:1715–1717. doi: 10.1096/fj.06-5843fje. [DOI] [PubMed] [Google Scholar]
- 187.Wilkins HM. Marquardt K. Lash LH. Linseman DA. Bcl-2 is a novel interacting partner for the 2-oxoglutarate carrier and a key regulator of mitochondrial glutathione. Free Radic Biol Med. 2012;52:410–419. doi: 10.1016/j.freeradbiomed.2011.10.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wonsey DR. Zeller KI. Dang CV. The c-Myc target gene PRDX3 is required for mitochondrial homeostasis and neoplastic transformation. Proc Natl Acad Sci USA. 2002;99:6649–6654. doi: 10.1073/pnas.102523299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Woo HA. Chae HZ. Hwang SC. Yang KS. Kang SW. Kim K. Rhee SG. Reversing the inactivation of peroxiredoxins caused by cysteine sulfinic acid formation. Science. 2003;300:653–656. doi: 10.1126/science.1080273. [DOI] [PubMed] [Google Scholar]
- 190.Woo HA. Jeong W. Chang TS. Park KJ. Park SJ. Yang JS. Rhee SG. Reduction of cysteine sulfinic acid by sulfiredoxin is specific to 2-cys peroxiredoxins. J Biol Chem. 2005;280:3125–3128. doi: 10.1074/jbc.C400496200. [DOI] [PubMed] [Google Scholar]
- 191.Woo HA. Yim SH. Shin DH. Kang D. Yu DY. Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 192.Wood-Allum CA. Barber SC. Kirby J. Heath P. Holden H. Mead R. Higginbottom A. Allen S. Beaujeux T. Alexson SE. Ince PG. Shaw PJ. Impairment of mitochondrial anti-oxidant defence in SOD1-related motor neuron injury and amelioration by ebselen. Brain. 2006;129:1693–1709. doi: 10.1093/brain/awl118. [DOI] [PubMed] [Google Scholar]
- 193.Wood ZA. Poole LB. Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- 194.Wood ZA. Schroder E. Robin Harris J. Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 195.Wüllner U. Seyfried J. Groscurth P. Beinroth S. Winter S. Gleichmann M. Heneka M. Löschmann PA. Schulz JB. Weller M. Klockgether T. Glutathione depletion and neuronal cell death: The role of reactive oxygen intermediates and mitochondrial function. Brain Res. 1999;826:53–62. doi: 10.1016/s0006-8993(99)01228-7. [DOI] [PubMed] [Google Scholar]
- 196.Yin F. Sancheti H. Cadenas E. Silencing of nicotinamide nucleotide transhydrogenase impairs cellular redox homeostasis and energy metabolism in PC12 cells. Biochim Biophys Acta. 2012;1817:401–409. doi: 10.1016/j.bbabio.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: Regulation and biological consequences. Antioxid Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 198.Zhang D. Lu C. Whiteman M. Chance B. Armstrong JS. The mitochondrial permeability transition regulates cytochrome c release for apoptosis during endoplasmic reticulum stress by remodeling the cristae junction. J Biol Chem. 2008;283:3476–3486. doi: 10.1074/jbc.M707528200. [DOI] [PubMed] [Google Scholar]
- 199.Zhang H. Go YM. Jones DP. Mitochondrial thioredoxin-2/peroxiredoxin-3 system functions in parallel with mitochondrial GSH system in protection against oxidative stress. Arch Biochem Biophys. 2007;465:119–126. doi: 10.1016/j.abb.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 200.Zhang R. Al-Lamki R. Bai L. Streb JW. Miano JM. Bradley J. Min W. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circ Res. 2004;94:1483–1491. doi: 10.1161/01.RES.0000130525.37646.a7. [DOI] [PubMed] [Google Scholar]
- 201.Zhao P. Kalhorn TF. Slattery JT. Selective mitochondrial glutathione depletion by ethanol enhances acetaminophen toxicity in rat liver. Hepatology. 2002;36:326–335. doi: 10.1053/jhep.2002.34943. [DOI] [PubMed] [Google Scholar]
- 202.Zhao P. Slattery JT. Effects of ethanol dose and ethanol withdrawal on rat liver mitochondrial glutathione: Implication of potentiated acetaminophen toxicity in alcoholics. Drug Metab Dispos. 2002;30:1413–1417. doi: 10.1124/dmd.30.12.1413. [DOI] [PubMed] [Google Scholar]
- 203.Zheng LM. Zychlinsky A. Liu CC. Ojcius DM. Young JD. Extracellular ATP as a trigger for apoptosis or programmed cell death. J Cell Biol. 1991;112:279–288. doi: 10.1083/jcb.112.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhong Q. Putt DA. Xu F. Lash LH. Hepatic mitochondrial transport of glutathione: Studies in isolated rat liver mitochondria and H4IIE rat hepatoma cells. Arch Biochem Biophys. 2008;474:119–127. doi: 10.1016/j.abb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhou Y. Kok KH. Chun AC. Wong CM. Wu HW. Lin MC. Fung PC. Kung H. Jin DY. Mouse peroxiredoxin V is a thioredoxin peroxidase that inhibits p53-induced apoptosis. Biochem Biophys Res Commun. 2000;268:921–927. doi: 10.1006/bbrc.2000.2231. [DOI] [PubMed] [Google Scholar]
- 206.Zimmermann AK. Loucks FA. Schroeder EK. Bouchard RJ. Tyler KL. Linseman DA. Glutathione binding to the Bcl-2 homology-3 domain groove: A molecular basis for Bcl-2 antioxidant function at mitochondria. J Biol Chem. 2007;282:29296–29304. doi: 10.1074/jbc.M702853200. [DOI] [PMC free article] [PubMed] [Google Scholar]