Abstract
Significance: Glutathione (GSH) depletion is a central signaling event that regulates the activation of cell death pathways. GSH depletion is often taken as a marker of oxidative stress and thus, as a consequence of its antioxidant properties scavenging reactive species of both oxygen and nitrogen (ROS/RNS). Recent Advances: There is increasing evidence demonstrating that GSH loss is an active phenomenon regulating the redox signaling events modulating cell death activation and progression. Critical Issues: In this work, we review the role of GSH depletion by its efflux, as an important event regulating alterations in the cellular redox balance during cell death independent from oxidative stress and ROS/RNS formation. We discuss the mechanisms involved in GSH efflux during cell death progression and the redox signaling events by which GSH depletion regulates the activation of the cell death machinery. Future Directions: The evidence summarized here clearly places GSH transport as a central mechanism mediating redox signaling during cell death progression. Future studies should be directed toward identifying the molecular identity of GSH transporters mediating GSH extrusion during cell death, and addressing the lack of sensitive approaches to quantify GSH efflux. Antioxid. Redox Signal. 17, 1694–1713.
Introduction
Cell death is generally classified by biochemical and morphological criteria. Accordingly, three distinct types of pathways can be defined which are apoptosis, necrosis, and autophagy, although there are numerous examples where cell death displays mixed features (84, 135). Apoptosis or programmed cell death is a ubiquitous homeostatic mechanism involved in many biological processes. Apoptotic cell death is critical not only in the turnover of cells in tissues but also during normal development and senescence. However, the deregulation of apoptosis also occurs as either a cause or a consequence of distinct pathologies including cancer, autoimmune and neurodegenerative disorders (64). Apoptosis is a highly organized program characterized by the progressive activation of selective signaling pathways conveying specific biochemical and morphological alterations. The initiator phase of apoptosis is characterized by initiator caspase (cysteine-dependent aspartate-directed protease) activation, cell shrinkage, loss of plasma membrane lipid asymmetry, and chromatin condensation, while the execution phase of apoptosis is characterized by activation of executioner caspases and endonucleases, apoptotic body formation, and ultimately cellular fragmentation (83).
Necrotic cell death is characterized by a gain in cell volume, swelling of organelles, plasma membrane rupture, and subsequent release of intracellular components. Although initially described as an accidental type of cell death, it is now proposed that the execution of necrotic cell death can also be finely regulated by specific signal transduction pathways and catabolic processes (necroptosis). Necrosis has been reported to occur in inflammatory and neurodegenerative disorders, heart disease, neuronal ischemia and toxicity, muscular dystrophy, diabetes, infections, and in apoptotic cells that fail to be engulfed by phagocytic cells (secondary necrosis) (135, 170).
Autophagy is a major catabolic pathway by which eukaryotic cells degrade and recycle macromolecules and organelles. It has an essential role in differentiation, development, and cellular response to stress. Autophagy is initiated by the selective or nonselective engulfment of cytoplasmic constituents by a phagophore, which forms a closed double-membrane structure, the autophagosome. The autophagosome subsequently fuses with a lysosome to become an autolysosome whose content is degraded by acidic lysosomal hydrolases (105). Autophagy is a homeostatic mechanism involved in both survival and cell death. Autophagic cell death is morphologically defined by massive autophagic vacuolization of the cytoplasm in the absence of chromatin condensation. Although autophagy deregulation has been associated with distinct pathologies, it is primarily regarded as a pro-survival mechanism and there are only a limited number of cases where increased autophagy has been established as the cause of cell death (55, 136, 218).
Redox signaling events are important regulators of cell death pathways (40, 115, 181, 209). Although oxidative stress and ROS/RNS formation have long been thought to be major players regulating cell death, other redox-dependent signaling mechanisms have been identified as key players in the activation of the cell death machinery. GSH depletion is an early hallmark in the progression of distinct cell death mechanisms (36, 74, 107, 234). We and others have extensively reviewed the mechanisms by which alterations in GSH homeostasis regulate the activation of the cell death machinery (39, 74, 77, 130). Other excellent reviews address the role of compartmentalized GSH pools (mitochondria and endoplasmic reticulum) (6, 166), specific GSH-dependent antioxidant systems (119, 159), and GSH-based protein modifications in cell death (2, 50). Furthermore, several review manuscripts address the role of GSH in regulating cell death pathways in distinct pathologies (14, 71, 77) such as neurodegenerative disorders (167), cancer (63), hepatotoxicity (252), autoimmunity (196), and pulmonary diseases (22). This review article aims at highlighting the role of plasma membrane GSH efflux in GSH depletion during apoptosis and the mechanisms by which GSH depletion, by its extrusion, might contribute to alterations in the cellular redox balance and cell death progression. Furthermore, this works aims at summarizing the current evidence regarding the molecular identity of plasma membrane GSH transporters.
Overview of GSH Homeostasis
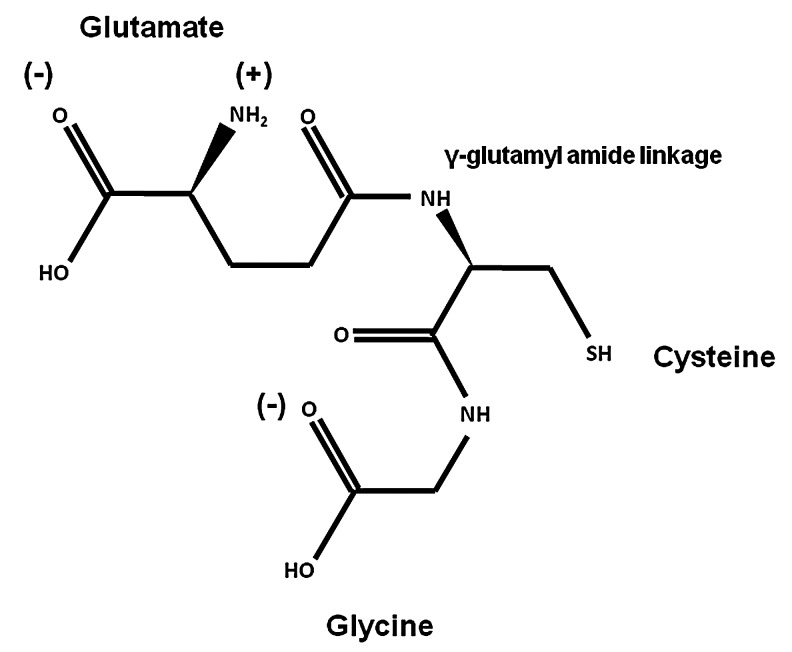
GSH (L-γ-glutamyl-L-cysteinyl-glycine) (Fig. 1) is the most abundant nonprotein thiol in mammalian cells acting as a major reducing agent and antioxidant defense by maintaining a tight control of the redox status. The peptidic γ-linkage between glutamate and cysteine protects GSH from hydrolysis by intracellular peptidases. The presence of the C-terminal glycine protects GSH against cleavage by intracellular γ-glutamylcyclotransferases. The cysteinyl moiety of GSH provides the reactive thiol group (-SH group) that mediates GSH biological functions, including oxidation-reduction (redox) and nucleophilic addition-type reactions (Fig. 1). GSH is also involved in the metabolism of xenobiotics, thiol disulfide exchange reactions, and acts as an important reservoir of cysteine. GSH synthesis is initiated by generation of γ-glutamylcysteine from glutamate and cysteine via the glutamate-cysteine ligase (GCL), and the subsequent addition of glycine by the activity of GSH synthetase (GS) (172, 219).
FIG. 1.
GSH (L-γ-glutamyl-L-cysteinyl-glycine) is a linear tripeptide (M.W. 307.4 g mol−1) formed from the amino acids glycine, cysteine, and glutamate. In solution, GSH possess a net negative charge of −1 at physiological pH, where the l-glutamic acid predominantly exists in its zwitterionic form, while the carboxyl group of the glycine fragment prefers to be deprotonated. GSH, glutathione.
Changes in the intracellular thiol-disulfide (GSH/GSSG) balance are considered major determinants in the redox status/signaling of the cell (123, 212). Almost all physiological oxidants react with thiols, and GSH has the ability to directly scavenge ROS/RNS. A large variety of unique GSH oxidation species can be generated on ROS/RNS formation, and their chemical profile depends on the magnitude and identity of the ROS/RNS generated. Similar to protein thiols (cysteines), GSH can be subject to one-electron oxidation by ROS such as superoxide anion (O2•−), which mediates derivatives with an unpaired electron, including the thiyl radical (glutathionyl radical [GS•]) and the thiyl peroxyl radical (GSOO•). Two-electron oxidation of GSH by ROS/RNS such as hydrogen peroxide (H2O2) and peroxynitrite (ONOO−) mediates the formation of other distinct oxidized states of GSH, which include the homo-disulfide glutathione disulfide (GSSG), glutathione sulfenic (GSOH), sulfinic (GSO2H) and sulfonic acids (GSO3H), glutathione disulfide S-oxide (GS(O)SG), glutathione disulfide S-dioxide (GS(O)2SG), glutathione thiosulfenamide (GSNHSG), glutathione N-hydroxysulfenamide (GSNHOH), and S-nitrosoglutathione (GSNO) (200, 246). Except for GSNO and GSSG, the physiological relevance of other oxidized GSH derivatives has not been studied in detail primarily due to the lack of accessible and selective techniques to quantify them, and their high instability/reactivity (116, 222, 230).
GSH Depletion During Cell Death: Where Does It Go?
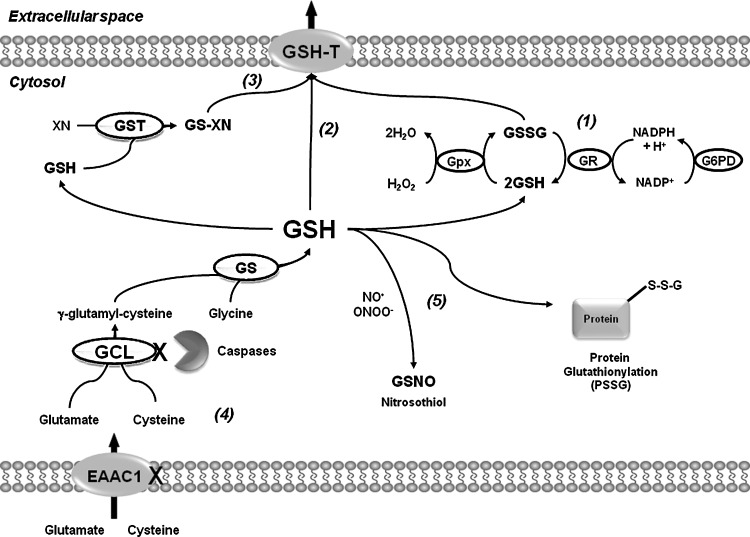
GSH is essential for cell survival as demonstrated by the observations that the GCL knockout mice die from massive apoptotic cell death (51), and that the knockdown of GCL in distinct cell types induces time-dependent apoptosis (58, 238). However, GSH itself is not required for survival, only the reducing equivalents provided by its reducing power (238). GSH depletion is a hallmark of the progression of cell death. More importantly, GSH depletion has been clearly shown to occur in apoptosis before the rupture of plasma membrane integrity (secondary necrosis) or cellular fragmentation, suggesting an active mechanism involved in its depletion (73). Distinct mechanisms have been reported to contribute to GSH depletion during cell death progression as summarized next (Table 1 and Fig. 2).
Table 1.
Potential Mechanisms Involved in Glutathione Depletion During Cell Death
| Mechanisms/transporters | Characteristics | References | |
|---|---|---|---|
| Impaired de novo synthesis | Inactivation of GCL. | Caspase-dependent cleavage. | (78, 79) |
| Impaired cysteine uptake by EAAC1 | Reduced EAAC1 translocation to the membrane and increased levels of oxidized EAAC1 in response to oxidative stress. | (5) | |
| Oxidation and adduct formation | Scavenging of ROS by GPx. | Induced by a wide variety of pro-oxidant conditions. Accumulation of GSSG can either be reduced by the GR/NADPH system, or extruded through MRP transporters. Inactivation of G6PD and depletion of NADPH impair GSSG recycling |
(41, 61, 62, 5, 88, 104, 151, 169, 177, 182, 188, 199, 255) |
| Incorporation into PSSG residues | Can be mediated by formation of mixed disulfides between: • Protein cysteines (SH) and GSSG • PSOH and GSH • Grx-catalyzed reaction of protein cysteines with GSSG or GS• • De-nitros(yl)ation of PSNO residues by GSH, or GSNO-mediated PSSG formation. |
(16, 19, 82, 122, 176, 179, 223) | |
| Formation of GSNO | Can be induced by: • Reaction of GS• with NO• • Reaction of GSH with NO2 or N2O3 • Cyt C mediated GSNO formation from NO• and GSH by acting as an electron acceptor. • Metal ions and metalloproteins |
(30, 120, 124, 131, 215, 226) | |
| GSH-electrophile adduct formation | Can be catalyzed by GSTs. Accumulation of adducts that are extruded through MRP transporters. | (23, 24, 43, 237, 244, 249) | |
| Plasma membrane efflux transport | MRP1 | Part of the ABCC subfamily of transporters. The MRP transporters act as ATP-dependent transporters. MRP1 is known to mediate: • Co-transport of OA− and GSH. • Transport of GSH-conjugated xenobiotics and metabolites • GSH efflux stimulated by xenobiotics (verapamil, apigenin) • GSSG efflux |
(21, 41, 43, 62, 67, 99, 106, 109, 110, 129, 139, 151, 177, 182, 188, 221) |
| Other MRPs: MRP2, MRP4, and MRP5 |
Although these MRPs have the ability to transport GSH, GSSG, or GSH adducts, their role in GSH depletion during apoptosis has not been determined. | (13, 206) | |
| CFTR | Belongs to the same family as MRPs, but acts as an ATP-gated chloride channel. | (94, 125, 138) | |
| ABGC2 | An ABC transporter, second member of the subfamily G (BCRP/ABCG2). ABCG2 and Cdrp1 (Candida albicans homologous protein) have been recently reported to mediate GSH transport. | (28, 256) | |
| OATP-like | OATPs have been proposed to mediate GSH efflux by a GSH/OA− exchange, where GSH efflux is driven by its electrochemical gradient across the plasma membrane and is trans-stimulated by the presence of a wide variety of structurally unrelated OA−. Pharmacolocial evidence suggests that GSH-depletion during apoptosis might be mediated by an OATP-like transport mechanism. However, recent evidence suggests that OATPs do not mediate GSH/OA− exchange. | (12, 29, 73, 76, 97, 152, 153, 160) | |
| Connexins | Unopposed gap junction hemichannels regulated by Ca2+ and voltage. Primarily reported to mediate GSH efflux in excitable cells. | (204, 224, 225) | |
| GLAST | Induced by glutamate in retinal cell cultures. | (87) | |
| OAT3 | In renal cells, but no association with cell death progression has been reported. | (144) | |
| RLIP76 | Proposed as an ATP-dependent multispecific transporter of GSH conjugates. | (10) | |
| VRAC/VSOAC | Cell swelling induces GSH depletion, and GSH depletion has also been shown to parallel apoptotic volume decrease. | (75, 148) | |
| Secretory pathway | Secretory granules | Thought to mediate GSSG transport from the ER. | (6, 112) |
ABC, ATP-binding cassette; ABCC, ATP-binding cassette (ABC) transporter, subfamily C; CFTR, cystic fibrosis transmembrane conductance regulator; Cyt C, cytochrome C; G6PD, glucose-6-phosphate dehydrogenase; GCL, glutamate-cysteine ligase; GLAST, glutamate/aspartate transporter; GPx, glutathione peroxidase; GR, glutathione reductase; Grx, glutaredoxin; GS•, glutathionyl radical; GSH, glutathione; GSNO, S-nitrosoglutathione; GST, glutathione-S-transferases; MRP, multidrug resistance protein; N2O3, dinitrogen trioxide; NADPH, nicotinamide adenine dinucleotide phosphate; NO•, nitric oxide; NO2, nitrogen dioxide; OA−, organic anion; OATP, organic anion transporting polypeptides; PSSG, protein glutathionylated; PSOH, protein sulfenic acid; PSNO, protein nitros(yl)ation; RLIP76 (RALBP1), Ral-binding, Rho/Rac-GAP and Ral effector; ER, endoplasmic reticulum; VRAC/VSOAC, volume-regulated/volume-sensitive organic osmolyte-anion channels.
FIG. 2.
Fates of GSH during cell death progression. GSH depletion during cell death can occur by distinct mechanisms. (1) Upon oxidative stress, GSH is used for the scavenging of peroxides by GPxs, which generate GSSG as a byproduct. GSSG can be reduced back to GSH by the GR/NADPH system. (2) GSH loss also occurs via its extrusion across the plasma membrane by the activation of GSH transporters or pumps (GSH-T). (3) GSH-Ts also mediate GSSG efflux and transport of GSH-conjugates (GS-XN) generated by xenobiotics in order to avoid deleterious effects of the accumulation of these toxins. (4) GSH depletion might also be associated with the impairment of GSH de novo synthesis as demonstrated by the impairment of the cysteine uptake transporters (EAAC1 in neurons) and the degradation of GCL by caspases. (5) Alterations in GSH/GSSG balance during apoptosis have been correlated with alterations in PSSG levels. In addition, other oxidative forms of GSH such as GSNO might also be formed by the direct interaction of GSH with distinct ROS/RNS. GCL, glutamate-cysteine ligase; GR, glutathione reductase; GSH, glutathione; GSSG, glutathione disulfide; GSNO, S-nitrosoglutathione; NADPH, nicotinamide adenine dinucleotide phosphate; PSSG, protein glutathionylated; ROS/RNS, reactive nitrogen species/reactive oxygen species.
GSH depletion during cell death progression has been largely ascribed to its oxidation in response to ROS/RNS formation. Indeed, during the apoptosis induced by cytotoxic agents, which by themselves induce oxidative stress such as pro-oxidants, xenobiotics, mitochondrial toxins, chemotherapeutics, and metals, GSH depletion is mediated by its oxidation to GSSG by ROS/RNS (61, 104, 169, 199, 237) (Table 1 and Fig. 2). Glutathione reductase (GR) reduces GSSG back to GSH using reduced nicotinamide adenine dinucleotide phosphate (NADPH) as the electron donor reductant, and glucose-6-phosphate dehydrogenase (G6PD) is indispensable for the regeneration of NADPH from NADP+ (Table 1 and Fig. 2). The depletion/oxidation of NADPH and the inactivation of G6PD occur during apoptotic cell death, which might impair GSH recycling and contribute to GSH depletion (54, 85, 88, 198, 255). Besides G6PD, other NADP+-dependent dehydrogenases can also regenerate NADPH in the cytoplasm, including the 6-phosphogluconate dehydrogenase, the cytosolic NADP+-dependent isocitrate dehydrogenase (IDPc), and the cytosolic NADP+-dependent malic enzyme. Knockdown of IDPc increases GSSG levels and augments the sensitivity of cells to cell death induced by oxidative stress (149).
Previous findings have shown that GCL is a direct target of caspase 3 (78, 79), which during apoptosis should not only prevent GSH replenishment but also contribute to GSH depletion, as GSH's half-life has been estimated to be between 2 and 5 h (18, 113, 207). Furthermore, the impairment of cysteine uptake during cell death induced by parkinsonian neurotoxins has also been suggested as contributing to GSH depletion (5) (Table 1 and Fig. 2).
Protein (S-)glutathionylation (PSSG, also known as [S-]glutathiolation) refers to the formation of a protein-mixed disulfide between the thiol group of GSH and a cysteine moiety of a protein. During cell death, increased PSSG has also been reported, which might also contribute to GSH depletion (1, 38, 57, 137, 201) (Table 1 and Fig. 2). GSH can also form other GSH derivatives on reaction with distinct ROS/RNS (Fig. 2). GSNO regulates apoptosis (72, 155, 174, 228), and a recent report suggests that released cytochrome C (Cyt C) during apoptosis has the ability to catalyze GSNO formation (17). GSNO is metabolized via the GSH-dependent formaldehyde dehydrogenase class III alcohol dehydrogenase, also known as GSNO reductase (GSNOR) (20). In thymus, GSNOR deficiency increases apoptosis, reducing the number of CD4 single-positive thymocytes (250).
GSH efflux also participates as a major contributor in the alterations of the cellular redox balance associated with cell death (Table 1 and Fig. 2). In addition, the formation of GSH-adducts by xenobiotics and electrophiles, and their subsequent extrusion by specific plasma membrane transporters, has also been reported to contribute to GSH depletion during apoptosis (23, 171, 185, 205, 244, 247) (Table 1 and Fig. 2). It is important to mention that multiple mechanisms are likely to participate in GSH loss during apoptosis (56, 61, 67). In the next section, we will review the mechanisms involved in GSH efflux during apoptosis.
Transport Mechanisms Involved in Plasma Membrane GSH Efflux During Cell Death
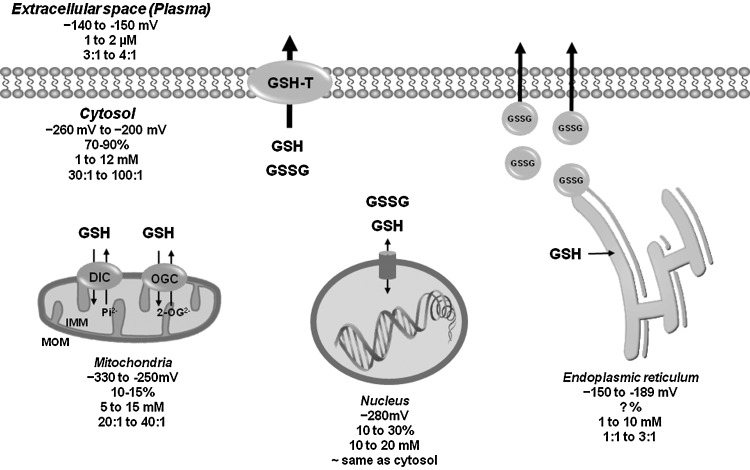
GSH is a ubiquitous tripeptide produced intracellularly that is not only 85%–90% freely distributed in the cytosol, but can also be found compartmentalized in mitochondria, peroxisomes, nuclear matrix, and endoplasmic reticulum (ER) (Table 1 and Fig. 3). Specific transport mechanisms have been evolved to maintain compartmentalized GSH/GSSG homeostasis. The concentration of mitochondrial GSH is similar to that of cytosol (10–14 mM). GSH can cross easily the outer mitochondrial membrane (OMM) through porin channels. A significant pool of GSH is compartmentalized in the mitochondria matrix by dicarboxylate carrier or 2-oxoglutarate transporters (OGC) [reviewed in this Forum and in Refs. (143, 166)] (Fig. 3). In contrast to mitochondria and cytosolic compartments, where GSH is predominantly found in its reduced form, in the ER, GSH exits mainly as GSSG acting as a source of oxidizing equivalents favoring disulfide bond formation for the proper folding of nascent proteins. Protein-dependent facilitated diffusion in the ER membrane is thought to mediate GSH permeation (Table 1 and Fig. 3). The ER is the initiating organelle of the secretory pathway, where secretory and membrane proteins are synthesized. In the cytosol, GSSG can be recycled back to GSH by GR or effluxed by specific transporters (discussed next). Since mitochondria lack a GSSG efflux mechanism, they rely on GR to counteract the pro-oxidant effects of GSSG. In contrast, the fate of the GSSG in the ER is unclear; it could be reduced within the ER by GR, transported to the cytosol for its reduction, or it could be secreted via the secretory pathway (6). Indeed, high levels of GSH have been found in secretory granules (112). GSSG extrusion through the secretory pathway can decrease GSH levels in the cell. However, if extracellular GSH is subject to recycle via the γ-glutamyl transpeptidase, its extrusion can promote cysteine recycling and de novo GSH synthesis. In this review, we focus only on the plasma membrane efflux mechanisms for GSH and GSSG and their role in cell death progression.
FIG. 3.
Compartmentalization of the GSH/GSSG redox couple. GSH is produced intracellularly and is found 70%–90% freely distributed in the cytosol, but also compartmentalized in mitochondria, nuclear matrix, and ER. Specific transport mechanisms maintain compartmentalized GSH/GSSG homeostasis. GSH diffuses through MOM via porin channels (not depicted here), and translocates through the IMM via DIC or OGC exchangers. In the nucleus, GSH is considered to diffuse freely through the nuclear pore. Protein-dependent facilitated diffusion is thought to mediate GSH permeation in the ER, but the molecular identify of the mechanism(s) involved remains unknown. Within the ER, GSH exits largely as GSSG due to its oxidation. It has been proposed that GSSG could be secreted via the secretory pathway for its recycle. A variety of protein transporters have been reported to act as plasma membrane GSH transporters (GSH-T), but their role in GSH depletion during cell death progression is still unclear. Values indicate redox potential for GSH/GSSG (mV), % of compartmentalized GSH with respect to total cellular levels, concentration of GSH (mM), and GSH/GSSG ratio for each subcellular compartment. Values were taken from (6, 95, 128, 212). IMM, inner mitochondrial membrane; DIC, dicarboxylate carrier; OGC, 2-oxoglutarate transporters.
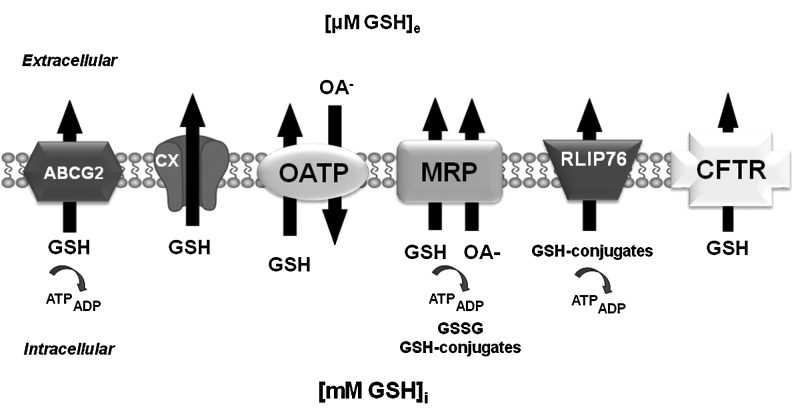
Apoptosis induced by distinct stimuli, particularly death receptors, has been reported to promote GSH depletion via the activation of a plasma membrane efflux transport (41, 67, 73, 90, 98, 106, 190, 220, 239). Inhibition of GSH depletion under these conditions rescues cells from apoptosis (73, 90, 96, 99). However, controversy still exists regarding the transport mechanism(s) involved in GSH depletion. A variety of protein transporters have been reported to act as GSH transporters (Table 1 and Fig. 4). Most studies to date have suggested that multidrug resistance proteins (MRPs) act as GSH efflux transporters during apoptosis (21, 67, 99, 106, 139). The human ATP-binding cassette (ABC) transporter, subfamily C (ABCC) subfamily of transporters contains 13 members from the ABC superfamily with sizes from 1325 to 1545 amino acids. The ABCC subfamily includes the cystic fibrosis transmembrane conductance regulator (CFTR, ABCC7), two sulfonylurea receptors SUR1 (ABCC8) and SUR2A/B (ABCC9), and nine MRPs. ABCC proteins are energy-dependent transporters, except for the CFTR that acts as a channel gated by ATP binding and hydrolysis, and SURs, which act as ATP-dependent potassium channel regulators. The MRP transporters have been demonstrated to act as cotransporters of organic anions (OA−) and GSH (12, 43). In addition, they also transport GSH-conjugated xenobiotics (GS-XN) and GSH-conjugated metabolites that must be exported to avoid deleterious effects. This efflux confers drug resistance to tumor cells and can protect normal cells from toxic insults. MRP1 functions as a GSH-conjugate transporter not only at the plasma membrane but also in intracellular secretory vesicles (240). The transport of organic anions, including drugs and conjugated OA−, by MRP, requires the hydrolysis of ATP (12, 43) (Table 1 and Fig. 4). Experimental conformation analysis has demonstrated that in solution, GSH is found as a mixture of different protonation states. Due to the presence of the two carboxylic acid groups, the thiol group, and the amino group, 16 different charged species of GSH with net charges ranging from +1 to −3 are found in solution. However, GSH has been found to possess a net negative charge of −1 at physiological pH (Fig. 1). Within GSH, the l-glutamic acid predominantly exists in its zwitterionic form, while the carboxyl group of the glycine fragment prefers to be deprotonated, and the cysteine moiety is in the neutral thiol form (142). Co-transport of two anions is an unusual mechanism. Thus, GSH transport by MRP1 transport might only target the GSH pool in a neutral or cationic state, which would explain the low affinity of MRP1 for GSH (see next). This pool could be slightly increased by acidification of the intracellular milieu during apoptosis (140). To date, there is no experimental evidence demonstrating that the co-transport of GSH and OA− molecules by MRP requires GSH to be in its anionic form. MRP1 can transport GSH alone, but this requires its stimulation by specific xenobiotics, for example, phenylalkylamines such as verapamil or bioflavonoids such as apigenin (43). Alterations in GSH levels reciprocally regulate MRP levels as shown by a recent report demonstrating that sustained GSH depletion prompts ubiquitin/proteosomal degradation of MRP2 (217).
FIG. 4.
Plasma membrane GSH efflux pumps. Distinct candidates have been proposed to act as GSH transporters. The MRPs act as ATP-dependent cotransporters of GSH (coupled to the extrusion of an OA−), GSSG, and GSH conjugates. MRP1 can transport GSH alone, but this requires its stimulation by xenobiotics. The OATPs were initially proposed to act as the GSH/OA− exchanger, where GSH efflux is thought to be driven by its electrochemical gradient across the plasma membrane, and stimulated by the presence of extracellular OA−. Other proposed candidates for GSH efflux are the members of the ABC family of transporter CFTR and BCRP/ABCG2, hemichannel connexins (CX), and RLIP76. Energy dependency of GSH transport by BCRP/ABCG2 has not yet been confirmed. ABC, ATP-binding cassette; MRP, multidrug resistance protein; OA−, organic anion; OATP, organic anion transporting polypeptide; RLIP76 (RALBP1), Ral-binding, Rho/Rac-GAP and Ral effector.
Pharmacological activation of MRPs induces apoptosis by GSH depletion (139, 197, 232, 235). However, contradictory results have been reported regarding the role of MRP1 in GSH efflux during apoptosis. We previously demonstrated that pharmacological inhibition of MRP1 with MK571 (10–50 μM) and probenecid (250–1000 μM) stimulated rather than inhibited GSH depletion and apoptosis induced by Fas ligand (FasL) (73). Interestingly, some reports have demonstrated that in some cell types, inhibitors of MRP1-mediated drug transport stimulate GSH-efflux via MRP1 (45, 177). Similarly, the inhibition (6.5–50 μM MK571) and genetic knockdown of MRP1 stimulates anti-Fas- and tumor necrosis factor-alpha (TNF-α)-induced apoptosis in human epithelial cells (25). In contrast, Hammond et al. (99), using the same experimental model (Jurkat lymphoid cells), reported that the inhibition of MRP1 using high concentrations of MK571 (75 μM) and probenecid (7 mM) resulted in a significant reduction of GSH loss induced by either intrinsic or extrinsic pathways (99). Unfortunately, neither of these inhibitors are specific, especially at high concentrations. In their study, probenecid, an MRP1 blocker with poor selectivity, almost completely abolished GSH depletion and apoptosis, while MK571, a more selective MRP1 inhibitor (15, 60), only marginally reduced GSH loss (99). Furthermore, although the authors demonstrated that siRNA knockdown of MRP1 decreased GSH loss induced by Fas activation, the effect of MRP1 knockdown on apoptosis was not evaluated (99).
Several other factors likely also contribute to the contradictory results presented by Hammond et al. (99) and in our study (73). Although in both studies GSH depletion and its extracellular accumulation were determined using the GSH recycling assay, we also corroborated our results with flow cytometry analysis, which allows the discrimination between dead cells, cellular debris, and cells at distinct stages during the apoptotic program. These studies represent a more accurate discrimination between early GSH loss (before the loss of plasma membrane integrity) and passive GSH depletion after the plasma membrane integrity has been compromised. In addition, some differences might exist regarding the signaling pathway triggered by the Fas receptor. While we used the physiological ligand (FasL) (73), Hammond et al. triggered apoptosis using anti-Fas antibodies (99), which do not reliably mimic FasL (114, 211). Finally, in a follow-up study, the same group recently reported that overexpression of MRP1 protects rather than stimulates Fas-induced apoptosis, contradicting their own published results (164).
In addition to GSH, GSSG has also been shown to be detoxified by its efflux across the plasma membrane through MRP transporters (41, 62, 109, 110, 129, 151, 177, 182, 188), suggesting that MRPs might play a role in the cellular response to oxidative stress. In fact, MRP1 affinity for GSSG (Km ∼100 μM) is significantly higher than that for GSH (Km ∼5–10 mM), which explains its protective role during apoptosis, as the accumulation of GSSG has deleterious effects in cells (43). GSSG directly induces or sensitizes cells to apoptosis by activation of stress-activated protein kinases JNK (c-jun-n-terminal kinase) and p38 (68, 70). A recent study demonstrates that MRP1 activity in retinal pigment epithelial cells mediates both GSH and GSSG efflux upon oxidative stress and that its inhibition protects against oxidative damage by facilitating the intracellular reduction of GSSG and preventing GSH depletion (221). In sickle cell disease erythorcytes, an increase in GSSG efflux by MRP1 is linked to GSH depletion and oxidative stress (188). Other MRP proteins have also been reported to mediate GSH and GSSG efflux, including MRP2, 4, and 5 (13, 206), but their role in apoptosis has not been studied (Table 1).
Bi-directional GSH/OA− has been reported in different cell types, including human cell lines (86, 118, 150, 152, 153, 186, 229), and organic anion transporting polypeptides (OATP) have been proposed to mediate GSH efflux by a GSH/OA− exchange (Table 1 and Fig. 4). GSH efflux by OATPs is stimulated by the presence of a wide range of structurally unrelated OA− substrates (trans-stimulation), demonstrating the wide nonspecificity of the OA− binding site in the OATP proteins. GSH is present at high concentrations within the cells (>1 mM), whereas blood plasma concentrations are at least two orders of magnitude lower (<0.01 mM). Furthermore, since GSH is negatively charged at physiological pH, there is a large negative intracellular potential (−30 to −60 mV) that facilitates its extrusion from the cell (12, 97). Since GSH transport by OATPs is driven by the outwardly directed electrochemical gradient across the plasma membrane, it is reversed by increases in the extracellular GSH concentration, demonstrating its bidirectionality. OATPs were initially reported to mediate this exchange transport (97, 152, 153). However recent studies suggest that GSH/OA− exchange is not mediated by this family of transporters (12, 29, 160). We previously proposed a role for an OATP-like transporter in GSH depletion based on the observation that not only a variety of structurally unrelated OA− stimulate GSH depletion, but also that GSH loss was paralleled by an increased uptake of OA− in the absence of plasma membrane permeabilization. However, there remains a possibility that GSH efflux and OA− uptake are also mediated by different and still uncharacterized molecular entities (73).
The CFTR has been suggested to mediate the transport of GSH during apoptosis (125). Recently, staurosporine-induced apoptosis and GSH/GSSG depletion (138), as well as cigarette smoke-induced GSH efflux in the lung were associated with CFTR activity (94) (Table 1 and Fig. 4). More recently, another ABC transporter, the subfamily G member 2 (BCRP/ABCG2), was identified in human epithelial cells as a GSH efflux transporter, but its role in apoptosis remains to be studied (28). In addition, it has been recently demonstrated that, in Candida albicans, the ABC transporter Cdrp1 mediates GSH depletion and apoptosis. Cdrp1 protein sequence shows a higher similarity to human BCRP/ABCG2 than other ABC transporters [BCRP/ABCG2>p-glycoprotein (ABCB1)>MRP1 (ABCC1)]. However, whether GSH depletion mediated by Cdrp1 is via efflux of the reduced or conjugated form of GSH has not been determined (256) (Table 1 and Fig. 4).
Several other proteins are proposed to mediate GSH transport. The organic anion transporter 3 (OAT3) has been suggested to mediate renal GSH transport (144) (Table 1). RLIP76 (RALBP1) is a 76 kDa Ral-binding, Rho/Rac-GAP, and Ral effector protein that was proposed to be a multispecific transporter of xenobiotics as well as GSH-conjugates with inherent ATPase activity (10) (Table 1 and Fig. 4). Connexins and glutamate/aspartate transporters (GLAST) have also been suggested to mediate the efflux of GSH in excitable cells (87, 204, 224, 225) (Table 1 and Fig. 4). Finally, cell swelling is reported to induce GSH depletion (148). Since volume-regulated/volume-sensitive organic osmolyte-anion channels (VRAC/VSOAC) are activated during apoptosis (27) (Table 1), GSH depletion might be mediated by these efflux pathways driven by the electrochemical gradient of GSH across the plasma membrane. Accordingly, we have recently demonstrated that GSH depletion regulates cell shrinkage during apoptosis (apoptotic volume decrease) and activation of ion fluxes (75).
It is clear that further studies are required to elucidate the molecular identity(ies) of the transporter(s) mediating GSH efflux during apoptosis. However, the study of GSH depletion by its efflux is hampered by the lack of more sensitive and accessible approaches to determine accumulation of extracellular GSH, as the GSH recycling assay commonly used to measure GSH and GSSG levels might not be sensitive enough (low μM detection limit) to accurately determine their presence extracellularly. In vitro, the extracellular medium is infinitely bigger compared with the intracellular space, and this would result in profound dilution of GSH levels (202). More sensitive methods to detect GSH and GSSG based on high-performance liquid chromatography combined with mass spectrometry analysis could provide a better means for evaluating GSH accumulation in the extracellular milieu, but the application of these approaches is limited by their accessibility (180).
Redox Signaling, GSH Depletion, and Cell Death Progression
GSH content is a determinant of cell death progression. Several studies have demonstrated that high intracellular GSH levels are associated with apoptotic-resistant phenotypes in several models of apoptosis (33, 80), while by itself, GSH depletion either induces or stimulates apoptosis (4, 9, 165). Conversely, GSH supplementation prevents the apoptosis induced by distinct stimuli (32, 46, 73, 76, 133). GSH depletion induced by inhibition of the GCL potentiates death receptor-induced apoptosis in T-cells (9, 80), but by itself, it does not trigger cell death. However, this might be attributed to the observation that pharmacological inhibition of GCL depletes only the cytosolic GSH pool, having little effect on mitochondrial GSH (91, 241, 253). The precise contribution of cytosolic versus mitochondrial GSH pools in apoptosis is not fully understood, although some reports suggest that apoptosis correlates directly with cytosolic rather than with mitochondrial GSH depletion (245). In contrast, other studies have shown that mitochondrial GSH depletion is essential in triggering the cell death cascade [reviewed in this Forum and in Refs. (143, 166)]. Another explanation to why GSH depletion might not induce cell death in some cell types is given by reports demonstrating that prolonged GSH depletion up-regulates antiapoptotic proteins such as B-cell lymphoma 2 (Bcl-2), heat shock proteins, and nuclear factor-kappa B (NF-κB) (47, 69, 236), as well as other antioxidant systems, including the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and heme oxygenase-1 that might inhibit cell death progression (41, 81, 108, 147, 183). Interestingly, excessive GSH overload has also recently been shown to mediate mitochondrial toxicity and cell death by reductive stress (254).
The signaling pathways that regulate the progression of apoptosis have been extensively studied and characterized (Fig. 5). Induction of apoptosis via the extrinsic pathway is triggered by the activation of the death receptors Fas (CD95/Apo-1), TNF-related apoptosis-inducing ligand (TRAIL) receptors 1 and 2 (DR4/DR5), and TNF receptor 1 (TNFR1) by their respective ligands FasL, TRAIL, and TNF-α. Activation of death receptors leads to the formation of the death-inducing signaling complex, which includes the Fas-associated death domain (FADD), initiator caspase 8 or 10, and the cellular FADD-like interleukin-1 beta-converting enzyme (FLICE)-inhibitory protein (FLIP). In contrast, TNFR1 signaling results in the formation of two signaling complexes. TNF-induced complex I formation lacks FADD and pro-caspase 8, but induces the recruitment of the receptor-interacting protein (RIP), TNFR-associated death domain protein (TRADD), and TNFR-associated factor (TRAF)-1/2, which translocate to the cytosol where FADD, caspase 8/10, and FLIP are recruited to form the traddosome or complex II, leading to the activation of initiator caspases (145). Activation of NF-κB antagonizes programmed cell death induced by TNFR1, and GSH depletion has been shown to down-regulate TNF-induced NF-κB activation and sensitize hepatocytes to apoptotic cell death (157).
FIG. 5.
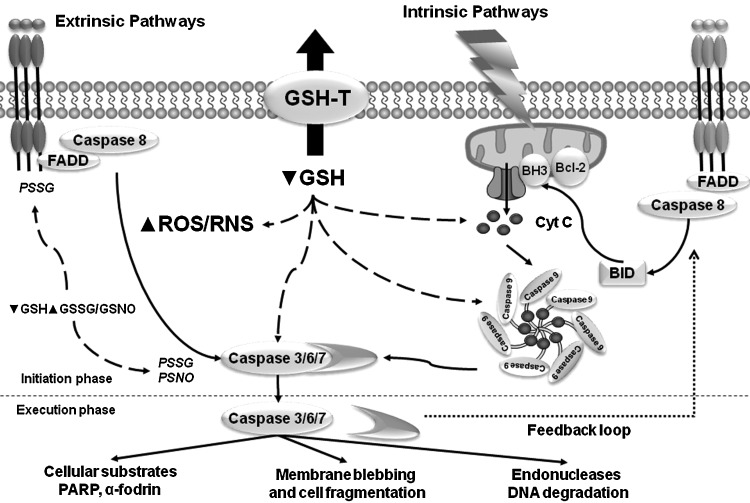
Molecular mechanisms involved in the regulation of apoptosis by GSH. GSH depletion regulates cell death progression by apoptosis through a variety of mechanisms. GSH depletion triggers the permeability transition pore of the mitochondria, the pro-apoptotic function of released Cyt C, the formation of the apoptosome, and the activation of executioner caspases. Furthermore, GSH depletion precedes oxidative stress and is necessary for ROS/RNS formation. Alterations in GSH, GSSG, GSNO, and ROS/RNS homeostasis can modify the levels of PSSG/PSNO residues. Aggregation of death receptors and caspase activation has been demonstrated to be regulated by protein glutathionylation and nitros(yl)ation. Cyt C, cytochrome C; BH3, Bcl-2 homology 3 ; Bcl-2, B-cell lymphoma 2; Bid, BH3 interacting-domain death agonist; FADD, Fas-associated death domain; GSNO, S-nitroglutathione; PSSG, protein glutathionylation; PSNO, protein nitros(yl)ation; ROS/RNS, reactive oxygen and nitrogen species.
The extrinsic/death receptor pathway has the ability to crosstalk to the intrinsic pathway of apoptosis by an amplification loop induced by caspase-dependent cleavage of the Bcl-2-family protein BH3 (Bcl-2 homology 3) interacting-domain death agonist (Bid), which translocates to the mitochondria and promotes the release of Cyt C. The intrinsic pathway of apoptosis is activated by a wide variety of stimuli, including chemotherapeutic/cytotoxic agents (environmental pollutants, xenobiotics, and drugs), stress (radiation, hyperglycemia, hypoxia, oxidative and osmotic stress), and cytokine withdrawal. Activation of the mitochondria pathway mediates the release of Cyt C that is regulated by the Bcl-2 protein family. The BH3-only Bcl-2 family members Bcl-2-associated death promoter (Bad), Bid, Bcl-2-like protein 11 (Bim), NOXA, and p53 upregulated modulator of apoptosis (PUMA) regulate the antiapoptotic Bcl-2 proteins Bcl-2 and Bcl-xl (B-cell lymphoma-extra large) to promote apoptosis. Bcl-2 and Bcl-xl inhibit Bcl-2 associated X protein (Bax) and Bcl-2 homologous antagonist/killer (Bak), and activation of BH3-only proteins derepresses Bax and Bak by direct inhibition of Bcl-2 and Bcl-xl. Bax and Bak are crucial for inducing the permeabilization of the OMM and the release of Cyt C. Subsequently, released Cyt C leads to the recruitment of Apaf1 into an apoptosome and activates caspase 9 (37).
GSH depletion is necessary for the formation of the apoptosome (210) and also triggers cell death by modulation of the permeability transition pore of the mitochondria and the activation of executioner caspases (3, 8, 42, 189, 238, 241) (Fig. 5). In addition, GSH depletion activates the intrinsic apoptotic pathway initiator Bax and Cyt C release (49, 96) (Fig. 5). Released Cyt C requires cytosolic GSH levels to be depleted for its pro-apoptotic action (31, 89, 103, 194). Depletion of intracellular GSH also overcomes Bcl-2-mediated resistance to apoptosis (8, 208). The antiapoptotic role of Bcl-2 has been linked to GSH content by several studies, where it was reported that Bcl-2 regulates GSH content and distribution in different cellular compartments (121, 126, 242). Bcl-2 overexpression also reduces GSH efflux, but the mechanism involved remains unclear (191, 192). A recent study suggests that Bcl-2 regulates mitochondrial GSH content by a direct interaction of the BH3 groove with GSH (257), while the antiapoptotic effect of Bcl-xl has also been attributed to the regulation of GSH homeostasis by preventing GSH loss (26). However, these effects appear to be cell-type specific and context -dependent (175, 214, 231).
GSH depletion might also be a prerequisite for oxidative stress and the activation of cell death pathways. By itself, GSH depletion promotes nistrosative stress and cell death, suggesting an important role of basal GSH levels in the maintenance of a homeostatic reductive environment and the buffering of ROS/RNS (7). GSH depletion occurs at earlier stages of the cell death program and is followed by a delayed accumulation of ROS, which requires GSH depletion (48, 76, 139). GSH depletion by its efflux has been shown to be independent from oxidative stress and ROS generation (76, 96). We and others have recently shown that GSH depletion is necessary for the generation of ROS during FasL-induced apoptosis (76, 139, 156), and that GSH content, but not the excess in ROS formation and oxidative stress, regulates apoptosis induced by Fas activation (76) (Fig. 5). Other studies have also shown that apoptosis seems to be actively regulated by GSH content and not by excessive oxidative stress and ROS generation (53, 101, 198). The role of ROS/RNS in apoptosis has been extensively studied (40, 209), and several GSH-dependent antioxidant enzymes protect cells from undergoing programmed cell death. However, protective effects of thiol compounds on apoptosis in the absence of excessive ROS formation are also observed (53, 102). Ceramide accumulation is induced by different pro-apoptotic signals, including Fas ligation, irradiation, and anticancer drugs. A recent report shows that GSH depletion independent of ROS mediates ceramide generation and apoptosis by inhibition of sphingomyelin synthase, which converts ceramide to sphingomyelin (134).
GSH catalytically detoxifies cells from peroxides such as H2O2, OONO−, and lipid peroxides (LOO•) by the action of GSH peroxidases (GPx), leading to the accumulation of GSSG (Figs. 2 and 6). The accumulation of GSSG upon oxidative stress has been observed to be toxic to the cell (68, 70). GPx has been shown to protect against apoptosis induced by Fas activation (92). However, death receptor- (Fas and TNF) induced cell death was shown to be similar in animals deficient in GPx compared with WT (11). GPx also protects against apoptosis induced by oxidative stress (127), ischemia/reperfusion injury (44), and doxorubicin (93), and reduces pro-apoptotic Bax expression (65). Phospholipid hydroperoxide glutathione peroxidase (PHGPx or GPx4) directly reduces phospholipid hydroperoxides. GPx4 overexpression has also been reported to protect against oxidative-stress induced apoptosis by preventing cardiolipin oxidation and Cyt C oxidation (154, 203), while its down-regulation induces apoptosis-inducing factor (AIF)-mediated cell death (216). Overexpression of the mitochondrial GPx4 was also shown to protect against apoptosis induced by the intrinsic mitochondrial pathway by reducing mitochondrial hydroperoxide accumulation (187).
FIG. 6.
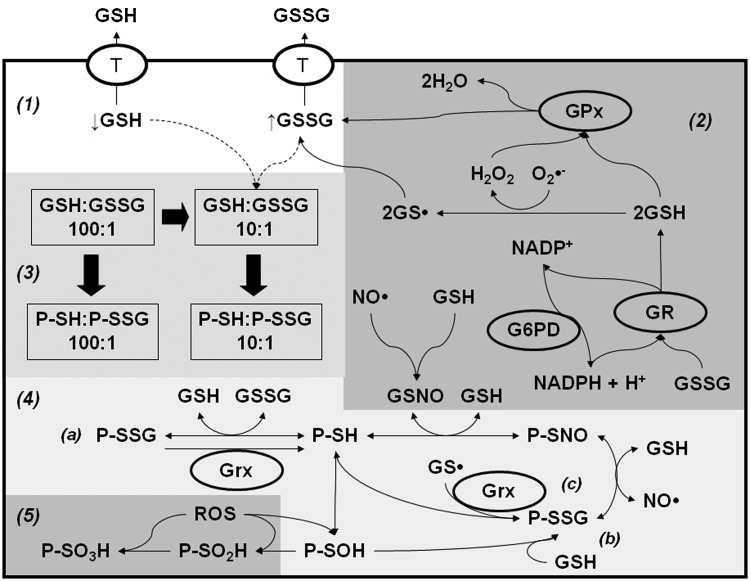
Redox alterations induced by GSH/GSSG transport. (1) Several protein transporters have been proposed to mediate GSH/GSSG transport, which in fact, can significantly impact cellular redox balance. (2) GSH levels maintain a reduced intracellular environment, even under normal conditions as evidenced by observations that by itself GSH depletion induces oxidative stress. GSH directly scavenges ROS/RNS or enzymatically, through the GPx/GR/NADPH/G6PD system. Thus, GSH efflux sensitizes cells to oxidative stress, while GSSG transport can serve as a protective mechanism. (3) Changes in the GSH:GSSG ratio directly result in alterations of oxidative post-translational modifications in protein thiols (PSH). (4) Both GSH and GSSG have the ability to promote PSSG formation via (a) GSSG reaction with PSH, (b) GSH reaction with PSOHs, the most commonly accepted mechanism, and (c) Grx-mediated transfer of thiyl radicals (GS•) to PSH residues. (5) PSSGs are known to regulate enzyme function and activity (redox signaling) and protect cysteines from irreversible oxidation to PSO2H and PSO3H residues, and subsequent degradation. Gpx, glutathione peroxidase; GR, glutathione reductase; G6PD, glucose-6-phosphate dehydrogenase; Grx, glutaredoxin; NADPH, nicotinamide adenine dinucleotide phosphate; PSOH, protein sulfenic acid; PSO3H, protein sulfonic acids; PSO2H, protein sulfinic acids.
As indicated previously, another fate of GSH during oxidative stress and apoptosis is the formation of mixed disulfides with protein cysteines or PSSG. Since this subject is also reviewed in detail in this Forum, we will only briefly describe some major findings in this area. GSH depletion induced by oxidative stress, or by its active efflux across the plasma membrane, exerts prefunds alterations in the GSH/GSSG redox balance that might regulate PSSG levels (Fig. 6). Both GSSG and GSH can induce PSSG formation, depending on the oxidized/reduced status of the cysteine residue and the redox potential of the protein. Apoptosis is accompanied by increased PSSG formation (1, 57, 227). TNF-α-induced apoptosis is reported to be paralleled by increased PSSG formation, which is inhibited by overexpression of Bcl-2 (227). Loss or suppression of NF-κB enhances sensitivity to apoptosis. Glutathionylation of NF-κB inhibits its DNA-binding capacity and enhances apoptosis induced by hypoxic conditions (201). FasL-induced apoptosis has also been reported to increase PSSG, which amplifies the apoptotic signaling cascade by glutathionylation of the Fas receptor (1) (Fig. 5). In contrast, caspases can be glutathionylated under basal conditions and become de-glutathionylated upon the induction of apoptosis (193) (Fig. 5). GSSG is commonly viewed as a byproduct of GSH metabolism, which is either recycled to GSH o or exported out of the cell (Fig. 6). However, pathophysiological significance of GSSG per se remains poorly studied. An early and transient rise in intracellular GSSG has been shown to precede Cyt C release and caspase 3 activation (39, 199). Interestingly, GSSG-induced caspase 3 glutathionylation inhibits its enzyme activity (117). A recent report shows that GSSG-induced toxicity is mediated by 12-lipoxygenase (12-LOX) activation via its glutathionylation (195). GSH depletion and GPx4 down-regulation induce cell death by the activation of 12-LOX (35, 146, 216, 243).
PSSG reductases glutaredoxins (Grxs) have been demonstrated to protect against apoptosis by decreasing PSSG formation. In contrast, knockdown of Grx1 significantly inhibits TNF-α-induced cell death via increased glutathionylation of caspase 3 and impaired activation of the enzyme (111, 193). GSNO is a well-known inducer of protein nitros(yl)ation (PSNO) (Fig. 6), which regulates apoptosis (161, 162, 173). Caspases have been shown to be nitrosylated under basal conditions, and their de-nitros(yl)ation is required for their activation during apoptosis (132, 163, 178). In addition, several other proteins whose signal transduction cascades modulate apoptosis have been demonstrated to be regulated by nitros(yl)ation including Bcl-2 and FLIP (34, 59).
Most of the evidence regarding the role of GSH in the activation of cell death pathways refers to apoptotic signaling cascades. However, recent reports also suggest a protective role of GSH in cell death processes other than apoptosis. For example, N-acetyl-L-cysteine (NAC) has been shown to prevent ROS-induced formation of autophagosomes and the subsequent degradation of proteins during starvation-induced autophagy (213). Lipopolysaccharide-induced autophagy is paralleled by ROS formation and GSH depletion, which was also prevented by NAC (251). Treatment with γ-glutamylcysteinyl ethyl ester, a precursor of de novo GSH formation, decreases autophagy after traumatic brain injury (141). Excessive GSH depletion and oxidative stress have been reported to switch apoptosis to necrotic cell death (66, 158, 233, 234). GSH-depleting agents at doses that decrease mitochondrial GSH levels induce necrosis. However, modest doses of these agents resulting in selective cytoplasmic GSH depletion sensitize hepatocytes to TNF-α -induced apoptosis (100, 168, 184). Ceramide has been implicated as a secondary messenger for TNF-α-induced cell necrosis, and NAC or GSH-monoethylester can delay the onset of ceramide-induced necrosis (52). Recently, necrostatin-1, an inhibitor of programmed cell necrosis or necroptosis, was shown to inhibit cell death in mouse hippocampal cells induced by GSH depletion (248).
Conclusions and Perspectives
GSH depletion has been observed to occur at early stages during the cell death progression. Although GSH depletion was initially associated mainly to its oxidation by ROS/RNS generated during oxidative stress, it is now recognized that GSH depletion occurs by a variety of distinct mechanisms. GSH depletion by its efflux has been described as an active process that in many cases is independent from oxidative stress and precedes ROS accumulation. More importantly, GSH depletion has also been demonstrated to directly regulate the cell death machinery independently from ROS accumulation and oxidative damage. Several protein transport mechanisms have been proposed to mediate GSH efflux, but controversy still exists regarding its role in GSH depletion during apoptosis. The understanding and identification of GSH tranpsorters involved in GSH depletion is hampered by the lack of sensitive and accessible approaches to determine extracellular GSH accumulation. More research is necessary to accurately determine the transporter or transporter entities regulating GSH depletion during cell death, and the signaling mechanisms regulating/activating them.
Abbreviations Used
- 12-LOX
12-lipoxygenase (EC 1.13.11.31)
- ABC
ATP-binding cassette
- ABCC
ATP-binding cassette (ABC) transporter, subfamily C
- ABCG2
ATP-binding cassette (ABC) transporter, subfamily G member 2
- AIF
apoptosis-inducing factor
- Bad
Bcl-2-associated death promoter
- Bak
Bcl-2 homologous antagonist/killer
- Bax
Bcl-2 associated X protein
- Bcl-2
B-cell lymphoma 2
- Bcl-xl
B-cell lymphoma-extra large
- BCRP
breast cancer resistance protein
- BH3
Bcl-2 homology 3
- Bid
BH3 interacting-domain death agonist
- Bim
Bcl-2 like protein 11
- Caspases
cysteine-dependent aspartate-directed proteases
- CFTR
cystic fibrosis transmembrane conductance regulator
- Cyt C
cytochrome C
- DIC
dicarboxylate carrier
- DR4
TRAIL receptor 1
- DR5
TRAIL receptor 2
- ER
endoplasmic reticulum
- FADD
Fas-associated death domain
- FasL
Fas ligand
- FLICE
FADD-like interleukin-1 beta-converting enzyme
- FLIP
FLICE-inhibitory protein
- G6PD
glucose-6-phosphate dehydrogenase (EC 1.1.1.49)
- GCL
glutamate-cysteine ligase (EC 6.3.2.2)
- GLAST
glutamate/aspartate transporter
- GPx
glutathione peroxidase (EC 1.11.1.9)
- GPx4
phospholipid hydroperoxide glutathione peroxidase or PHGPx (EC 1.11.1.12)
- GR
glutathione reductase (EC 1.8.1.7)
- Grx
glutaredoxin (EC 1.20.4.1)
- GS(O)2SG
glutathione disulfide S-dioxide
- GS(O)SG
glutathione disulfide S-oxide
- GS•
glutathionyl radical
- GSH
glutathione
- GSNHOH
glutathione N-hydroxysulfenamide
- GSNHSG
glutathione thiosulfenamide
- GSNO
S-nitrosoglutathione
- GSNOR
GSH-dependent formaldehyde dehydrogenase class III alcohol dehydrogenase (ADH3) or GSNO reductase (EC 1.1.1.284)
- GSO2H
glutathione sulfinic acid
- GSO3H
glutathione sulfonic acid
- GSOH
glutathione sulfenic acid
- GSOO•
thiyl peroxyl radical
- GSSG
glutathione disulfide
- GST
glutathione-S-transferases
- H2O2
hydrogen peroxide
- IDPc
cytosolic NADP+-dependent isocitrate dehydrogenase
- IMM
inner mitocondrial membrane
- LOO•
lipid peroxides
- MRP
multidrug resistance protein
- N2O3
dinitrogen trioxide
- NAC
N-acetyl-L-cysteine
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor-kappa B
- NO•
nitric oxide
- NO2
nitrogen dioxide
- Nrf2
nuclear factor (erythroid-derived 2)-like 2
- O2•−
superoxide anion
- OATP
organic anion transporting polypeptides
- OA−
organic anion
- OGC
2-oxoglutarate transporters
- OMM
outer mitochondrial membrane
- ONOO−
peroxynitrite
- PHGPx or GPx4
phospholipid hydroperoxide glutathione peroxidase
- PSNO
protein nitros(yl)ation
- PSO2H
protein sulfinic acids
- PSO3H
protein sulfonic acids
- PSOH
protein sulfenic acid
- PSSG
protein glutathionylated
- PUMA
p53 upregulated modulator of apoptosis
- RIP
receptor-interacting protein
- RLIP76 (RALBP1)
Ral-binding, Rho/Rac-GAP and Ral effector
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- TNF-α
tumor necrosis factor-alpha
- TNFR
TNF receptor 1
- TRADD
TNFR-associated death domain protein
- TRAF
TNFR-associated factor
- TRAIL
TNF-related apoptosis-inducing ligand
- VRAC/VSOAC
volume-regulated/volume-sensitive organic osmolyte-anion channels
Acknowledgments
This work was supported by the Intramural Research Program of the NIH/National Institute of Environmental Health Sciences 1Z01ES090079 (J.A.C.), P20RR17675 Centers of Biomedical Research Excellence (COBRE), and an Interdisciplinary Grant from the Research Council and the Life Sciences Grant Program of the University of Nebraska-Lincoln (R.F).
References
- 1.Anathy V. Aesif SW. Guala AS. Havermans M. Reynaert NL. Ho YS. Budd RC. Janssen-Heininger YM. Redox amplification of apoptosis by caspase-dependent cleavage of glutaredoxin 1 and S-glutathionylation of Fas. J Cell Biol. 2009;184:241–252. doi: 10.1083/jcb.200807019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anathy V. Roberson EC. Guala AS. Godburn KE. Budd RC. Janssen-Heininger YM. Redox-based regulation of apoptosis: S-glutathionylation as a regulatory mechanism to control cell death. Antioxid Redox Signal. 2012;16:496–505. doi: 10.1089/ars.2011.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aon MA. Cortassa S. Maack C. O'Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem. 2007;282:21889–21900. doi: 10.1074/jbc.M702841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoshiba K. Yasui S. Nishimura K. Nagai A. Thiol depletion induces apoptosis in cultured lung fibroblasts. Am J Respir Cell Mol Biol. 1999;21:54–64. doi: 10.1165/ajrcmb.21.1.3411. [DOI] [PubMed] [Google Scholar]
- 5.Aoyama K. Matsumura N. Watabe M. Nakaki T. Oxidative stress on EAAC1 is involved in MPTP-induced glutathione depletion and motor dysfunction. Eur J Neurosci. 2008;27:20–30. doi: 10.1111/j.1460-9568.2007.05979.x. [DOI] [PubMed] [Google Scholar]
- 6.Appenzeller-Herzog C. Glutathione- and non-glutathione-based oxidant control in the endoplasmic reticulum. J Cell Sci. 2011;124:847–855. doi: 10.1242/jcs.080895. [DOI] [PubMed] [Google Scholar]
- 7.Aquilano K. Baldelli S. Cardaci S. Rotilio G. Ciriolo MR. Nitric oxide is the primary mediator of cytotoxicity induced by GSH depletion in neuronal cells. J Cell Sci. 2011;124:1043–1054. doi: 10.1242/jcs.077149. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong JS. Jones DP. Glutathione depletion enforces the mitochondrial permeability transition and causes cell death in Bcl-2 overexpressing HL60 cells. FASEB J. 2002;16:1263–1265. doi: 10.1096/fj.02-0097fje. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong JS. Steinauer KK. Hornung B. Irish JM. Lecane P. Birrell GW. Peehl DM. Knox SJ. Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death Differ. 2002;9:252–263. doi: 10.1038/sj.cdd.4400959. [DOI] [PubMed] [Google Scholar]
- 10.Awasthi S. Singhal SS. Sharma R. Zimniak P. Awasthi YC. Transport of glutathione conjugates and chemotherapeutic drugs by RLIP76 (RALBP1): a novel link between G-protein and tyrosine kinase signaling and drug resistance. Int J Cancer. 2003;106:635–646. doi: 10.1002/ijc.11260. [DOI] [PubMed] [Google Scholar]
- 11.Bajt ML. Ho YS. Vonderfecht SL. Jaeschke H. Reactive oxygen as modulator of TNF and fas receptor-mediated apoptosis in vivo: studies with glutathione peroxidase-deficient mice. Antioxid Redox Signal. 2002;4:733–740. doi: 10.1089/152308602760598873. [DOI] [PubMed] [Google Scholar]
- 12.Ballatori N. Hammond CL. Cunningham JB. Krance SM. Marchan R. Molecular mechanisms of reduced glutathione transport: role of the MRP/CFTR/ABCC and OATP/SLC21A families of membrane proteins. Toxicol Appl Pharmacol. 2005;204:238–255. doi: 10.1016/j.taap.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Ballatori N. Krance SM. Marchan R. Hammond CL. Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology. Mol Aspects Med. 2009;30:13–28. doi: 10.1016/j.mam.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballatori N. Krance SM. Notenboom S. Shi S. Tieu K. Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009;390:191–214. doi: 10.1515/BC.2009.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baltes S. Fedrowitz M. Tortos CL. Potschka H. Loscher W. Valproic acid is not a substrate for P-glycoprotein or multidrug resistance proteins 1 and 2 in a number of in vitro and in vivo transport assays. J Pharmacol Exp Ther. 2007;320:331–343. doi: 10.1124/jpet.106.102491. [DOI] [PubMed] [Google Scholar]
- 16.Barrett WC. DeGnore JP. Konig S. Fales HM. Keng YF. Zhang ZY. Yim MB. Chock PB. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry. 1999;38:6699–6705. doi: 10.1021/bi990240v. [DOI] [PubMed] [Google Scholar]
- 17.Basu S. Keszler A. Azarova NA. Nwanze N. Perlegas A. Shiva S. Broniowska KA. Hogg N. Kim-Shapiro DB. A novel role for cytochrome c: efficient catalysis of S-nitrosothiol formation. Free Radic Biol Med. 2010;48:255–263. doi: 10.1016/j.freeradbiomed.2009.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baudouin-Cornu P. Lagniel G. Kumar C. Huang ME. Labarre J. Glutathione degradation is a key determinant of glutathione homeostasis. J Biol Chem. 2012;287:4552–4561. doi: 10.1074/jbc.M111.315705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beer SM. Taylor ER. Brown SE. Dahm CC. Costa NJ. Runswick MJ. Murphy MP. Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: implications for mitochondrial redox regulation and antioxidant DEFENSE. J Biol Chem. 2004;279:47939–47951. doi: 10.1074/jbc.M408011200. [DOI] [PubMed] [Google Scholar]
- 20.Benhar M. Forrester MT. Stamler JS. Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat Rev Mol Cell Biol. 2009;10:721–732. doi: 10.1038/nrm2764. [DOI] [PubMed] [Google Scholar]
- 21.Benlloch M. Ortega A. Ferrer P. Segarra R. Obrador E. Asensi M. Carretero J. Estrela JM. Acceleration of glutathione efflux and inhibition of gamma-glutamyltranspeptidase sensitize metastatic B16 melanoma cells to endothelium-induced cytotoxicity. J Biol Chem. 2005;280:6950–6959. doi: 10.1074/jbc.M408531200. [DOI] [PubMed] [Google Scholar]
- 22.Biswas SK. Rahman I. Environmental toxicity, redox signaling and lung inflammation: the role of glutathione. Mol Aspects Med. 2009;30:60–76. doi: 10.1016/j.mam.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blair IA. Endogenous glutathione adducts. Curr Drug Metab. 2006;7:853–872. doi: 10.2174/138920006779010601. [DOI] [PubMed] [Google Scholar]
- 24.Blair IA. Analysis of endogenous glutathione-adducts and their metabolites. Biomed Chromatogr. 2010;24:29–38. doi: 10.1002/bmc.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blokzijl H. van Steenpaal A. Vander Borght S. Bok LI. Libbrecht L. Tamminga M. Geuken M. Roskams TA. Dijkstra G. Moshage H. Jansen PL. Faber KN. Upregulation and cytoprotective role of epithelial multidrug resistance-associated protein 1 in inflammatory bowel disease. J Biol Chem. 2008;283:35630–35637. doi: 10.1074/jbc.M804374200. [DOI] [PubMed] [Google Scholar]
- 26.Bojes HK. Datta K. Xu J. Chin A. Simonian P. Nunez G. Kehrer JP. Bcl-xL overexpression attenuates glutathione depletion in FL5.12 cells following interleukin-3 withdrawal. Biochem J. 1997;325(Pt 2):315–319. doi: 10.1042/bj3250315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bortner CD. Cidlowski JA. The role of apoptotic volume decrease and ionic homeostasis in the activation and repression of apoptosis. Pflugers Arch. 2004;448:313–318. doi: 10.1007/s00424-004-1266-5. [DOI] [PubMed] [Google Scholar]
- 28.Brechbuhl HM. Gould N. Kachadourian R. Riekhof WR. Voelker DR. Day BJ. Glutathione transport is a unique function of the ATP-binding cassette protein ABCG2. J Biol Chem. 2010;285:16582–16587. doi: 10.1074/jbc.M109.090506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Briz O. Romero MR. Martinez-Becerra P. Macias RI. Perez MJ. Jimenez F. San Martin FG. Marin JJ. OATP8/1B3-mediated cotransport of bile acids and glutathione: an export pathway for organic anions from hepatocytes? J Biol Chem. 2006;281:30326–30335. doi: 10.1074/jbc.M602048200. [DOI] [PubMed] [Google Scholar]
- 30.Broniowska KA. Keszler A. Basu S. Kim-Shapiro DB. Hogg N. Cytochrome c-mediated formation of S-nitrosothiol in cells. Biochem J. 2012;442:191–197. doi: 10.1042/BJ20111294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown GC. Borutaite V. Regulation of apoptosis by the redox state of cytochrome c. Biochim Biophys Acta. 2008;1777:877–881. doi: 10.1016/j.bbabio.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 32.Bush JA. Ho VC. Mitchell DL. Tron VA. Li G. Effect of N-acetylcysteine on UVB-induced apoptosis and DNA repair in human and mouse keratinocytes. Photochem Photobiol. 1999;70:329–333. [PubMed] [Google Scholar]
- 33.Cazanave S. Berson A. Haouzi D. Vadrot N. Fau D. Grodet A. Letteron P. Feldmann G. El-Benna J. Fromenty B. Robin MA. Pessayre D. High hepatic glutathione stores alleviate Fas-induced apoptosis in mice. J Hepatol. 2007;46:858–868. doi: 10.1016/j.jhep.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Chanvorachote P. Nimmannit U. Wang L. Stehlik C. Lu B. Azad N. Rojanasakul Y. Nitric oxide negatively regulates Fas CD95-induced apoptosis through inhibition of ubiquitin-proteasome-mediated degradation of FLICE inhibitory protein. J Biol Chem. 2005;280:42044–42050. doi: 10.1074/jbc.M510080200. [DOI] [PubMed] [Google Scholar]
- 35.Chen CJ. Huang HS. Chang WC. Depletion of phospholipid hydroperoxide glutathione peroxidase up-regulates arachidonate metabolism by 12S-lipoxygenase and cyclooxygenase 1 in human epidermoid carcinoma A431 cells. FASEB J. 2003;17:1694–1696. doi: 10.1096/fj.02-0847fje. [DOI] [PubMed] [Google Scholar]
- 36.Chiang HS. Maric M. Lysosomal thiol reductase negatively regulates autophagy by altering glutathione synthesis and oxidation. Free Radic Biol Med. 2011;51:688–699. doi: 10.1016/j.freeradbiomed.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 37.Chipuk JE. Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chrestensen CA. Starke DW. Mieyal JJ. Acute cadmium exposure inactivates thioltransferase (Glutaredoxin), inhibits intracellular reduction of protein-glutathionyl-mixed disulfides, and initiates apoptosis. J Biol Chem. 2000;275:26556–26565. doi: 10.1074/jbc.M004097200. [DOI] [PubMed] [Google Scholar]
- 39.Circu ML. Aw TY. Glutathione and apoptosis. Free Radic Res. 2008;42:689–706. doi: 10.1080/10715760802317663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Circu ML. Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Circu ML. Stringer S. Rhoads CA. Moyer MP. Aw TY. The role of GSH efflux in staurosporine-induced apoptosis in colonic epithelial cells. Biochem Pharmacol. 2009;77:76–85. doi: 10.1016/j.bcp.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coffey RN. Watson RW. Hegarty NJ. O'Neill A. Gibbons N. Brady HR. Fitzpatrick JM. Thiol-mediated apoptosis in prostate carcinoma cells. Cancer. 2000;88:2092–2104. doi: 10.1002/(sici)1097-0142(20000501)88:9<2092::aid-cncr15>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 43.Cole SP. Deeley RG. Transport of glutathione and glutathione conjugates by MRP1. Trends Pharmacol Sci. 2006;27:438–446. doi: 10.1016/j.tips.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Crack PJ. Taylor JM. Flentjar NJ. de Haan J. Hertzog P. Iannello RC. Kola I. Increased infarct size and exacerbated apoptosis in the glutathione peroxidase-1 (Gpx-1) knockout mouse brain in response to ischemia/reperfusion injury. J Neurochem. 2001;78:1389–1399. doi: 10.1046/j.1471-4159.2001.00535.x. [DOI] [PubMed] [Google Scholar]
- 45.Cullen KV. Davey RA. Davey MW. Verapamil-stimulated glutathione transport by the multidrug resistance-associated protein (MRP1) in leukaemia cells. Biochem Pharmacol. 2001;62:417–424. doi: 10.1016/s0006-2952(01)00681-5. [DOI] [PubMed] [Google Scholar]
- 46.Custodio JB. Cardoso CM. Almeida LM. Thiol protecting agents and antioxidants inhibit the mitochondrial permeability transition promoted by etoposide: implications in the prevention of etoposide-induced apoptosis. Chem Biol Interact. 2002;140:169–184. doi: 10.1016/s0009-2797(02)00020-0. [DOI] [PubMed] [Google Scholar]
- 47.D'Alessio M. Cerella C. Amici C. Pesce C. Coppola S. Fanelli C. De Nicola M. Cristofanon S. Clavarino G. Bergamaschi A. Magrini A. Gualandi G. Ghibelli L. Glutathione depletion up-regulates Bcl-2 in BSO-resistant cells. FASEB J. 2004;18:1609–1611. doi: 10.1096/fj.04-1813fje. [DOI] [PubMed] [Google Scholar]
- 48.D'Alessio M. Cerella C. De Nicola M. Bergamaschi A. Magrini A. Gualandi G. Alfonsi AM. Ghibelli L. Apoptotic GSH extrusion is associated with free radical generation. Ann N Y Acad Sci. 2003;1010:449–452. doi: 10.1196/annals.1299.082. [DOI] [PubMed] [Google Scholar]
- 49.D'Alessio M. De Nicola M. Coppola S. Gualandi G. Pugliese L. Cerella C. Cristofanon S. Civitareale P. Ciriolo MR. Bergamaschi A. Magrini A. Ghibelli L. Oxidative Bax dimerization promotes its translocation to mitochondria independently of apoptosis. FASEB J. 2005;19:1504–1506. doi: 10.1096/fj.04-3329fje. [DOI] [PubMed] [Google Scholar]
- 50.Dalle-Donne I. Colombo G. Gagliano N. Colombo R. Giustarini D. Rossi R. Milzani A. S-glutathiolation in life and death decisions of the cell. Free Radic Res. 2011;45:3–15. doi: 10.3109/10715762.2010.515217. [DOI] [PubMed] [Google Scholar]
- 51.Dalton TP. Chen Y. Schneider SN. Nebert DW. Shertzer HG. Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic Biol Med. 2004;37:1511–1526. doi: 10.1016/j.freeradbiomed.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 52.Davis MA. Flaws JA. Young M. Collins K. Colburn NH. Effect of ceramide on intracellular glutathione determines apoptotic or necrotic cell death of JB6 tumor cells. Toxicol Sci. 2000;53:48–55. doi: 10.1093/toxsci/53.1.48. [DOI] [PubMed] [Google Scholar]
- 53.Deas O. Dumont C. Mollereau B. Metivier D. Pasquier C. Bernard-Pomier G. Hirsch F. Charpentier B. Senik A. Thiol-mediated inhibition of FAS and CD2 apoptotic signaling in activated human peripheral T cells. Int Immunol. 1997;9:117–125. doi: 10.1093/intimm/9.1.117. [DOI] [PubMed] [Google Scholar]
- 54.Delgado-Esteban M. Almeida A. Bolanos JP. D-Glucose prevents glutathione oxidation and mitochondrial damage after glutamate receptor stimulation in rat cortical primary neurons. J Neurochem. 2000;75:1618–1624. doi: 10.1046/j.1471-4159.2000.0751618.x. [DOI] [PubMed] [Google Scholar]
- 55.Denton D. Nicolson S. Kumar S. Cell death by autophagy: facts and apparent artefacts. Cell Death Differ. 2012;19:87–95. doi: 10.1038/cdd.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Monte D. Sandy MS. Smith MT. Increased efflux rather than oxidation is the mechanism of glutathione depletion by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Biochem Biophys Res Commun. 1987;148:153–160. doi: 10.1016/0006-291x(87)91089-8. [DOI] [PubMed] [Google Scholar]
- 57.Di Stefano A. Frosali S. Leonini A. Ettorre A. Priora R. Di Simplicio FC. Di Simplicio P. GSH depletion, protein S-glutathionylation and mitochondrial transmembrane potential hyperpolarization are early events in initiation of cell death induced by a mixture of isothiazolinones in HL60 cells. Biochim Biophys Acta. 2006;1763:214–225. doi: 10.1016/j.bbamcr.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 58.Diaz-Hernandez JI. Almeida A. Delgado-Esteban M. Fernandez E. Bolanos JP. Knockdown of glutamate-cysteine ligase by small hairpin RNA reveals that both catalytic and modulatory subunits are essential for the survival of primary neurons. J Biol Chem. 2005;280:38992–39001. doi: 10.1074/jbc.M507065200. [DOI] [PubMed] [Google Scholar]
- 59.Dimmeler S. Haendeler J. Nehls M. Zeiher AM. Suppression of apoptosis by nitric oxide via inhibition of interleukin-1beta-converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like proteases. J Exp Med. 1997;185:601–607. doi: 10.1084/jem.185.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dogan AL. Legrand O. Faussat AM. Perrot JY. Marie JP. Evaluation and comparison of MRP1 activity with three fluorescent dyes and three modulators in leukemic cell lines. Leuk Res. 2004;28:619–622. doi: 10.1016/j.leukres.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 61.Drechsel DA. Liang LP. Patel M. 1-methyl-4-phenylpyridinium-induced alterations of glutathione status in immortalized rat dopaminergic neurons. Toxicol Appl Pharmacol. 2007;220:341–348. doi: 10.1016/j.taap.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ellison I. Richie JP., Jr Mechanisms of glutathione disulfide efflux from erythrocytes. Biochem Pharmacol. 2012;83:164–169. doi: 10.1016/j.bcp.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 63.Estrela JM. Ortega A. Obrador E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci. 2006;43:143–181. doi: 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- 64.Fadeel B. Orrenius S. Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J Intern Med. 2005;258:479–517. doi: 10.1111/j.1365-2796.2005.01570.x. [DOI] [PubMed] [Google Scholar]
- 65.Faucher K. Rabinovitch-Chable H. Cook-Moreau J. Barriere G. Sturtz F. Rigaud M. Overexpression of human GPX1 modifies Bax to Bcl-2 apoptotic ratio in human endothelial cells. Mol Cell Biochem. 2005;277:81–87. doi: 10.1007/s11010-005-5075-8. [DOI] [PubMed] [Google Scholar]
- 66.Fernandes RS. Cotter TG. Apoptosis or necrosis: intracellular levels of glutathione influence mode of cell death. Biochem Pharmacol. 1994;48:675–681. doi: 10.1016/0006-2952(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 67.Fico A. Manganelli G. Cigliano L. Bergamo P. Abrescia P. Franceschi C. Martini G. Filosa S. 2-deoxy-d-ribose induces apoptosis by inhibiting the synthesis and increasing the efflux of glutathione. Free Radic Biol Med. 2008;45:211–217. doi: 10.1016/j.freeradbiomed.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 68.Filomeni G. Aquilano K. Civitareale P. Rotilio G. Ciriolo MR. Activation of c-Jun-N-terminal kinase is required for apoptosis triggered by glutathione disulfide in neuroblastoma cells. Free Radic Biol Med. 2005;39:345–354. doi: 10.1016/j.freeradbiomed.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 69.Filomeni G. Aquilano K. Rotilio G. Ciriolo MR. Antiapoptotic response to induced GSH depletion: involvement of heat shock proteins and NF-kappaB activation. Antioxid Redox Signal. 2005;7:446–455. doi: 10.1089/ars.2005.7.446. [DOI] [PubMed] [Google Scholar]
- 70.Filomeni G. Rotilio G. Ciriolo MR. Glutathione disulfide induces apoptosis in U937 cells by a redox-mediated p38 MAP kinase pathway. FASEB J. 2003;17:64–66. doi: 10.1096/fj.02-0105fje. [DOI] [PubMed] [Google Scholar]
- 71.Forman HJ. Zhang H. Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fortenberry JD. Owens ML. Brown LA. S-nitrosoglutathione enhances neutrophil DNA fragmentation and cell death. Am J Physiol. 1999;276:L435–L442. doi: 10.1152/ajplung.1999.276.3.L435. [DOI] [PubMed] [Google Scholar]
- 73.Franco R. Cidlowski JA. SLCO/OATP-like transport of glutathione in FasL-induced apoptosis: glutathione efflux is coupled to an organic anion exchange and is necessary for the progression of the execution phase of apoptosis. J Biol Chem. 2006;281:29542–29557. doi: 10.1074/jbc.M602500200. [DOI] [PubMed] [Google Scholar]
- 74.Franco R. Cidlowski JA. Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ. 2009;16:1303–1314. doi: 10.1038/cdd.2009.107. [DOI] [PubMed] [Google Scholar]
- 75.Franco R. DeHaven WI. Sifre M. Bortner CD. Cidlowski JA. Glutathione depletion and disruption of intracellular ionic homeostasis regulate lymphoid cell apoptosis. J Biol Chem. 2008;283:36071–36087. doi: 10.1074/jbc.M807061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Franco R. Panayiotidis MI. Cidlowski JA. Glutathione depletion is necessary for apoptosis in lymphoid cells independent of reactive oxygen species formation. J Biol Chem. 2007;282:30452–30465. doi: 10.1074/jbc.M703091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Franco R. Schoneveld OJ. Pappa A. Panayiotidis MI. The central role of glutathione in the pathophysiology of human diseases. Arch Physiol Biochem. 2007;113:234–258. doi: 10.1080/13813450701661198. [DOI] [PubMed] [Google Scholar]
- 78.Franklin CC. Krejsa CM. Pierce RH. White CC. Fausto N. Kavanagh TJ. Caspase-3-dependent cleavage of the glutamate-l-cysteine ligase catalytic subunit during apoptotic cell death. Am J Pathol. 2002;160:1887–1894. doi: 10.1016/S0002-9440(10)61135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Franklin CC. Rosenfeld-Franklin ME. White C. Kavanagh TJ. Fausto N. TGFbeta1-induced suppression of glutathione antioxidant defenses in hepatocytes: caspase-dependent post-translational and caspase-independent transcriptional regulatory mechanisms. FASEB J. 2003;17:1535–1537. doi: 10.1096/fj.02-0867fje. [DOI] [PubMed] [Google Scholar]
- 80.Friesen C. Kiess Y. Debatin KM. A critical role of glutathione in determining apoptosis sensitivity and resistance in leukemia cells. Cell Death Differ. 2004;11(Suppl 1):S73–S85. doi: 10.1038/sj.cdd.4401431. [DOI] [PubMed] [Google Scholar]
- 81.Furfaro AL. Macay JR. Marengo B. Nitti M. Parodi A. Fenoglio D. Marinari UM. Pronzato MA. Domenicotti C. Traverso N. Resistance of neuroblastoma GI-ME-N cell line to glutathione depletion involves Nrf2 and heme oxygenase-1. Free Radic Biol Med. 2012;52:488–496. doi: 10.1016/j.freeradbiomed.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 82.Gallogly MM. Starke DW. Mieyal JJ. Mechanistic and kinetic details of catalysis of thiol-disulfide exchange by glutaredoxins and potential mechanisms of regulation. Antioxid Redox Signal. 2009;11:1059–1081. doi: 10.1089/ars.2008.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Galluzzi L. Maiuri MC. Vitale I. Zischka H. Castedo M. Zitvogel L. Kroemer G. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 2007;14:1237–1243. doi: 10.1038/sj.cdd.4402148. [DOI] [PubMed] [Google Scholar]
- 84.Galluzzi L. Vitale I. Abrams JM. Alnemri ES. Baehrecke EH. Blagosklonny MV. Dawson TM. Dawson VL. El-Deiry WS. Fulda S. Gottlieb E. Green DR. Hengartner MO. Kepp O. Knight RA. Kumar S. Lipton SA. Lu X. Madeo F. Malorni W. Mehlen P. Nunez G. Peter ME. Piacentini M. Rubinsztein DC. Shi Y. Simon HU. Vandenabeele P. White E. Yuan J. Zhivotovsky B. Melino G. Kroemer G. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garcia-Nogales P. Almeida A. Bolanos JP. Peroxynitrite protects neurons against nitric oxide-mediated apoptosis. A key role for glucose-6-phosphate dehydrogenase activity in neuroprotection. J Biol Chem. 2003;278:864–874. doi: 10.1074/jbc.M206835200. [DOI] [PubMed] [Google Scholar]
- 86.Garcia-Ruiz C. Fernandez-Checa JC. Kaplowitz N. Bidirectional mechanism of plasma membrane transport of reduced glutathione in intact rat hepatocytes and membrane vesicles. J Biol Chem. 1992;267:22256–22264. [PubMed] [Google Scholar]
- 87.Garcia TB. Oliveira KR. do Nascimento JL. Crespo-Lopez ME. Picanco-Diniz DL. Mota TC. Herculano AM. Glutamate induces glutathione efflux mediated by glutamate/aspartate transporter in retinal cell cultures. Neurochem Res. 2011;36:412–418. doi: 10.1007/s11064-010-0356-3. [DOI] [PubMed] [Google Scholar]
- 88.Gendron MC. Schrantz N. Metivier D. Kroemer G. Maciorowska Z. Sureau F. Koester S. Petit PX. Oxidation of pyridine nucleotides during Fas- and ceramide-induced apoptosis in Jurkat cells: correlation with changes in mitochondria, glutathione depletion, intracellular acidification and caspase 3 activation. Biochem J. 2001;353:357–367. [PMC free article] [PubMed] [Google Scholar]
- 89.Ghibelli L. Coppola S. Fanelli C. Rotilio G. Civitareale P. Scovassi AI. Ciriolo MR. Glutathione depletion causes cytochrome c release even in the absence of cell commitment to apoptosis. FASEB J. 1999;13:2031–2036. doi: 10.1096/fasebj.13.14.2031. [DOI] [PubMed] [Google Scholar]
- 90.Ghibelli L. Fanelli C. Rotilio G. Lafavia E. Coppola S. Colussi C. Civitareale P. Ciriolo MR. Rescue of cells from apoptosis by inhibition of active GSH extrusion. FASEB J. 1998;12:479–486. doi: 10.1096/fasebj.12.6.479. [DOI] [PubMed] [Google Scholar]
- 91.Ghosh S. Pulinilkunnil T. Yuen G. Kewalramani G. An D. Qi D. Abrahani A. Rodrigues B. Cardiomyocyte apoptosis induced by short-term diabetes requires mitochondrial GSH depletion. Am J Physiol Heart Circ Physiol. 2005;289:H768–H776. doi: 10.1152/ajpheart.00038.2005. [DOI] [PubMed] [Google Scholar]
- 92.Gouaze V. Andrieu-Abadie N. Cuvillier O. Malagarie-Cazenave S. Frisach MF. Mirault ME. Levade T. Glutathione peroxidase-1 protects from CD95-induced apoptosis. J Biol Chem. 2002;277:42867–42874. doi: 10.1074/jbc.M203067200. [DOI] [PubMed] [Google Scholar]
- 93.Gouaze V. Mirault ME. Carpentier S. Salvayre R. Levade T. Andrieu-Abadie N. Glutathione peroxidase-1 overexpression prevents ceramide production and partially inhibits apoptosis in doxorubicin-treated human breast carcinoma cells. Mol Pharmacol. 2001;60:488–496. [PubMed] [Google Scholar]
- 94.Gould NS. Min E. Martin RJ. Day BJ. CFTR is the primary known apical glutathione transporter involved in cigarette smoke-induced adaptive responses in the lung. Free Radic Biol Med. 2012;52:1201–1206. doi: 10.1016/j.freeradbiomed.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Green RM. Graham M. O'Donovan MR. Chipman JK. Hodges NJ. Subcellular compartmentalization of glutathione: correlations with parameters of oxidative stress related to genotoxicity. Mutagenesis. 2006;21:383–390. doi: 10.1093/mutage/gel043. [DOI] [PubMed] [Google Scholar]
- 96.Guha P. Dey A. Sen R. Chatterjee M. Chattopadhyay S. Bandyopadhyay SK. Intracellular GSH depletion triggered mitochondrial Bax translocation to accomplish resveratrol-induced apoptosis in the U937 cell line. J Pharmacol Exp Ther. 2011;336:206–214. doi: 10.1124/jpet.110.171983. [DOI] [PubMed] [Google Scholar]
- 97.Hagenbuch B. Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447:653–665. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- 98.Hammond CL. Madejczyk MS. Ballatori N. Activation of plasma membrane reduced glutathione transport in death receptor apoptosis of HepG2 cells. Toxicol Appl Pharmacol. 2004;195:12–22. doi: 10.1016/j.taap.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 99.Hammond CL. Marchan R. Krance SM. Ballatori N. Glutathione export during apoptosis requires functional multidrug resistance-associated proteins. J Biol Chem. 2007;282:14337–14347. doi: 10.1074/jbc.M611019200. [DOI] [PubMed] [Google Scholar]
- 100.Han D. Hanawa N. Saberi B. Kaplowitz N. Hydrogen peroxide and redox modulation sensitize primary mouse hepatocytes to TNF-induced apoptosis. Free Radic Biol Med. 2006;41:627–639. doi: 10.1016/j.freeradbiomed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 101.Han YH. Kim SH. Kim SZ. Park WH. Apoptosis in arsenic trioxide-treated Calu-6 lung cells is correlated with the depletion of GSH levels rather than the changes of ROS levels. J Cell Biochem. 2008;104:862–878. doi: 10.1002/jcb.21673. [DOI] [PubMed] [Google Scholar]
- 102.Han YH. Kim SZ. Kim SH. Park WH. Apoptosis in pyrogallol-treated Calu-6 cells is correlated with the changes of intracellular GSH levels rather than ROS levels. Lung Cancer. 2008;59:301–314. doi: 10.1016/j.lungcan.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 103.Hancock JT. Desikan R. Neill SJ. Does the redox status of cytochrome C act as a fail-safe mechanism in the regulation of programmed cell death? Free Radic Biol Med. 2001;31:697–703. doi: 10.1016/s0891-5849(01)00646-3. [DOI] [PubMed] [Google Scholar]
- 104.Hansen JM. Zhang H. Jones DP. Differential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal ions. Free Radic Biol Med. 2006;40:138–145. doi: 10.1016/j.freeradbiomed.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 105.He C. Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.He YY. Huang JL. Ramirez DC. Chignell CF. Role of reduced glutathione efflux in apoptosis of immortalized human keratinocytes induced by UVA. J Biol Chem. 2003;278:8058–8064. doi: 10.1074/jbc.M207781200. [DOI] [PubMed] [Google Scholar]
- 107.Hentze H. Gantner F. Kolb SA. Wendel A. Depletion of hepatic glutathione prevents death receptor-dependent apoptotic and necrotic liver injury in mice. Am J Pathol. 2000;156:2045–2056. doi: 10.1016/S0002-9440(10)65076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hentze H. Schmitz I. Latta M. Krueger A. Krammer PH. Wendel A. Glutathione dependence of caspase-8 activation at the death-inducing signaling complex. J Biol Chem. 2002;277:5588–5595. doi: 10.1074/jbc.M110766200. [DOI] [PubMed] [Google Scholar]
- 109.Hirrlinger J. Dringen R. Multidrug resistance protein 1-mediated export of glutathione and glutathione disulfide from brain astrocytes. Methods Enzymol. 2005;400:395–409. doi: 10.1016/S0076-6879(05)00023-6. [DOI] [PubMed] [Google Scholar]
- 110.Hirrlinger J. Konig J. Keppler D. Lindenau J. Schulz JB. Dringen R. The multidrug resistance protein MRP1 mediates the release of glutathione disulfide from rat astrocytes during oxidative stress. J Neurochem. 2001;76:627–636. doi: 10.1046/j.1471-4159.2001.00101.x. [DOI] [PubMed] [Google Scholar]
- 111.Ho YS. Xiong Y. Ho DS. Gao J. Chua BH. Pai H. Mieyal JJ. Targeted disruption of the glutaredoxin 1 gene does not sensitize adult mice to tissue injury induced by ischemia/reperfusion and hyperoxia. Free Radic Biol Med. 2007;43:1299–1312. doi: 10.1016/j.freeradbiomed.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hofken T. Linder D. Kleene R. Goke B. Wagner AC. Membrane dipeptidase and glutathione are major components of pig pancreatic zymogen granules. Exp Cell Res. 1998;244:481–490. doi: 10.1006/excr.1998.4233. [DOI] [PubMed] [Google Scholar]
- 113.Hu HL. Forsey RJ. Blades TJ. Barratt ME. Parmar P. Powell JR. Antioxidants may contribute in the fight against ageing: an in vitro model. Mech Ageing Dev. 2000;121:217–230. doi: 10.1016/s0047-6374(00)00212-8. [DOI] [PubMed] [Google Scholar]
- 114.Huang DC. Hahne M. Schroeter M. Frei K. Fontana A. Villunger A. Newton K. Tschopp J. Strasser A. Activation of Fas by FasL induces apoptosis by a mechanism that cannot be blocked by Bcl-2 or Bcl-x(L) Proc Natl Acad Sci U S A. 1999;96:14871–14876. doi: 10.1073/pnas.96.26.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huang J. Lam GY. Brumell JH. Autophagy signaling through reactive oxygen species. Antioxid Redox Signal. 2011;14:2215–2231. doi: 10.1089/ars.2010.3554. [DOI] [PubMed] [Google Scholar]
- 116.Huang KP. Huang FL. Glutathionylation of proteins by glutathione disulfide S-oxide. Biochem Pharmacol. 2002;64:1049–1056. doi: 10.1016/s0006-2952(02)01175-9. [DOI] [PubMed] [Google Scholar]
- 117.Huang Z. Pinto JT. Deng H. Richie JP., Jr Inhibition of caspase-3 activity and activation by protein glutathionylation. Biochem Pharmacol. 2008;75:2234–2244. doi: 10.1016/j.bcp.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Iantomasi T. Favilli F. Marraccini P. Magaldi T. Bruni P. Vincenzini MT. Glutathione transport system in human small intestine epithelial cells. Biochim Biophys Acta. 1997;1330:274–283. doi: 10.1016/s0005-2736(97)00097-7. [DOI] [PubMed] [Google Scholar]
- 119.Imai H. Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med. 2003;34:145–169. doi: 10.1016/s0891-5849(02)01197-8. [DOI] [PubMed] [Google Scholar]
- 120.Inoue K. Akaike T. Miyamoto Y. Okamoto T. Sawa T. Otagiri M. Suzuki S. Yoshimura T. Maeda H. Nitrosothiol formation catalyzed by ceruloplasmin. Implication for cytoprotective mechanism in vivo. J Biol Chem. 1999;274:27069–27075. doi: 10.1074/jbc.274.38.27069. [DOI] [PubMed] [Google Scholar]
- 121.Jang JH. Surh YJ. Bcl-2 attenuation of oxidative cell death is associated with up-regulation of gamma-glutamylcysteine ligase via constitutive NF-kappaB activation. J Biol Chem. 2004;279:38779–38786. doi: 10.1074/jbc.M406371200. [DOI] [PubMed] [Google Scholar]
- 122.Johansson M. Lundberg M. Glutathionylation of beta-actin via a cysteinyl sulfenic acid intermediary. BMC Biochem. 2007;8:26. doi: 10.1186/1471-2091-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 124.Jourd'heuil D. Jourd'heuil FL. Feelisch M. Oxidation and nitrosation of thiols at low micromolar exposure to nitric oxide. Evidence for a free radical mechanism. J Biol Chem. 2003;278:15720–15726. doi: 10.1074/jbc.M300203200. [DOI] [PubMed] [Google Scholar]
- 125.Jungas T. Motta I. Duffieux F. Fanen P. Stoven V. Ojcius DM. Glutathione levels and BAX activation during apoptosis due to oxidative stress in cells expressing wild-type and mutant cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2002;277:27912–27918. doi: 10.1074/jbc.M110288200. [DOI] [PubMed] [Google Scholar]
- 126.Kane DJ. Sarafian TA. Anton R. Hahn H. Gralla EB. Valentine JS. Ord T. Bredesen DE. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993;262:1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- 127.Kayanoki Y. Fujii J. Islam KN. Suzuki K. Kawata S. Matsuzawa Y. Taniguchi N. The protective role of glutathione peroxidase in apoptosis induced by reactive oxygen species. J Biochem. 1996;119:817–822. doi: 10.1093/oxfordjournals.jbchem.a021313. [DOI] [PubMed] [Google Scholar]
- 128.Kemp M. Go YM. Jones DP. Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Radic Biol Med. 2008;44:921–937. doi: 10.1016/j.freeradbiomed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Keppler D. Leier I. Jedlitschky G. Konig J. ATP-dependent transport of glutathione S-conjugates by the multidrug resistance protein MRP1 and its apical isoform MRP2. Chem Biol Interact. 1998;111–112:153–161. doi: 10.1016/s0009-2797(97)00158-0. [DOI] [PubMed] [Google Scholar]
- 130.Kern JC. Kehrer JP. Free radicals and apoptosis: relationships with glutathione, thioredoxin, and the BCL family of proteins. Front Biosci. 2005;10:1727–1738. doi: 10.2741/1656. [DOI] [PubMed] [Google Scholar]
- 131.Keszler A. Zhang Y. Hogg N. Reaction between nitric oxide, glutathione, and oxygen in the presence and absence of protein: How are S-nitrosothiols formed? Free Radic Biol Med. 2010;48:55–64. doi: 10.1016/j.freeradbiomed.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim JE. Tannenbaum SR. S-Nitrosation regulates the activation of endogenous procaspase-9 in HT-29 human colon carcinoma cells. J Biol Chem. 2004;279:9758–9764. doi: 10.1074/jbc.M312722200. [DOI] [PubMed] [Google Scholar]
- 133.Kirkland RA. Franklin JL. Evidence for redox regulation of cytochrome C release during programmed neuronal death: antioxidant effects of protein synthesis and caspase inhibition. J Neurosci. 2001;21:1949–1963. doi: 10.1523/JNEUROSCI.21-06-01949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kizhakkayil J. Thayyullathil F. Chathoth S. Hago A. Patel M. Galadari S. Glutathione regulates caspase-dependent ceramide production and curcumin-induced apoptosis in human leukemic cells. Free Radic Biol Med. 2012;52:1854–1864. doi: 10.1016/j.freeradbiomed.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 135.Kroemer G. Galluzzi L. Vandenabeele P. Abrams J. Alnemri ES. Baehrecke EH. Blagosklonny MV. El-Deiry WS. Golstein P. Green DR. Hengartner M. Knight RA. Kumar S. Lipton SA. Malorni W. Nunez G. Peter ME. Tschopp J. Yuan J. Piacentini M. Zhivotovsky B. Melino G. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kroemer G. Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kuipers I. Guala AS. Aesif SW. Konings G. Bouwman FG. Mariman EC. Wouters EF. Janssen-Heininger YM. Reynaert NL. Cigarette smoke targets glutaredoxin 1, increasing s-glutathionylation and epithelial cell death. Am J Respir Cell Mol Biol. 2011;45:931–937. doi: 10.1165/rcmb.2010-0249OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.l'Hoste S. Chargui A. Belfodil R. Corcelle E. Duranton C. Rubera I. Poujeol C. Mograbi B. Tauc M. Poujeol P. CFTR mediates apoptotic volume decrease and cell death by controlling glutathione efflux and ROS production in cultured mice proximal tubules. Am J Physiol Renal Physiol. 2010;298:F435–F453. doi: 10.1152/ajprenal.00286.2009. [DOI] [PubMed] [Google Scholar]
- 139.Laberge RM. Karwatsky J. Lincoln MC. Leimanis ML. Georges E. Modulation of GSH levels in ABCC1 expressing tumor cells triggers apoptosis through oxidative stress. Biochem Pharmacol. 2007;73:1727–1737. doi: 10.1016/j.bcp.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 140.Lagadic-Gossmann D. Huc L. Lecureur V. Alterations of intracellular pH homeostasis in apoptosis: origins and roles. Cell Death Differ. 2004;11:953–961. doi: 10.1038/sj.cdd.4401466. [DOI] [PubMed] [Google Scholar]
- 141.Lai Y. Hickey RW. Chen Y. Bayir H. Sullivan ML. Chu CT. Kochanek PM. Dixon CE. Jenkins LW. Graham SH. Watkins SC. Clark RS. Autophagy is increased after traumatic brain injury in mice and is partially inhibited by the antioxidant gamma-glutamylcysteinyl ethyl ester. J Cereb Blood Flow Metab. 2008;28:540–550. doi: 10.1038/sj.jcbfm.9600551. [DOI] [PubMed] [Google Scholar]
- 142.Lampela O. Juffer AH. Rauk A. Conformational analysis of glutathione in aqueous solution with molecular dynamics. J Phys Chem A. 2003;107:9208–9220. [Google Scholar]
- 143.Lash LH. Mitochondrial glutathione transport: physiological, pathological and toxicological implications. Chem Biol Interact. 2006;163:54–67. doi: 10.1016/j.cbi.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lash LH. Putt DA. Xu F. Matherly LH. Role of rat organic anion transporter 3 (Oat3) in the renal basolateral transport of glutathione. Chem Biol Interact. 2007;170:124–134. doi: 10.1016/j.cbi.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lavrik I. Golks A. Krammer PH. Death receptor signaling. J Cell Sci. 2005;118:265–267. doi: 10.1242/jcs.01610. [DOI] [PubMed] [Google Scholar]
- 146.Le Foll I. Duval DP. Programmed cell death induced by glutathione depletion in PC 12 cells is blocked by inhibitors of 12 lipoxygenase, but does not appear to be mediated through the formation of 12 HETE derivatives. Free Radic Biol Med. 2001;30:793–802. doi: 10.1016/s0891-5849(01)00467-1. [DOI] [PubMed] [Google Scholar]
- 147.Lee HR. Cho JM. Shin DH. Yong CS. Choi HG. Wakabayashi N. Kwak MK. Adaptive response to GSH depletion and resistance to L: -buthionine-(S,R)-sulfoximine: involvement of Nrf2 activation. Mol Cell Biochem. 2008;318:23–31. doi: 10.1007/s11010-008-9853-y. [DOI] [PubMed] [Google Scholar]
- 148.Lee JW. Ko YE. Lee IH. Lee HK. Kim HW. Kim YH. Osmotic stress induces loss of glutathione and increases the sensitivity to oxidative stress in H9c2 cardiac myocytes. Free Radic Res. 2009;43:262–271. doi: 10.1080/10715760802691471. [DOI] [PubMed] [Google Scholar]
- 149.Lee SM. Koh HJ. Park DC. Song BJ. Huh TL. Park JW. Cytosolic NADP(+)-dependent isocitrate dehydrogenase status modulates oxidative damage to cells. Free Radic Biol Med. 2002;32:1185–1196. doi: 10.1016/s0891-5849(02)00815-8. [DOI] [PubMed] [Google Scholar]
- 150.Lee TK. Hammond CL. Ballatori N. Intracellular glutathione regulates taurocholate transport in HepG2 cells. Toxicol Appl Pharmacol. 2001;174:207–215. doi: 10.1006/taap.2001.9208. [DOI] [PubMed] [Google Scholar]
- 151.Leier I. Jedlitschky G. Buchholz U. Center M. Cole SP. Deeley RG. Keppler D. ATP-dependent glutathione disulphide transport mediated by the MRP gene-encoded conjugate export pump. Biochem J. 1996;314(Pt 2):433–437. doi: 10.1042/bj3140433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li L. Lee TK. Meier PJ. Ballatori N. Identification of glutathione as a driving force and leukotriene C4 as a substrate for oatp1, the hepatic sinusoidal organic solute transporter. J Biol Chem. 1998;273:16184–16191. doi: 10.1074/jbc.273.26.16184. [DOI] [PubMed] [Google Scholar]
- 153.Li L. Meier PJ. Ballatori N. Oatp2 mediates bidirectional organic solute transport: a role for intracellular glutathione. Mol Pharmacol. 2000;58:335–340. doi: 10.1124/mol.58.2.335. [DOI] [PubMed] [Google Scholar]
- 154.Liang H. Ran Q. Jang YC. Holstein D. Lechleiter J. McDonald-Marsh T. Musatov A. Song W. Van Remmen H. Richardson A. Glutathione peroxidase 4 differentially regulates the release of apoptogenic proteins from mitochondria. Free Radic Biol Med. 2009;47:312–320. doi: 10.1016/j.freeradbiomed.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lin DY. Ma WY. Duan SJ. Zhang Y. Du LY. Real-time imaging of viable-apoptotic switch in GSNO-induced mouse thymocyte apoptosis. Apoptosis. 2006;11:1289–1298. doi: 10.1007/s10495-006-7804-1. [DOI] [PubMed] [Google Scholar]
- 156.Liuzzi F. Fanelli C. Ciriolo MR. Cerella C. D'Alessio M. Denicola M. Magrini A. Bergamaschi A. Ghibelli L. Rescue of cells from apoptosis by antioxidants occurs downstream from GSH extrusion. Ann N Y Acad Sci. 2003;1010:441–445. doi: 10.1196/annals.1299.080. [DOI] [PubMed] [Google Scholar]
- 157.Lou H. Kaplowitz N. Glutathione depletion down-regulates tumor necrosis factor alpha-induced NF-kappaB activity via IkappaB kinase-dependent and -independent mechanisms. J Biol Chem. 2007;282:29470–29481. doi: 10.1074/jbc.M706145200. [DOI] [PubMed] [Google Scholar]
- 158.Lu Y. Cederbaum A. The mode of cisplatin-induced cell death in CYP2E1-overexpressing HepG2 cells: modulation by ERK, ROS, glutathione, and thioredoxin. Free Radic Biol Med. 2007;43:1061–1075. doi: 10.1016/j.freeradbiomed.2007.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lubos E. Loscalzo J. Handy DE. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011;15:1957–1997. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mahagita C. Grassl SM. Piyachaturawat P. Ballatori N. Human organic anion transporter 1B1 (OATP1B1/OATP-C) and 1B3 (OATP1B3/OATP-8) function as bidirectional carriers and do not mediate GSH-bile acid co-transport. Am J Physiol Gastrointest Liver Physiol. 2007;293:G271–G278. doi: 10.1152/ajpgi.00075.2007. [DOI] [PubMed] [Google Scholar]
- 161.Mannick JB. Hausladen A. Liu L. Hess DT. Zeng M. Miao QX. Kane LS. Gow AJ. Stamler JS. Fas-induced caspase denitrosylation. Science. 1999;284:651–654. doi: 10.1126/science.284.5414.651. [DOI] [PubMed] [Google Scholar]
- 162.Mannick JB. Miao XQ. Stamler JS. Nitric oxide inhibits Fas-induced apoptosis. J Biol Chem. 1997;272:24125–24128. doi: 10.1074/jbc.272.39.24125. [DOI] [PubMed] [Google Scholar]
- 163.Mannick JB. Schonhoff C. Papeta N. Ghafourifar P. Szibor M. Fang K. Gaston B. S-Nitrosylation of mitochondrial caspases. J Cell Biol. 2001;154:1111–1116. doi: 10.1083/jcb.200104008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Marchan R. Hammond CL. Ballatori N. Multidrug resistance-associated protein 1 as a major mediator of basal and apoptotic glutathione release. Biochim Biophys Acta. 2008;1778:2413–2420. doi: 10.1016/j.bbamem.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Marengo B. De Ciucis C. Verzola D. Pistoia V. Raffaghello L. Patriarca S. Balbis E. Traverso N. Cottalasso D. Pronzato MA. Marinari UM. Domenicotti C. Mechanisms of BSO (L-buthionine-S,R-sulfoximine)-induced cytotoxic effects in neuroblastoma. Free Radic Biol Med. 2008;44:474–482. doi: 10.1016/j.freeradbiomed.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 166.Mari M. Morales A. Colell A. Garcia-Ruiz C. Fernandez-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal. 2009;11:2685–2700. doi: 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Martin HL. Teismann P. Glutathione—a review on its role and significance in Parkinson's disease. FASEB J. 2009;23:3263–3272. doi: 10.1096/fj.08-125443. [DOI] [PubMed] [Google Scholar]
- 168.Matsumaru K. Ji C. Kaplowitz N. Mechanisms for sensitization to TNF-induced apoptosis by acute glutathione depletion in murine hepatocytes. Hepatology. 2003;37:1425–1434. doi: 10.1053/jhep.2003.50230. [DOI] [PubMed] [Google Scholar]
- 169.Mattson DM. Ahmad IM. Dayal D. Parsons AD. Aykin-Burns N. Li L. Orcutt KP. Spitz DR. Dornfeld KJ. Simons AL. Cisplatin combined with zidovudine enhances cytotoxicity and oxidative stress in human head and neck cancer cells via a thiol-dependent mechanism. Free Radic Biol Med. 2009;46:232–237. doi: 10.1016/j.freeradbiomed.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.McCall K. Genetic control of necrosis—another type of programmed cell death. Curr Opin Cell Biol. 2010;22:882–888. doi: 10.1016/j.ceb.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.McNeely SC. Belshoff AC. Taylor BF. Fan TW. McCabe MJ., Jr. Pinhas AR. States JC. Sensitivity to sodium arsenite in human melanoma cells depends upon susceptibility to arsenite-induced mitotic arrest. Toxicol Appl Pharmacol. 2008;229:252–261. doi: 10.1016/j.taap.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Meister A. Glutathione biosynthesis and its inhibition. Methods Enzymol. 1995;252:26–30. doi: 10.1016/0076-6879(95)52005-8. [DOI] [PubMed] [Google Scholar]
- 173.Melino G. Bernassola F. Knight RA. Corasaniti MT. Nistico G. Finazzi-Agro A. S-nitrosylation regulates apoptosis. Nature. 1997;388:432–433. doi: 10.1038/41237. [DOI] [PubMed] [Google Scholar]
- 174.Messmer UK. Lapetina EG. Brune B. Nitric oxide-induced apoptosis in RAW 264.7 macrophages is antagonized by protein kinase C- and protein kinase A-activating compounds. Mol Pharmacol. 1995;47:757–765. [PubMed] [Google Scholar]
- 175.Meurette O. Lefeuvre-Orfila L. Rebillard A. Lagadic-Gossmann D. Dimanche-Boitrel MT. Role of intracellular glutathione in cell sensitivity to the apoptosis induced by tumor necrosis factor {alpha}-related apoptosis-inducing ligand/anticancer drug combinations. Clin Cancer Res. 2005;11:3075–3083. doi: 10.1158/1078-0432.CCR-04-1764. [DOI] [PubMed] [Google Scholar]
- 176.Mieyal JJ. Gallogly MM. Qanungo S. Sabens EA. Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Minich T. Riemer J. Schulz JB. Wielinga P. Wijnholds J. Dringen R. The multidrug resistance protein 1 (Mrp1), but not Mrp5, mediates export of glutathione and glutathione disulfide from brain astrocytes. J Neurochem. 2006;97:373–384. doi: 10.1111/j.1471-4159.2006.03737.x. [DOI] [PubMed] [Google Scholar]
- 178.Mitchell DA. Morton SU. Fernhoff NB. Marletta MA. Thioredoxin is required for S-nitrosation of procaspase-3 and the inhibition of apoptosis in Jurkat cells. Proc Natl Acad Sci U S A. 2007;104:11609–11614. doi: 10.1073/pnas.0704898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mohr S. Hallak H. de Boitte A. Lapetina EG. Brune B. Nitric oxide-induced S-glutathionylation and inactivation of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1999;274:9427–9430. doi: 10.1074/jbc.274.14.9427. [DOI] [PubMed] [Google Scholar]
- 180.Monostori P. Wittmann G. Karg E. Turi S. Determination of glutathione and glutathione disulfide in biological samples: an in-depth review. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:3331–3346. doi: 10.1016/j.jchromb.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 181.Morgan MJ. Kim YS. Liu ZG. TNFalpha and reactive oxygen species in necrotic cell death. Cell Res. 2008;18:343–349. doi: 10.1038/cr.2008.31. [DOI] [PubMed] [Google Scholar]
- 182.Mueller CF. Widder JD. McNally JS. McCann L. Jones DP. Harrison DG. The role of the multidrug resistance protein-1 in modulation of endothelial cell oxidative stress. Circ Res. 2005;97:637–644. doi: 10.1161/01.RES.0000183734.21112.b7. [DOI] [PubMed] [Google Scholar]
- 183.Musallam L. Ethier C. Haddad PS. Bilodeau M. EGF mediates protection against Fas-induced apoptosis by depleting and oxidizing intracellular GSH stocks. J Cell Physiol. 2004;198:62–72. doi: 10.1002/jcp.10389. [DOI] [PubMed] [Google Scholar]
- 184.Nagai H. Matsumaru K. Feng G. Kaplowitz N. Reduced glutathione depletion causes necrosis and sensitization to tumor necrosis factor-alpha-induced apoptosis in cultured mouse hepatocytes. Hepatology. 2002;36:55–64. doi: 10.1053/jhep.2002.33995. [DOI] [PubMed] [Google Scholar]
- 185.Nepravishta R. Sabelli R. Iorio E. Micheli L. Paci M. Melino S. Oxidative species and S-glutathionyl conjugates in the apoptosis induction by allyl thiosulfate. FEBS J. 2012;279:154–167. doi: 10.1111/j.1742-4658.2011.08407.x. [DOI] [PubMed] [Google Scholar]
- 186.Ng KH. Lim BG. Wong KP. Sulfate conjugating and transport functions of MDCK distal tubular cells. Kidney Int. 2003;63:976–986. doi: 10.1046/j.1523-1755.2003.00818.x. [DOI] [PubMed] [Google Scholar]
- 187.Nomura K. Imai H. Koumura T. Kobayashi T. Nakagawa Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis. Biochem J. 2000;351:183–193. doi: 10.1042/0264-6021:3510183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nur E. Verwijs M. de Waart DR. Schnog JJ. Otten HM. Brandjes DP. Biemond BJ. Elferink RP. Increased efflux of oxidized glutathione (GSSG) causes glutathione depletion and potentially diminishes antioxidant defense in sickle erythrocytes. Biochim Biophys Acta. 2011;1812:1412–1417. doi: 10.1016/j.bbadis.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 189.O'Neill AJ. O'Neill S. Hegarty NJ. Coffey RN. Gibbons N. Brady H. Fitzpatrick JM. Watson RW. Glutathione depletion-induced neutrophil apoptosis is caspase 3 dependent. Shock. 2000;14:605–609. doi: 10.1097/00024382-200014060-00006. [DOI] [PubMed] [Google Scholar]
- 190.Oda T. Sadakata N. Komatsu N. Muramatsu T. Specific efflux of glutathione from the basolateral membrane domain in polarized MDCK cells during ricin-induced apoptosis. J Biochem. 1999;126:715–721. doi: 10.1093/oxfordjournals.jbchem.a022508. [DOI] [PubMed] [Google Scholar]
- 191.Ortega A. Ferrer P. Carretero J. Obrador E. Asensi M. Pellicer JA. Estrela JM. Down-regulation of glutathione and Bcl-2 synthesis in mouse B16 melanoma cells avoids their survival during interaction with the vascular endothelium. J Biol Chem. 2003;278:39591–39599. doi: 10.1074/jbc.M303753200. [DOI] [PubMed] [Google Scholar]
- 192.Osbild S. Brault L. Battaglia E. Bagrel D. Resistance to cisplatin and adriamycin is associated with the inhibition of glutathione efflux in MCF-7-derived cells. Anticancer Res. 2006;26:3595–3600. [PubMed] [Google Scholar]
- 193.Pan S. Berk BC. Glutathiolation regulates tumor necrosis factor-alpha-induced caspase-3 cleavage and apoptosis: key role for glutaredoxin in the death pathway. Circ Res. 2007;100:213–219. doi: 10.1161/01.RES.0000256089.30318.20. [DOI] [PubMed] [Google Scholar]
- 194.Pan Z. Voehringer DW. Meyn RE. Analysis of redox regulation of cytochrome c-induced apoptosis in a cell-free system. Cell Death Differ. 1999;6:683–688. doi: 10.1038/sj.cdd.4400544. [DOI] [PubMed] [Google Scholar]
- 195.Park HA. Khanna S. Rink C. Gnyawali S. Roy S. Sen CK. Glutathione disulfide induces neural cell death via a 12-lipoxygenase pathway. Cell Death Differ. 2009;16:1167–1179. doi: 10.1038/cdd.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Perricone C. De Carolis C. Perricone R. Glutathione: a key player in autoimmunity. Autoimmun Rev. 2009;8:697–701. doi: 10.1016/j.autrev.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 197.Perrotton T. Trompier D. Chang XB. Di Pietro A. Baubichon-Cortay H. (R)- and (S)-verapamil differentially modulate the multidrug-resistant protein MRP1. J Biol Chem. 2007;282:31542–31548. doi: 10.1074/jbc.M703964200. [DOI] [PubMed] [Google Scholar]
- 198.Pias EK. Aw TY. Apoptosis in mitotic competent undifferentiated cells is induced by cellular redox imbalance independent of reactive oxygen species production. FASEB J. 2002;16:781–790. doi: 10.1096/fj.01-0784com. [DOI] [PubMed] [Google Scholar]
- 199.Pias EK. Aw TY. Early redox imbalance mediates hydroperoxide-induced apoptosis in mitotic competent undifferentiated PC-12 cells. Cell Death Differ. 2002;9:1007–1016. doi: 10.1038/sj.cdd.4401064. [DOI] [PubMed] [Google Scholar]
- 200.Poole LB. Nelson KJ. Discovering mechanisms of signaling-mediated cysteine oxidation. Curr Opin Chem Biol. 2008;12:18–24. doi: 10.1016/j.cbpa.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Qanungo S. Starke DW. Pai HV. Mieyal JJ. Nieminen AL. Glutathione supplementation potentiates hypoxic apoptosis by S-glutathionylation of p65-NFkappaB. J Biol Chem. 2007;282:18427–18436. doi: 10.1074/jbc.M610934200. [DOI] [PubMed] [Google Scholar]
- 202.Rahman I. Kode A. Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 203.Ran Q. Liang H. Gu M. Qi W. Walter CA. Roberts LJ., 2nd Herman B. Richardson A. Van Remmen H. Transgenic mice overexpressing glutathione peroxidase 4 are protected against oxidative stress-induced apoptosis. J Biol Chem. 2004;279:55137–55146. doi: 10.1074/jbc.M410387200. [DOI] [PubMed] [Google Scholar]
- 204.Rana S. Dringen R. Gap junction hemichannel-mediated release of glutathione from cultured rat astrocytes. Neurosci Lett. 2007;415:45–48. doi: 10.1016/j.neulet.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 205.Ray SD. Kamendulis LM. Gurule MW. Yorkin RD. Corcoran GB. Ca2+ antagonists inhibit DNA fragmentation and toxic cell death induced by acetaminophen. FASEB J. 1993;7:453–463. doi: 10.1096/fasebj.7.5.8462787. [DOI] [PubMed] [Google Scholar]
- 206.Rius M. Hummel-Eisenbeiss J. Hofmann AF. Keppler D. Substrate specificity of human ABCC4 (MRP4)-mediated cotransport of bile acids and reduced glutathione. Am J Physiol Gastrointest Liver Physiol. 2006;290:G640–G649. doi: 10.1152/ajpgi.00354.2005. [DOI] [PubMed] [Google Scholar]
- 207.Rouzer CA. Scott WA. Griffith OW. Hamill AL. Cohn ZA. Glutathione metabolism in resting and phagocytizing peritoneal macrophages. J Biol Chem. 1982;257:2002–2008. [PubMed] [Google Scholar]
- 208.Rudin CM. Yang Z. Schumaker LM. VanderWeele DJ. Newkirk K. Egorin MJ. Zuhowski EG. Cullen KJ. Inhibition of glutathione synthesis reverses Bcl-2-mediated cisplatin resistance. Cancer Res. 2003;63:312–318. [PubMed] [Google Scholar]
- 209.Ryter SW. Kim HP. Hoetzel A. Park JW. Nakahira K. Wang X. Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- 210.Sato T. Machida T. Takahashi S. Iyama S. Sato Y. Kuribayashi K. Takada K. Oku T. Kawano Y. Okamoto T. Takimoto R. Matsunaga T. Takayama T. Takahashi M. Kato J. Niitsu Y. Fas-mediated apoptosome formation is dependent on reactive oxygen species derived from mitochondrial permeability transition in Jurkat cells. J Immunol. 2004;173:285–296. doi: 10.4049/jimmunol.173.1.285. [DOI] [PubMed] [Google Scholar]
- 211.Sawai H. Domae N. Transfer of Fas (CD95) protein from the cell surface to the surface of polystyrene beads coated with anti-Fas antibody clone CH-11. Eur J Histochem. 2010;54:e8. doi: 10.4081/ejh.2010.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Schafer FQ. Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 213.Scherz-Shouval R. Shvets E. Fass E. Shorer H. Gil L. Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Schor NF. Rudin CM. Hartman AR. Thompson CB. Tyurina YY. Kagan VE. Cell line dependence of Bcl-2-induced alteration of glutathione handling. Oncogene. 2000;19:472–476. doi: 10.1038/sj.onc.1203324. [DOI] [PubMed] [Google Scholar]
- 215.Schrammel A. Gorren AC. Schmidt K. Pfeiffer S. Mayer B. S-nitrosation of glutathione by nitric oxide, peroxynitrite, and (*)NO/O(2)(*-) Free Radic Biol Med. 2003;34:1078–1088. doi: 10.1016/s0891-5849(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 216.Seiler A. Schneider M. Forster H. Roth S. Wirth EK. Culmsee C. Plesnila N. Kremmer E. Radmark O. Wurst W. Bornkamm GW. Schweizer U. Conrad M. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8:237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 217.Sekine S. Mitsuki K. Ito K. Kugioka S. Horie T. Sustained intrahepatic glutathione depletion causes proteasomal degradation of multidrug resistance-associated protein 2 in rat liver. Biochim Biophys Acta. 2012;1822:980–987. doi: 10.1016/j.bbadis.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 218.Shen S. Kepp O. Kroemer G. The end of autophagic cell death? Autophagy. 2012;8 doi: 10.4161/auto.8.1.16618. [DOI] [PubMed] [Google Scholar]
- 219.Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999;27:916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 220.Slater AF. Stefan C. Nobel I. van den Dobbelsteen DJ. Orrenius S. Intracellular redox changes during apoptosis. Cell Death Differ. 1996;3:57–62. [PubMed] [Google Scholar]
- 221.Sreekumar PG. Spee C. Ryan SJ. Cole SP. Kannan R. Hinton DR. Mechanism of RPE cell death in alpha-crystallin deficient mice: a novel and critical role for MRP1-mediated GSH efflux. PLoS One. 2012;7:e33420. doi: 10.1371/journal.pone.0033420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Staab CA. Alander J. Brandt M. Lengqvist J. Morgenstern R. Grafstrom RC. Hoog JO. Reduction of S-nitrosoglutathione by alcohol dehydrogenase 3 is facilitated by substrate alcohols via direct cofactor recycling and leads to GSH-controlled formation of glutathione transferase inhibitors. Biochem J. 2008;413:493–504. doi: 10.1042/BJ20071666. [DOI] [PubMed] [Google Scholar]
- 223.Starke DW. Chock PB. Mieyal JJ. Glutathione-thiyl radical scavenging and transferase properties of human glutaredoxin (thioltransferase). Potential role in redox signal transduction. J Biol Chem. 2003;278:14607–14613. doi: 10.1074/jbc.M210434200. [DOI] [PubMed] [Google Scholar]
- 224.Stridh MH. Correa F. Nodin C. Weber SG. Blomstrand F. Nilsson M. Sandberg M. Enhanced glutathione efflux from astrocytes in culture by low extracellular Ca2+ and curcumin. Neurochem Res. 2010;35:1231–1238. doi: 10.1007/s11064-010-0179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Stridh MH. Tranberg M. Weber SG. Blomstrand F. Sandberg M. Stimulated efflux of amino acids and glutathione from cultured hippocampal slices by omission of extracellular calcium: likely involvement of connexin hemichannels. J Biol Chem. 2008;283:10347–10356. doi: 10.1074/jbc.M704153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Stubauer G. Giuffre A. Sarti P. Mechanism of S-nitrosothiol formation and degradation mediated by copper ions. J Biol Chem. 1999;274:28128–28133. doi: 10.1074/jbc.274.40.28128. [DOI] [PubMed] [Google Scholar]
- 227.Sullivan DM. Wehr NB. Fergusson MM. Levine RL. Finkel T. Identification of oxidant-sensitive proteins: TNF-alpha induces protein glutathiolation. Biochemistry. 2000;39:11121–11128. doi: 10.1021/bi0007674. [DOI] [PubMed] [Google Scholar]
- 228.Sumbayev VV. S-nitrosylation of thioredoxin mediates activation of apoptosis signal-regulating kinase 1. Arch Biochem Biophys. 2003;415:133–136. doi: 10.1016/s0003-9861(03)00199-1. [DOI] [PubMed] [Google Scholar]
- 229.Sze G. Kaplowitz N. Ookhtens M. Lu SC. Bidirectional membrane transport of intact glutathione in Hep G2 cells. Am J Physiol. 1993;265:G1128–G1134. doi: 10.1152/ajpgi.1993.265.6.G1128. [DOI] [PubMed] [Google Scholar]
- 230.Tao L. English AM. Protein S-glutathiolation triggered by decomposed S-nitrosoglutathione. Biochemistry. 2004;43:4028–4038. doi: 10.1021/bi035924o. [DOI] [PubMed] [Google Scholar]
- 231.Thomson SJ. Cox AG. Cuddihy SL. Pullar JM. Hampton MB. Inhibition of receptor-mediated apoptosis upon Bcl-2 overexpression is not associated with increased antioxidant status. Biochem Biophys Res Commun. 2008;375:145–150. doi: 10.1016/j.bbrc.2008.07.133. [DOI] [PubMed] [Google Scholar]
- 232.Trompier D. Chang XB. Barattin R. du Moulinet D'Hardemare A. Di Pietro A. Baubichon-Cortay H. Verapamil and its derivative trigger apoptosis through glutathione extrusion by multidrug resistance protein MRP1. Cancer Res. 2004;64:4950–4956. doi: 10.1158/0008-5472.CAN-04-0143. [DOI] [PubMed] [Google Scholar]
- 233.Troyano A. Fernandez C. Sancho P. de Blas E. Aller P. Effect of glutathione depletion on antitumor drug toxicity (apoptosis and necrosis) in U-937 human promonocytic cells. The role of intracellular oxidation. J Biol Chem. 2001;276:47107–47115. doi: 10.1074/jbc.M104516200. [DOI] [PubMed] [Google Scholar]
- 234.Troyano A. Sancho P. Fernandez C. de Blas E. Bernardi P. Aller P. The selection between apoptosis and necrosis is differentially regulated in hydrogen peroxide-treated and glutathione-depleted human promonocytic cells. Cell Death Differ. 2003;10:889–898. doi: 10.1038/sj.cdd.4401249. [DOI] [PubMed] [Google Scholar]
- 235.Tulpule K. Dringen R. Formaldehyde stimulates Mrp1-mediated glutathione deprivation of cultured astrocytes. J Neurochem. 2011;116:626–635. doi: 10.1111/j.1471-4159.2010.07150.x. [DOI] [PubMed] [Google Scholar]
- 236.Vahrmeijer AL. Hoetelmans RW. Mulder GJ. Schutrups J. van Vlierberghe RL. van de Velde CJ. van Dierendonck JH. Development of resistance to glutathione depletion-induced cell death in CC531 colon carcinoma cells: association with increased expression of bcl-2. Biochem Pharmacol. 2000;59:1557–1562. doi: 10.1016/s0006-2952(00)00286-0. [DOI] [PubMed] [Google Scholar]
- 237.Valko M. Morris H. Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 238.Valverde M. Rojas E. Kala SV. Kala G. Lieberman MW. Survival and cell death in cells constitutively unable to synthesize glutathione. Mutat Res. 2006;594:172–180. doi: 10.1016/j.mrfmmm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 239.van den Dobbelsteen DJ. Nobel CS. Schlegel J. Cotgreave IA. Orrenius S. Slater AF. Rapid and specific efflux of reduced glutathione during apoptosis induced by anti-Fas/APO-1 antibody. J Biol Chem. 1996;271:15420–15427. doi: 10.1074/jbc.271.26.15420. [DOI] [PubMed] [Google Scholar]
- 240.Van Luyn MJ. Muller M. Renes J. Meijer C. Scheper RJ. Nienhuis EF. Mulder NH. Jansen PL. De Vries EG. Transport of glutathione conjugates into secretory vesicles is mediated by the multidrug-resistance protein 1. Int J Cancer. 1998;76:55–62. doi: 10.1002/(sici)1097-0215(19980330)76:1<55::aid-ijc10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 241.Varghese J. Khandre NS. Sarin A. Caspase-3 activation is an early event and initiates apoptotic damage in a human leukemia cell line. Apoptosis. 2003;8:363–370. doi: 10.1023/a:1024121017841. [DOI] [PubMed] [Google Scholar]
- 242.Voehringer DW. Meyn RE. Redox aspects of Bcl-2 function. Antioxid Redox Signal. 2000;2:537–550. doi: 10.1089/15230860050192314. [DOI] [PubMed] [Google Scholar]
- 243.Wang H. Li J. Follett PL. Zhang Y. Cotanche DA. Jensen FE. Volpe JJ. Rosenberg PA. 12-Lipoxygenase plays a key role in cell death caused by glutathione depletion and arachidonic acid in rat oligodendrocytes. Eur J Neurosci. 2004;20:2049–2058. doi: 10.1111/j.1460-9568.2004.03650.x. [DOI] [PubMed] [Google Scholar]
- 244.Wang W. Ballatori N. Endogenous glutathione conjugates: occurrence and biological functions. Pharmacol Rev. 1998;50:335–356. [PubMed] [Google Scholar]
- 245.Will Y. Kaetzel RS. Brown MK. Fraley TS. Reed DJ. In vivo reversal of glutathione deficiency and susceptibility to in vivo dexamethasone-induced apoptosis by N-acetylcysteine and L-2-oxothiazolidine-4-carboxylic acid, but not ascorbic acid, in thymocytes from gamma-glutamyltranspeptidase-deficient knockout mice. Arch Biochem Biophys. 2002;397:399–406. doi: 10.1006/abbi.2001.2662. [DOI] [PubMed] [Google Scholar]
- 246.Winterbourn CC. Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 247.Xu K. Thornalley PJ. Involvement of glutathione metabolism in the cytotoxicity of the phenethyl isothiocyanate and its cysteine conjugate to human leukaemia cells in vitro. Biochem Pharmacol. 2001;61:165–177. doi: 10.1016/s0006-2952(00)00526-8. [DOI] [PubMed] [Google Scholar]
- 248.Xu X. Chua CC. Kong J. Kostrzewa RM. Kumaraguru U. Hamdy RC. Chua BH. Necrostatin-1 protects against glutamate-induced glutathione depletion and caspase-independent cell death in HT-22 cells. J Neurochem. 2007;103:2004–2014. doi: 10.1111/j.1471-4159.2007.04884.x. [DOI] [PubMed] [Google Scholar]
- 249.Yang J. Bogni A. Schuetz EG. Ratain M. Dolan ME. McLeod H. Gong L. Thorn C. Relling MV. Klein TE. Altman RB. Etoposide pathway. Pharmacogenet Genomics. 2009;19:552–553. doi: 10.1097/FPC.0b013e32832e0e7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Yang Z. Wang ZE. Doulias PT. Wei W. Ischiropoulos H. Locksley RM. Liu L. Lymphocyte development requires S-nitrosoglutathione reductase. J Immunol. 2010;185:6664–6669. doi: 10.4049/jimmunol.1000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Yuan H. Perry CN. Huang C. Iwai-Kanai E. Carreira RS. Glembotski CC. Gottlieb RA. LPS-induced autophagy is mediated by oxidative signaling in cardiomyocytes and is associated with cytoprotection. Am J Physiol Heart Circ Physiol. 2009;296:H470–H479. doi: 10.1152/ajpheart.01051.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Yuan L. Kaplowitz N. Glutathione in liver diseases and hepatotoxicity. Mol Aspects Med. 2009;30:29–41. doi: 10.1016/j.mam.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 253.Zamzami N. Marzo I. Susin SA. Brenner C. Larochette N. Marchetti P. Reed J. Kofler R. Kroemer G. The thiol crosslinking agent diamide overcomes the apoptosis-inhibitory effect of Bcl-2 by enforcing mitochondrial permeability transition. Oncogene. 1998;16:1055–1063. doi: 10.1038/sj.onc.1201864. [DOI] [PubMed] [Google Scholar]
- 254.Zhang H. Limphong P. Pieper J. Liu Q. Rodesch CK. Christians E. Benjamin IJ. Glutathione-dependent reductive stress triggers mitochondrial oxidation and cytotoxicity. FASEB J. 2012;26:1442–1451. doi: 10.1096/fj.11-199869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhang Z. Liew CW. Handy DE. Zhang Y. Leopold JA. Hu J. Guo L. Kulkarni RN. Loscalzo J. Stanton RC. High glucose inhibits glucose-6-phosphate dehydrogenase, leading to increased oxidative stress and beta-cell apoptosis. FASEB J. 2010;24:1497–1505. doi: 10.1096/fj.09-136572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhu J. Krom BP. Sanglard D. Intapa C. Dawson CC. Peters BM. Shirtliff ME. Jabra-Rizk MA. Farnesol-induced apoptosis in Candida albicans is mediated by Cdr1-p extrusion and depletion of intracellular glutathione. PLoS One. 2011;6:e28830. doi: 10.1371/journal.pone.0028830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zimmermann AK. Loucks FA. Schroeder EK. Bouchard RJ. Tyler KL. Linseman DA. Glutathione binding to the Bcl-2 homology-3 domain groove: a molecular basis for Bcl-2 antioxidant function at mitochondria. J Biol Chem. 2007;282:29296–29304. doi: 10.1074/jbc.M702853200. [DOI] [PMC free article] [PubMed] [Google Scholar]