Abstract
Significance: Glutaredoxin (Grx) is the primary enzyme responsible for catalysis of deglutathionylation of protein-mixed disulfides with glutathione (GSH) (protein-SSG). This reversible post-translational modification alters the activity and function of many proteins important in regulation of critical cellular processes. Aberrant regulation of protein glutathionylation/deglutathionylation reactions due to changes in Grx activity can disrupt both apoptotic and survival signaling pathways. Recent Advances: Grx is known to regulate the activity of many proteins through reversible glutathionylation, such as Ras, Fas, ASK1, NFκB, and procaspase-3, all of which play important roles in control of apoptosis. Reactive oxygen species and/or reactive nitrogen species mediate oxidative modifications of critical Cys residues on these apoptotic mediators, facilitating protein-SSG formation and thereby altering protein function and apoptotic signaling. Critical Issues: Much of what is known about the regulation of apoptotic mediators by Grx and reversible glutathionylation has been gleaned from in vitro studies of discrete apoptotic pathways. To relate these results to events in vivo it is important to examine changes in protein-SSG status in situ under natural cellular conditions, maintaining relevant GSH:GSSG ratios and using appropriate inducers of apoptosis. Future Directions: Apoptosis is a highly complex, tightly regulated process involving many different checks and balances. The influence of Grx activity on the interconnectivity among these various pathways remains unknown. Knowledge of the effects of Grx is essential for developing novel therapeutic approaches for treating diseases involving dysregulated apoptosis, such as cancer, heart disease, diabetes, and neurodegenerative diseases, where alterations in redox homeostasis are hallmarks for pathogenesis. Antioxid. Redox Signal. 17, 1748–1763.
Overview
The well-known glutaredoxins (Grxs) are small, heat-stable proteins responsible for specific catalysis of removal of the glutathionyl moiety from protein-mixed disulfides (deglutathionylation). This review examines potential mechanisms of glutathionylation and deglutathionylation of protein cysteine residues implicated in the regulation of apoptosis. In particular, the regulatory role of the Grxs in apoptosis is addressed, focusing on critical protein mediators in cell death pathways whose function is affected by reversible glutathionylation and the thiol-disulfide oxidoreductase activity of the Grxs.
In relevant studies of apoptosis there is considerable variability in model systems, Grx isoforms, and cellular insults. It is clear that Grx is important for regulating cell death signaling; however, it is still uncertain which components of apoptosis signaling pathways are subjected to regulation by the Grxs in different contexts. Future research should aim to fill in the gaps, clarifying the Grx isoforms important in each circumstance; investigating multiple cellular insults; deciphering the mechanisms of activation, induction, and degradation of Grx; and identifying continuity in Grx regulation targets across multiple cell types.
The majority of research concerning the role of Grxs in apoptosis has been completed in mammalian systems, but Grx is likely very important for regulating apoptosis in many other organisms. A few studies investigating the influence of Grxs on apoptosis in yeast and plants have been reported (see section on Grx Substrate Specificity and Isoforms), but more research is needed to better understand the regulatory role of the Grxs in cell death in these organisms.
Protein Glutathionylation and Deglutathionylation: Role in Cellular Function
Reversible, covalent modifications of proteins are critical for signal transduction and cellular homeostasis. These modifications are often capable of altering highly controlled cellular processes, such as transcription, protein expression, protein degradation, and pro-apoptotic and anti-apoptotic signaling. There are many different types of reversible protein modifications, such as phosphorylation, acetylation, ubiquitination, and so on. Particularly pertinent to this review is the regulatory covalent modification, S-glutathionylation; that is, the formation of this mixed disulfide bond between glutathione (GSH) and a Cys residue (protein-SSG). S-glutathionylation plays a critical role in regulation of thiol/disulfide homeostasis within the cell and functions in protecting Cys residues from irreversible oxidative damage (i.e., formation of sulfinic or sulfonic acid). Reversible formation of specific protein-SSG intermediates is recognized as an important mechanism for the transduction of redox signaling, serving to regulate many key cellular processes. However, overproduction of glutathionylated proteins is an indicator of oxidative stress, potentially leading to cell death. Thus, alterations in cellular sulfhydryl homeostasis, particularly in the steady-state glutathionylation of specific regulatory proteins, have been shown to modulate signaling in various pathways, ultimately shifting the balance from cell survival to cell death.
Mechanisms of protein glutathionylation
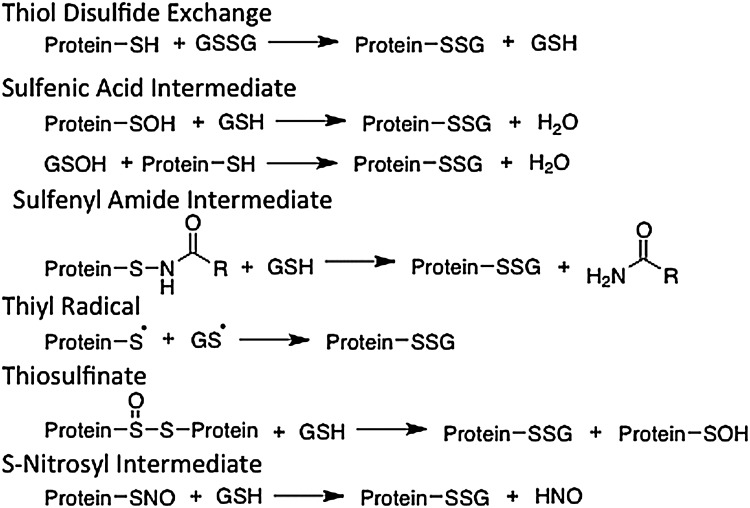
Reversible S-glutathionylation has been implicated in regulation of the structure and function of many proteins (21–24, 30, 68, 69, 94); however, the mechanisms of formation of protein-SSG are yet to be established. Based upon previous studies, there is evidence supporting several mechanisms for protein glutathionylation (27, 68). These mechanisms include thiol-disulfide exchange, sulfenic acid intermediates, sulfenylamide intermediates, thiyl radicals, thiosulfinates, and S-nitrosylated intermediates (Fig. 1). It is also possible that certain enzymes like glutathione-S-transferase or peroxidase may catalyze protein glutathionylation; however, documentation of such catalysis has been very limited to date (35, 106).
FIG. 1.
Potential mechanisms of protein glutathionylation. There are multiple possible mechanisms for the formation of protein-SS-glutathione mixed disulfides. These mechanisms involve the activation of a free thiol, either of the protein or glutathione to a sulfenic acid, or formation of sulfenylamide, thiyl radical, thiosulfinates, or S-nitrosyl intermediates. These potential mechanisms are discussed in previous reviews (27,68).
The typical pKa of protein Cys residues is ∼8.5, but cysteines with unusually low pKas are favored sites for glutathionylation. The thiol group of these residues exists primarily as a thiolate, and is likely to undergo nucleophilic substitution reactions with oxidized glutathione (GSSG) if the relative concentrations are sufficient, resulting in formation of a glutathionylated protein. However, the typical redox potential of protein-Cys moieties precludes substantial formation of protein-SSG via thiol-disulfide exchange with GSSG unless the GSSG concentration is unusually high. Thus, for most protein-SH the intracellular GSH/GSSG ratio would have to be shifted from ∼100:1 to ∼1:1 in order for 50% of the protein to be converted to protein-SSG (32). A notable exception is c-Jun whose redox potential would favor glutathionylation at lower GSSG concentrations, but the reaction must also be kinetically favorable for this to be a significant regulatory modification (52). In order for most Cys residues to be glutathionylated in a timely fashion, there must first be an activated intermediate formed, such as sulfenic acid (protein-SOH+GSH) or thiyl radical (protein-S.+GS.). These reactive thiols result from local increases in reactive oxygen species (ROS) or reactive nitrogen species (RNS), and may occur on either protein or nonprotein thiols, such as GSH. The oxidized thiols (i.e., GS-OH, GS-NO, and protein-SOH) participate in the aforementioned reaction mechanisms resulting in protein glutathionylation, as depicted in Figure 1. The mechanisms and relative susceptibility of thiols to undergo glutathionylation have been reviewed in more detail previously (21).
According to these proposed mechanisms, reversible protein glutathionylation is dependent upon the redox status of the cell. Factors that affect the redox status include ROS and RSN and disruptions of the relative ratio of GSH:GSSG (typically >100:1). At high levels of oxidative insult, however, irreversible modification of reactive thiols may occur, that is, sulfinic acid (protein-SO2H) or sulfonic acid (protein-SO3H). These irreversibly modified thiols cannot be protected by glutathionylation and result in protein damage and accelerated degradation.
Mechanisms of protein deglutathionylation by Grx
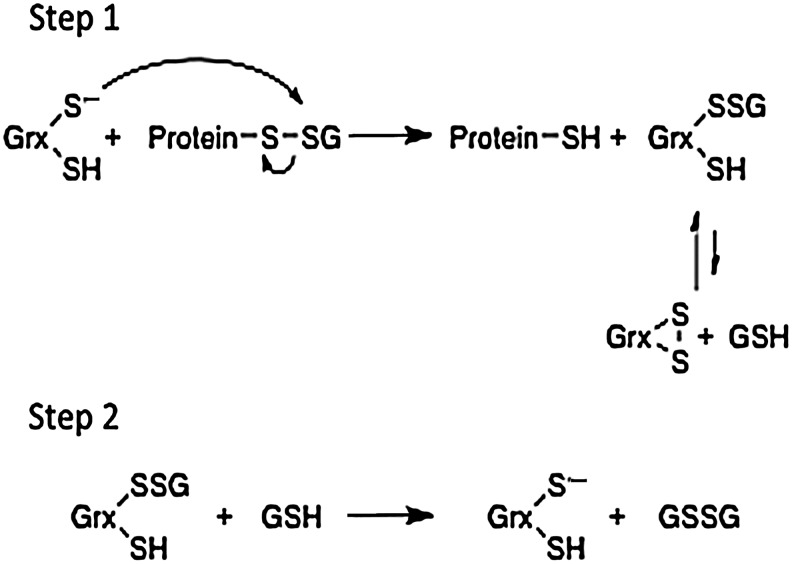
An important characteristic of glutathionylation is that it is a reversible modification of proteins. While the mechanism of the formation of glutathionylated proteins is not well understood, deglutathionylation has been well studied and known to be catalyzed by many members of the Grx family of enzymes. Typically, the Grx enzymes are heat stable, and low-molecular-weight proteins (∼10–16 kDa), although a number of multifunctional, multidomain proteins are also known to contain the Grx domain (102). The mechanism of Grx catalysis has been characterized most extensively for human Grx1, which has been thoroughly discussed in previous reviews, along with nuances in the catalytic properties of other forms of Grx (27, 29, 30, 68, 69), and involves the removal of GSH moieties from Cys residues of proteins. This deglutathionylation occurs through a thiol-disulfide exchange reaction involving sequential nucleophilic displacements. In this reaction, the thiolate moiety of Grx attacks the mixed disulfide on a protein (protein-SSG), resulting in glutathionylated Grx (Grx-SSG) and the reduced protein (protein-SH) (Step 1). This is followed by displacement of the reduced Grx enzyme by GSH in the rate-limiting step of the overall reaction (Step 2). This reaction produces oxidized glutathione (GSSG) as the second product (Fig. 2).
FIG. 2.
Mechanism of protein deglutathionylation catalyzed by glutaredoxin (Grx). The first step of the Grx deglutathionylation reaction is nucleophilic attack of the enzyme's thiolate anion on the glutathionyl sulfur atom of the mixed disulfide of the glutathionylated protein. This results in the formation of a reduced protein and a glutathionylated Grx intermediate. The enzyme intermediate then can form either an intramolecular disulfide (side reaction) or be reduced by glutathione (GSH) (step 2), regenerating Grx and forming glutathione disulfide (GSSG).
The active site of many Grxs, represented by human Grx1, contains the sequence CPYC. Even though there are two Cys residues present, the efficient deglutathionylation mechanism, as outlined in Figure 2, only uses one Cys, Cys22, acting through a monothiol mechanism. The low pKa of Cys22 (3.5) means that this thiol exists as a thiolate anion at physiological pH, making it a good nucleophile and an especially good leaving group in the catalytic reaction. A dithiol mechanism (utilizing both the N-terminal and C-terminal cysteines of the CXXC active site) has been proposed in other contexts (8,30), but is not as efficient as the monothiol mechanism for deglutathionylation, as confirmed with site-directed mutagenesis. In fact, the presence of both Cys residues enables formation of an intramolecular disulfide bond that diverts the catalytic cycle and hinders activity of Grx (illustrated in Fig. 2). Thus, the Ser mutation of the second Cys renders the corresponding human Grx1 and Grx2 more efficient as deglutathionylation catalysts than the wild-type enzymes (111). However, this is not a universal phenomenon because the corresponding mutant of E. coli Grx1 does not display enhanced catalytic activity (89).
Grx Substrate Specificity and Isoforms
Grxs, initially referred to as “thioltransferases,” were first studied in 1974 (6). The first enzyme bearing the name “glutaredoxin” was reported in E. coli by Holmgren et al. in 1976 (42). It was later determined that Grxs and thioltransferases belonged in the same family, according to homologies of sequence, structure, and function. While “thioltransferase” more accurately describes the function of this family of enzymes, “glutaredoxin” has become the universally utilized name. There are many isoforms of Grx characterized in a variety of organisms, including yeast, plants, and mammals. These enzymes are responsible for deglutathionylating proteins, thereby mediating sulfhydryl homeostasis under oxidative stress, as well as controlling redox signaling pathways that mediate critical cellular functions like proliferation and apoptosis. Certain Grx isoforms are also implicated in other cellular functions, such as redox cycling of ascorbate and iron homeostasis.
Mammalian Grxs
There are two prominent and well-characterized isoforms of mammalian Grx, namely Grx1 and Grx2, as reviewed in (68). These isoforms share ∼30% homology and differ in their localization and substrate specificity (68, 94). Grx1 is primarily localized to the cytosol, but has also been documented to reside in the intermembrane space (IMS) of the mitochondria, and possibly the nucleus in certain cells. Grx1 has been extensively characterized and is ∼10 times more active than Grx2 whose catalytic mechanism mirrors that of Grx1. The lower catalytic efficiency of Grx2 is explained in part by the higher active site pKa for Grx2 (pKa=4.5) compared with Grx1 (pKa=3.5). Additionally, Grx2 is less effective at enhancing the nucleophilicity of GSH for turning over the enzyme-SSG intermediate (Fig. 1, Step 2). The Grx2 isoform is primarily found in the mitochondrial matrix where its estimated concentration is ∼10-fold higher than the concentration of Grx1 in the mitochondrial IMS, potentially compensating for their difference in catalytic activity (19). However, there is the additional complication that Grx2 may in large part be bound as a catalytically inactive dimer to an Fe2S2 iron sulfur cluster. Studies of the isolated dimeric Grx2–iron sulfur complex suggest that active, monomeric Grx2 could be dissociated under oxidative stress conditions (60), thereby triggering its deglutathionylase function (the potential roles of the mitochondrial Grx1 and Grx2 enzymes in regulating apoptosis are considered in the next paragraph). Besides the mitochondrial location, there is evidence that Grx2 is also found in the nucleus, but this localization has been established only for cells of the reproductive organs. Nevertheless, nuclear location of active Grx2 would suggest a regulatory role in deglutathionylation of transcription factors (see the NFκB section).
Grx1 and monomeric Grx2 are capable of catalyzing both the reduction (deglutathionylation) and formation of S-glutathionylated proteins, depending on the redox conditions and the relative concentrations of GSH, GSSG, protein-SH, and protein-SSG. Enzymes catalyze the approach to equilibrium but do not alter the position of equilibrium; therefore, in order for the Grx enzymes to accelerate formation of protein-SSG, the concentration of GSSG must be high relative to GSH, a situation not likely in vivo. A more likely scenario for protein-SSG formation by Grx is through a thiyl radical mechanism, as described previously (29, 80). Additional discussion of the catalytic mechanisms of mammalian Grx1 and Grx2 is provided in a previous review (30). Also described in that review are the less well-known Grx3 (PICOT) and Grx5 isoforms that belong to a different Grx family and are not known to display thiol-disulfide oxidoreductase activity. Mammalian Grx3 and Grx5 correspond to the yeast isoforms Grx3/4 and Grx5 that are described in more detail in the next section. The mammalian CSYC-type Grx2 and CGFS-type Grx5 both bind iron-sulfur clusters and have been implicated in iron homeostasis, analogously to particular yeast Grx isoforms that have been well characterized in this role (71; see the Yeast Grxs section). To more fully understand the cellular functions of the mammalian Grx isoforms, additional studies are necessary to distinguish their specificities for different protein-SSG substrates in different cellular locations, as well as to understand their relative contributions to other functions, such as iron transport and maintenance of iron-sulfur clusters. In this regard, recent studies implicated Grx5, which is naturally expressed at relatively high levels in bone cells, as playing an anti-apoptotic regulatory role in osteoblasts (62). However, it was not determined whether the manipulations of Grx5 content (knockdown or overexpression), which corresponded to changes in oxidant-induced apoptosis of the osteoblasts, were due to alterations in thiol-disulfide homeostasis or iron-sulfur cluster homeostasis.
Yeast Grxs
Grxs are found in many other organisms in addition to mammals. In Saccharomyces cerevisiae, there are 8 isoforms of Grx classified into three subfamilies, as discussed in (17, 37, 66, 71). The first subfamily contains the dithiol isoforms Grx1p, Grx2p, and Grx8p. Grx1p and Grx2p contain the common Grx active site CPYC, whereas Grx8p has a CPDC active site. Monothiol forms of Grx are grouped in the second subfamily, and contain only a single active-site Cys residue. In these isoforms, a Ser residue replaces the second, C-terminal Cys residue in the active site. Grx3p, Grx4p, Grx5p, Grx6p, and Grx7p are in this second subfamily. Grx3p and Grx4p are also members of the third family, classified separately because they have an extra thioredoxin domain. Grx isoforms in yeast also vary in localization. Many are cytosolic, such as Grx1p and Grx2p, but Grx2p can also be found in the mitochondria along with Grx5p. The Grx3p and 4p isoforms have been found mainly in the nuclear fraction of S. cerevisiae (22, 29). While further research is necessary to fully characterize the function and protein targets of the Grxs in yeast, these enzymes are known to be important for antioxidant defenses and iron homeostasis (37, 49, 71). Notably, the isoform that displays the highest deglutathionylase activity is Grx2p, whereas the monothiol Grxs typically do not catalyze deglutathionylation (25, 38).
Plant Grxs
In plants, Grxs comprise a very complex and diverse family of enzymes that are found in both photosynthetic prokaryotes and eukaryotes. The number of Grx genes found in different types of plants varies greatly, with only a few Grx genes found in photosynthetic prokaryotes and lower eukaryotes (17). In higher plants, over 30 genes coding for Grxs have been identified, and they have been categorized into six classes, as reviewed in (84, 115). The first two classes (I and II) are found in all plants from cyanobacteria to higher plants. Class I includes Grxs that contain an active site motif of CXXC or CXXS, and these can be divided further into five subclasses: GrxC1, C2, C3, C4, and C5/S12. The letter following “Grx” (C or S) indicates the identity of the amino acid at the fourth position of the active site (CXXC or CXXS). The active site of Class II Grxs is CGFS, and these are divided into four subclasses: GrxS14, S15, S16, and S17. Class III and Class IV Grxs are found primarily in land plants and photosynthetic eukaryotes (i.e., algae), whereas Class V and Class VI are found in cyanobacteria.
Many plants code for several Grxs; for example, Arabidopsis thaliana has 14 Grx genes with a CXXC active site and 17 genes for Grxs with a CXXS active site (86). The cellular localization of the Grxs is also diverse with Grxs found in chloroplasts, the nucleus, cytosol, apoplasm, mitochondrial matrix, and stroma. While most research has focused on genetic analysis, there have been a few studies on the function of Grxs within plants. The well-documented functions of Grx include regulation of the stress response, iron-sulfur cluster formation (83, 84, 88), plant development (12, 59, 67, 72, 110), and catalysis of deglutathionylation (18, 31, 104, 116). GrxS12 has been shown to operate via a catalytic deglutathionylation mechanism fully analogous to the mammalian Grxs (29, 114).
The function of Grxs has not been completely elucidated in plants. This family of enzymes is thought to be involved in processes, such as iron homeostasis, the formation of iron-sulfur clusters, oxidative stress response, petal development, and transcription factor regulation (84, 86–88). A recent review is focused particularly on protein S-glutathionylation in plants, implicating the Grxs in regulatory roles (115). The cell death mechanisms in plants are not as well defined as they are in mammals. This is due primarily to the lack of protein orthologs between mammals and plants for apoptosis signaling (39). It is likely that Grxs play a role in regulating apoptosis in plants; however, additional research on apoptotic mechanisms and the interaction between critical proteins and Grx in plants is compulsory before any definitive conclusions can be made.
Glutathionylation and Apoptosis in Mammals
Apoptosis is a complex and highly regulated cellular process that is responsible for programmed cell death. It removes severely damaged, senescent, transformed, infected, or precursor cells to allow for functionally advanced cells. The apoptotic process involves cell shrinkage, nuclear condensation, nuclear fragmentation, cleavage of chromosomal DNA, and the formation of apoptotic bodies. Apoptosis may be initiated from exposure to extracellular factors, such as environmental toxicants, growth factors, and cytokines, or from intracellular signals, including ER or mitochondrial stress, protein/DNA damage, and hypoxia. Oxidative and nitrosative stress can serve as both intrinsic and extrinsic factors.
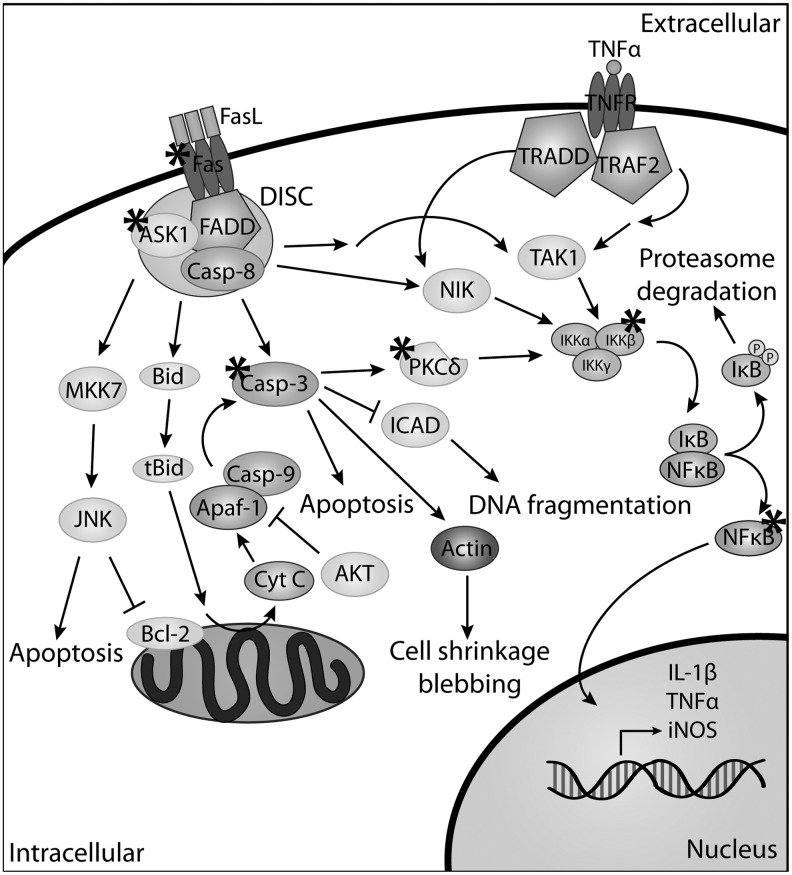
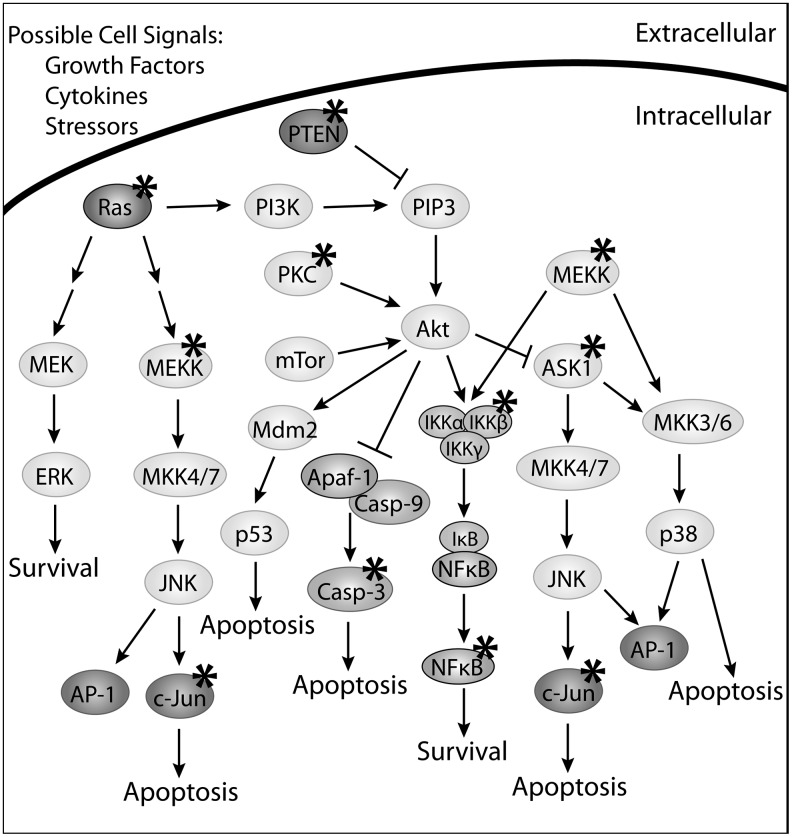
Cells have the innate ability to detect, interpret, and respond to intrinsic and extrinsic stressors allowing them to adapt and maintain homeostasis. When the stress is beyond compensatory survival mechanisms, the cellular response may involve the upregulation of apoptotic proteins or changes in post-translational modifications of specific regulatory proteins. Generalized overviews of several apoptotic pathways are outlined in Figures 3 and 4. Figure 3 highlights the downstream effectors of Fas ligand (FasL) and tumor necrosis factor α (TNFα) signaling. Figure 4 focuses on the downstream mediators in the rat sarcoma (Ras) and Akt (protein kinase B) pathways. While these figures do not include all of the proteins involved in the regulation of apoptosis, they feature proteins that are affected, directly or indirectly, by Grx.
FIG. 3.
Overview of apoptotic signaling through tumor necrosis factor superfamily receptor 6 (Fas) and tumor necrosis factor receptor (TNFR). When Fas ligand (FasL) (CD95L) binds to Fas it initiates the formation of the death-inducing signaling complex (DISC) involving the association of Fas-associated death domain (FADD) and pro-caspase-8 (pro-casp-8). In the absence of caspase-8-FADD-like interleukin-1β-converting enzyme inhibitory protein (c-FLIP), pro-caspase-8 is activated and initiates the caspase proteolytic cascade, leading to apoptosis. DISC formation can also lead to the activation of the MKK7 pathway and jun N-terminal kinase (JNK) phosphorylation, resulting in apoptosis. There are other cell-type-specific pathways that are affected by FasL. One of these pathways involves Bid, which can become truncated Bid (tBID) leading to the release of cytochrome C, and subsequently caspase activation. FasL can also stimulate the IκB kinase (IKK)/NFκB pathway resulting in the phosphorylation of IκB, leading to proteasome degradation and the translocation of NFκB to the nucleus. NFκB is responsible for initiating transcription of proteins, such as Grx, iNOS, and TNFα. The NFκB pathway can also be activated through the binding of TNFα to the TNFR, acting through NIK or TAK1. Proteins in these pathways for which there is experimental evidence implicating that they are regulated by Grx and protein glutathionylation are indicated by an asterisk (*).
FIG. 4.
Signaling pathways mediated by rat sarcoma (Ras) and protein kinase B (Akt). Ras and Akt are key apoptosis mediators responsible for several regulatory pathways. There is experimental evidence to implicate that many of the proteins in these pathways as being regulated by Grx and protein glutathionylation, as indicated by an asterisk (*). Depending on the cellular insult, cell type, and protein target, alterations in protein glutathionylation may result in cell survival or apoptosis. Ras is responsible for mediating the Akt, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase (MEKK)/extracellular signal-regulated kinase (ERK), and MKK/JNK pathways. Glutathionylated Ras is an active form of this protein that can lead to increased Akt activity and therefore cell survival via the nuclear factor kappa-light chain enhancer of activated B cells (NFκB) cascade. When Grx deglutathionylates Ras, this can result in activation of the MEKK pathway and inhibition of Akt, resulting in apoptosis. An upstream regulator of Akt is phosphatase and tensin homolog (PTEN), which is responsible for dephosphorylating PIP3, preventing the activation of Akt. PTEN-SSG is the inactive form of this protein, and is no longer able to dephosphorylate PIP3. Therefore, in the absence of Grx, PIP3 is phosphorylated and capable of activating Akt, resulting in cell survival. Like PTEN-SSG, protein kinase C (PKC) is also inhibited when it is glutathionylated. However, this protein modification prevents PKC's ability to phosphorylate and activate Akt, causing apoptosis. The downstream effectors of Akt include apoptosis signaling kinase (ASK1), Apaf-1/caspase-9, and the NFκB pathway. Many of these proteins can also be regulated by Grx and protein glutathionylation. Grx is capable of binding to ASK1 resulting in the inhibition of this protein and ultimately cell survival. Grx regulates the NFκB pathway via deglutathionylation of IKK and NFκB. Grx is implicated in many of the cellular survival/death signaling mechanisms, evident by the number of proteins potentially regulated by Grx and glutathionylation in these pathways.
A more recent discovery concerning apoptosis is the importance of Grx and the diverse role protein glutathionylation has in regulating pathways involved in cell death. Just as apoptosis is influenced by intrinsic and extrinsic factors, such as oxidative stress or cytokines, so can the activity and expression of Grx. Protein glutathionylation is a consequence of oxidative/nitrosative stress, where an increase in ROS and/or RNS can lead to the activation of Cys residues, leaving them more susceptible to S-glutathionylation. The effects of changes in Grx activity on critical proteins involved in apoptosis are described in detail in the sections immediately following, particularly focusing on the interdependence of Grx and apoptosis susceptibility and how alterations to protein glutathionylation/deglutathionylation status affect apoptotic signaling.
Fas and FasL
TNF receptor 6 (Fas, CD95, and Apo-1) is a constitutively expressed member of the TNF receptor (TNFR) superfamily containing a death domain (DD). Fas-mediated apoptosis, discussed in more detail in (57), is initiated by the release of FasL (a TNF cytokine) from lymphocyte lysosomes. When FasL (CD95L) binds to Fas it initiates the formation of the death-inducing signaling complex (DISC). DISC formation involves the association of Fas-associated death domain (FADD), followed by the binding of pro-caspase-8/10. In the absence of caspase-8-FADD-like interleukin-1β-converting enzyme inhibitory protein (c-FLIP), pro-caspase-8/10 is activated and initiates the caspase proteolytic cascade (Fig. 3). This downstream process involves the activation of effector caspases (i.e., pro-caspase-3, 6, and 7), resulting in the proteolytic cleavage of their cellular protein targets, thereby promoting apoptosis. However, when c-FLIP is bound to the death effector domain of Fas, pro-caspase-8 activation is inhibited. This prevents the caspase cascade and prevents apoptosis that would have otherwise been mediated by FasL.
In addition to regulation by c-FLIP, Fas-mediated apoptosis was also found to be dependent on the activity of Grx1 and the glutathionylation status of Fas. In epithelial cells (C10) and lung fibroblasts, Grx1 was shown to play an anti-apoptotic role by maintaining deglutathionylated Fas (2). Accordingly, decreased content and activity of Grx1 was observed upon exposure of the epithelial cells to FasL, which corresponded to an increase of glutathionylated Fas (Fas-SSG) and increased apoptosis.
The glutathionylation of Fas occurs at Cys294, located at the C-terminus of the DD (2). How this modification occurs and how the glutathionylation status of Fas affects the interaction of this receptor with c-FLIP is unknown, so further investigation is necessary to elucidate the detailed molecular mechanism. The glutathionylation appears to be initiated by an increase in ROS, although independently of NADPH oxidase action (2). Glutathionylation of Cys294 is not influenced by the status of Cys194, a known palmitoylation site. While these two cysteinyl post-translational modifications occur independently, either modification results in an increased localization of Fas into lipid rafts. This localization facilitates FasL binding and DISC assembly, leading to the propagation of the initial apoptosis signal (Fig. 5). As noted, Grx1 functions in this pathway to prevent apoptosis through the deglutathionylation of Fas at Cys294 (3). It is possible that an analogous mechanism may occur in other receptors containing a DD, such as TNF-related apoptosis-inducing ligand receptor 1 (TRAIL R1), a member of the TNFR superfamily. In addition, changes in Grx1 content/activity are reported to affect other apoptotic control mechanisms, such as modulation of caspase-3 activity, described in the next section, Caspase-3 and pro-caspase-3.
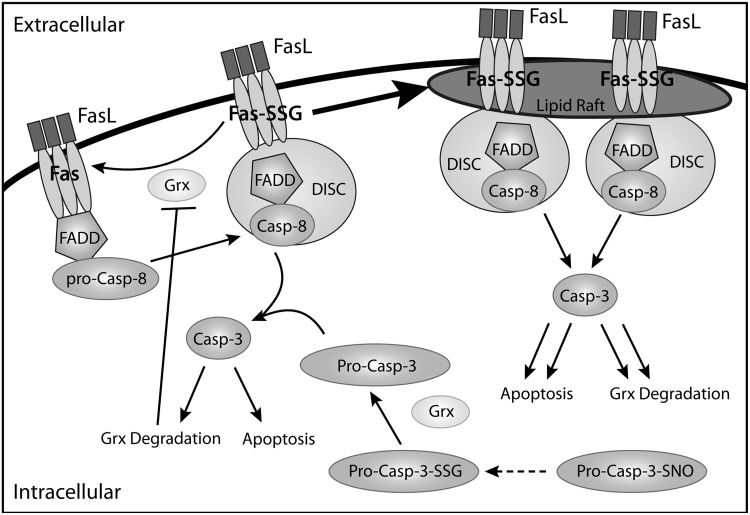
FIG. 5.
Role of glutathionylation in the Fas/FasL pathway. In C10 epithelial cells, pro-caspase-8 is activated when c-FLIP dissociates from Fas, resulting in the formation of DISC and the activation of pro-caspase-3 (pro-casp-3). Caspase-3 (Casp-3) is capable of degrading Grx, which can increase the number of glutathionylated Fas proteins. Fas-SSG is more readily recruited into lipid rafts, facilitating and propagating this apoptotic signal leading to enhanced Grx degradation and apoptosis, as described in (3). It is important to note that an alternative role of Grx in promoting apoptosis was reported (76); namely, in response to TNFα stimulation of endothelial cells, Grx is activated and catalyzes deglutathionylation of pro-caspase-3, thereby enabling proteolytic cleavage and release of the active caspase 3. Pro-caspase-3-SSG, which may be formed from pro-caspase-3-SNO, is unable to be cleaved, preventing Grx degradation and Fas deglutathionylation, thereby preventing apoptosis.
Caspase-3 and pro-caspase-3
Caspase 3, a cysteine protease, plays a pivotal role in the execution of most apoptotic cascades, operating at a point of convergence for several signaling pathways. It is activated by caspase-8-mediated proteolysis of pro-caspase-3, and is responsible for the proteolysis of many important proteins, eventually resulting in cell death. Due to its critical role, caspase 3 activity is subjected to redox regulation by several mechanisms, including reversible glutathionylation and corresponding regulation by Grx (76). In addition, S-nitrosylation of pro-caspase-3 has been reported to prevent its cleavage and activation (3). This modification can be reversed by thioredoxin (61), the other principal thiol-disulfide oxidoreductase that plays a complementary role with Grx in sulfhydryl homeostasis. S-nitrosylation may also be a precursor to protein glutathionylation as observed in other contexts [reviewed in (27), see Fig. 1].
While the mechanism(s) of S-glutathionylation of pro-caspase-3 remains hypothetical, evidence supports the deglutathionylation being catalyzed by Grx (76). In this study, treatment of endothelial cells with TNFα led to an increase in Grx activity and concomitant decrease in pro-caspase-3-SSG, resulting in increased pro-caspase-3 cleavage and cell death. Accordingly, Grx knockdown was associated with a decrease in caspase-3-mediated cellular death (76). Grx was also found to co-precipitate with caspase-3 after treatment of the endothelial cells with TNFα, further supporting the interaction of these two proteins. These correlations imply that Grx functions in this context to propagate apoptotic signals, and that glutathionylation of pro-caspase-3 protects against apoptosis.
The studies of pro-caspase 3-SSG (76) and Fas-SSG [described above (3)] suggest opposing functions of Grx-catalyzed deglutathionylation. Whereas Grx catalysis of pro-caspase-3 deglutathionylation would promote apoptosis, Grx catalysis of deglutathionylation of Fas-SSG would inhibit apoptosis. In addition, the Grx-mediated activation of pro-caspase-3 by deglutathionylation, facilitating its proteolytic conversion to caspase 3, appears to be self-limiting because caspase-3 can in turn mediate degradation of Grx1, at least in murine alveolar type II epithelial cells (C10 cells) (3). The initial decrease of Grx protein content in that study was correlated with increased caspase-3 content, and the direct proteolytic action of caspase 3 on Grx was confirmed with the isolated human recombinant caspase 3 and Grx proteins. Based on these results and the role Grx plays in deglutathionylating Fas to prevent apoptosis, the degradation of Grx would reinforce the commitment of the cell to apoptosis in this particular context, but it would inhibit apoptosis in the context where glutathionylation of pro-caspase-3 appears to be a prominent mechanism of limiting apoptosis (76).
These competing regulatory mechanisms for apoptosis may be due to differences in cell type (C10 vs. endothelial cells) or stimuli (FasL vs. TNFα), or they may be representative of the complexity and interconnectivity of many of the apoptotic pathways. To better discern the relationship between Grx and pro-caspase-3, it will be important to determine whether Grx is degraded after the activation of pro-caspase-3 in the C10 cells, as was observed in the endothelial cells. This observation would suggest a negative feedback loop between caspase-3 and Grx, such that Grx activity promotes the activation of pro-caspase-3, which upon conversion to caspase-3 is capable of degrading Grx. To fully understand the interaction of Grx with caspase-3, it will also be important to identify the specific residues on pro-caspase-3 that are subjected to glutathionylation/deglutathionylation. The factors affecting the redox status of pro-caspase-3 as it relates to cell death pathways should also be investigated further. Further, caspase-3 itself is susceptible to inactivation by glutathionylation of its active site cysteine residue, as demonstrated in vitro (43). Hence, the deglutathionylation activity of Grx may play a critical role in regulation of cell survival/proliferation versus apoptosis at different control points in various cell signaling pathways in different cellular contexts. Examples are delineated below under the headings of specific proteins that mediate regulation of cell viability.
Ras
Ras is a small GTPase that is important for signal transduction and the regulation of cell proliferation, differentiation, and apoptosis (9). As illustrated in Figure 4, Ras mediates the mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase (MEKK), Akt, and MAPK-activated protein kinase (MKK)/Jun N-terminal protein kinase (JNK) pathways. These pathways are initiated by a number of different cellular signals, including changes in ROS, cytokines, or growth factors. Ras has been shown to be susceptible to glutathionylation in response to mechanical strain imposed on cells or treatment of cells with angiotensin II (1, 77). The glutathionylation of Ras at Cys118 was confirmed to activate this protein, resulting in an increase in p38 and Akt activity. This was also associated with hypertrophy and augmented protein synthesis in both vascular smooth muscle and ventricular myocytes. The formation of Ras-SSG was found to be reversible with dithiothreitol treatment. More specifically, overexpression of Grx was found to attenuate the effects of mechanical strain and oxidative stress consistent with deglutathionylation of Ras-SSG (1, 77). Changes to Ras activity in two types of cardiac cells were shown to alter the activity of several proteins critical in regulating the balance between survival and apoptosis. While apoptosis was not directly measured in these studies, the balance between hypertrophy and apoptosis is highly interdependent and tightly regulated. Additional studies are warranted to determine the role of Ras-SSG in initiating apoptosis in other cell types and the corresponding regulatory role of Grx in these contexts. This pursuit is important to distinguish how changes to the regulation of Ras-SSG may influence cellular commitment to survival/proliferation/hypertrophy versus apoptosis.
PTEN
Phosphatase and tensin homolog (PTEN) is responsible for the dephosphorylation of phosphatidylinositol-3,4,5-triphosphate (PIP3), thereby preventing the activation of Akt (a serine/threonine kinase). This action of PTEN also affects the downstream effectors in the Akt pathway [i.e., apoptosis signaling kinase (ASK1), IκB kinase (IKK), or murine double minute 2 (Mdm2)], as illustrated in Figure 4. PTEN has been reported to be sensitive to inactivation by ROS and RNS in neuronal cells (54) and cardiomyocytes (107), likely due to modification of the active site cysteine residue, but the nature of the modification was not determined in these studies. Previously, however, PTEN cysteine modification/inactivation in A431 cells by exogenously added nitrosothiols was interpreted to occur by stepwise conversion to glutathionylated PTEN (113). This interpretation is reinforced by a study of NR8383 macrophages, where ATP-stimulated increases in ROS were shown to result in reversible glutathionylation of PTEN, according to western blot analysis of immunoprecipitated PTEN with anti-GSH antibody (20). This modification disrupts downstream signaling, and PTEN-SSG is no longer able to dephosphorylate PIP3. This effect results in an increase in Akt kinase activity, which can promote cell survival in two ways (Fig. 4). Activation of the Akt pathway is a cell survival mechanism, and inhibition of ASK1 is an anti-apoptotic mechanism. According to this scenario, deglutathionylation of PTEN-SSG would promote apoptosis.
In a recent study, human PTEN was found to be susceptible to reversible protein modification upon treatment with H2O2 (50). This protein modification on human PTEN is normally reversed quickly in yeast; however, when various Grx genes, particularly grx5, are mutated, this reversibility is delayed. These results imply that human PTEN is a substrate for yeast Grx5 (50). It will be important to confirm that PTEN-SSG is a substrate for Grx, and to carry out studies of the glutathionylation status of PTEN and the apoptotic susceptibility of cells in which Grx1 has been knocked down. These studies would provide insight regarding the relative importance of alterations in the activities of Grx and/or PTEN under conditions (e.g., various diseases) where apoptosis of specific cells is increased. This type of systematic investigation has been applied to the NFκB signaling pathway in several contexts (see NFκB section below).
PKC
As depicted in Figure 4, protein kinase C (PKC) can act as an upstream modulator of Akt and thus affect cell survival in a fashion analogous to that described for PTEN (see the PTEN section). PKC activity is important in cell growth and differentiation, with aberrant activation implicated in cancer. This protein is differentially regulated by oxidative modification at both the regulatory and catalytic domains (34). PKC is activated by superoxide anions at the regulatory domain, potentially leading to aberrant growth and cancer. However, in the presence of nitric oxide, S-nitrosylating agents, or thiol-containing substrates (including GSH), the catalytic domain is susceptible to oxidative modification and enzyme inhibition (15,34). The active site of PKC within the catalytic domain contains 2 Cys residues that are susceptible to oxidative modification (15,108). It was determined that diamide is capable of causing S-glutathionylation on PKC isolated from rat brains as well as in NIH3T3 fibroblast cells. Diamide is a compound that reacts with free thiols making them more reactive and susceptible to S-thiolation, particularly S-glutathionylation (94). The formation of PKC-SSG was reversible by dithiothreitol and Grx, consistent with a potential intracellular regulatory mechanism (15,108). Glutathionylation of PKC resulted in enzyme inhibition, and presumably apoptosis, although this was not directly monitored. The regulation of PKC by Grx may have important implications for cancer treatments, where decreased Grx activity would result in greater PKC-SSG, and a decrease in cell growth and proliferation.
MEKK
Mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase (MEKK) is responsible for mediating cell survival signals by activating the NFκB pathway at IKK; alternatively, MEKK can signal apoptosis by activating MKK3/6 (Fig. 4). Overexpression of MEKK in HEK293 cells increases activation of the transcription factor activator factor 1 (AP-1). This effect is further enhanced when Grx is overexpressed (41). MEKK is inhibited in fibroblasts and lymph node carcinoma cells with increased oxidative stress, such as treatment with H2O2 (19). This inhibition of MEKK was determined to be reversible upon the addition of dithiothreitol. Upon further investigation, MEKK inhibition was attributed to glutathionylation of Cys1238 (19). Based on the MEKK pathway depicted in Figure 4, inhibition of MEKK by glutathionylation would be expected to result in an increased susceptibility to apoptosis by activating through the NFκB pathway. Based on this model, deglutathionylation of MEKK-SSG with either increased activity or overexpression of Grx would presumably attenuate apoptosis signaling. However, MEKK can also induce apoptosis through the MKK3/6 pathway, with MEKK-SSG expected to lead to cell proliferation. Additional research is necessary to delineate the effects and interconnectivity of the MKK3/6 and NFκB pathways as mediated by MEKK glutathionylation.
ASK1
Apoptosis signal-regulating kinase 1 (ASK1) is a mitogen-activated protein kinase kinase kinase. ASK1 is similar to MEKK with respect to structure, and they may activate the same downstream apoptotic effector proteins (Fig. 4). Despite their similarities, the interactions of MEKK and ASK1 with Grx appear to be distinctly different (56, 98). As discussed earlier, it is likely that Grx functions to activate MEKK through its deglutathionylation. In contrast, direct binding of Grx to ASK1 has been linked to inactivation of ASK1 in human prostate cancer cells (98) and pancreatic cancer cells (56). Grx has been shown to dissociate from ASK1 under oxidative stress conditions, leading to ASK1 activation and apoptosis. Hence, Grx appears to serve as a negative effector/anti-apoptotic factor in these contexts rather than a pro-apoptotic catalyst of deglutathionylation. A similar interaction has also been observed between ASK1 and thioredoxin, another thiol-disulfide oxidoreductase (97, 117). Thioredoxin interacts with ASK1 preventing its activation; however, under oxidative/nitrosative stress conditions, thioredoxin dissociates from ASK1 leading to its activation and apoptosis (97, 101, 112, 117). Although ASK1 activity is sensitive to oxidative stress, glutathionylation of ASK1 leading to inactivation has not been reported; therefore, it remains an open question whether Grx may regulate ASK1 activity by more than one mechanism. Much has been learned about the oxidant-sensitive reversible binding of Grx1 to the C-terminus of ASK1 under various conditions. The interaction between Grx1 and ASK1 has been shown to promote apoptosis in different cell types (56, 98–100), but both Grx1 and ASK1 were overexpressed in these studies, so caution should be exercised in assuming that the mechanistic conclusions apply to normal cellular conditions.
In another context, SHSY5Y neuronal cells treated with L-3,4-dihydroxyphenylalanine (L-DOPA) showed increased apoptosis concomitant with deactivation of Grx. Knockdown of Grx in these cells without other treatment also showed increased apoptosis, while knockdown of ASK1 protected against L-DOPA-induced apoptosis (90, 92). These results are suggestive of Grx regulation of oxidant-induced apoptosis in neurons mediated through ASK1. However, since there are many potential effectors of ASK1 activation, which are also sensitive to oxidative modification and potentially regulated by Grx, further study is necessary to delineate the specific role of Grx in this situation. More discussion of the putative role of Grx regulation of various effectors of ASK1 is provided in a recent review focused on neurodegeneration (91).
c-Jun
c-Jun is a transcription factor activated through phosphorylation by JNK, and is a mediator of apoptosis signaling downstream of ASK1 (Fig. 4). The activity of c-Jun and other apoptotic transcription factors, such as p38, is sensitive to the relative concentrations of GSH and GSSG (109). Diminution of GSH content causes an increase in JNK activity and transcription via c-Jun and p38. More specifically, an increase in GSSG concentration in vitro decreases c-Jun's ability to bind to DNA. This DNA binding is dependent upon Cys269, located in the DNA binding domain of c-Jun. This thiol is reversibly glutathionylated with increased GSSG content (52). Cys269 has an unusually low redox potential, and would therefore be susceptible to inactivation by glutathionylation at relatively low concentrations of GSSG (52), so long as the kinetics of formation of c-Jun-SSG are favorable. Glutathionylation was also found when c-Jun was incubated with S-nitrosoglutathione, implying that NO signaling may facilitate formation of c-Jun-SSG (53). These results were obtained from in vitro studies, and the ability of Grx to reverse c-Jun glutathionylation has not yet been documented. Nevertheless, the collective results imply that c-Jun is susceptible to regulation by S-glutathionylation and prompts follow-up studies to document this mechanism in a cellular context. There are also other transcription factors with similar DNA binding domains, including p38, which may also be susceptible to regulation by S-glutathionylation, warranting further investigation.
NFκB
The nuclear factor kappa-light chain enhancer of activated B cells (NFκB) pathway of transcriptional regulation is well known for its role in producing anti-apoptotic factors, and several mediators in this pathway are subjected to reversible glutathionylation and regulation by Grx. The NFκB transcription factor consists of hetero- or homodimer complexes consisting of various combinations of five different subunits, p50, p52, p65, Rel-B, or cRel. NFκB promotes cell survival by inducing expression of anti-apoptotic proteins, such as c-FLIP, X-linked inhibitor of apoptosis protein (XIAP), B-cell lymphoma 2 (Bcl-2), and B-cell lymphoma extra large (Bcl-xL) (13). Under hypoxic conditions in murine embryonic fibroblasts, NFκB was activated, preventing apoptosis. However, when these cells were treated with N-acetyl Cys (capable of increasing GSH content within the cell), NFκB activity was diminished, and the cells were more susceptible to apoptosis (81). The molecular mechanism of this altered NFκB activity was not delineated. In later studies where the analogous approach was applied to murine pancreatic cancer cells, the p65 subunit of NFκB was found to be vulnerable to S-glutathionylation. This corresponded to a decrease in DNA binding by the p65 subunit, particularly under hypoxic conditions and treatment with N-acetyl Cys or GSH ester (80). The glutathionylation of p65 was diminished in cells where Grx1 was knocked down by shRNA, resulting in a corresponding increase in NFκB activity. This result, considering the catalytic properties of Grx (20) and the cellular conditions conducive to thiyl radical formation, is consistent with Grx catalysis of thiyl radical-mediated S-glutathionylation of p65-NFκB. When the inactivation of NFκB was further investigated with isolated nuclear extracts, DNA binding of NFκB was restored upon the addition of Grx, GR, and NADPH to facilitate deglutathionylation, thus confirming that S-glutathionylation of p65 results in the inactivation of NFκB activity (80). In other studies, the recombinant p50 subunit of NFκB also was found to be susceptible to glutathionylation at the Cys62 position, particularly under conditions where the GSSG:GSH ratio was high (78). However, it is currently unknown what the basal glutathionylation state of NFκB is and how other apoptotic stimuli (e.g., TNFα and FasL) influence the regulation of NFκB activity by Grx.
Another instance of interdependence between NFκB pro-survival activity and Grx was observed in a study of primary cardiomyocytes from young adult and elderly rats as well as with immortalized rat cardiomyocytes (H9c2 cells) (13). Cardiomyocytes from elderly rats in comparison to young rats displayed diminished Grx activity, NFκB transcriptional activity, and Bcl-xL content, corresponding to increased susceptibility to oxidant-induced apoptosis. This increased sensitivity to apoptosis was recapitulated with H9c2 cells in which Grx1 was selectively knocked down. Remarkably, in the Grx knockdown cells, the basal NFκB activity was diminished and Bcl-xL expression was decreased concomitantly, accounting for the increased apoptotic susceptibility (28).
Another aspect of interdependent regulation of NFκB and Grx1 activities was reported recently (2). It was discovered that NFκB could regulate expression of Grx1 transcriptionally, with Grx content being increased in macrophages (RAW264.7) treated with lipopolysaccharide. NFκB transcriptional activity was also found to be increased in C10 epithelial cells overexpressing constitutively active IKK. IKK is responsible for phosphorylating IκB that leads to translocation of active NFκB dimers to the nucleus (Figs. 3 and 4). Putative binding sites for NFκB were identified in a 2 kb region of the Glrx1 promoter, implying that NFκB directly affects transcription of Glrx1. This interpretation was reinforced by a chromatin immunoprecipitation assay, showing association of NFκB with the Glrx1 promoter. The increased content and activity of Grx1 resulting from activation of NFκB could serve to deglutathionylate components of the NFκB pathway [e.g., p65-SSG or IKK-SSG (see IKKβ section below)] in a feed-forward mechanism resulting in prolonged activation of the NFκB anti-apoptotic pathway (2).
IKKβ
Grx can regulate the NFκB pathway through reversible glutathionylation of NFκB and at control points upstream of DNA binding, for example, at the level of IKK. IKK has three subunits (α, β, and γ), with the β subunit being the most susceptible to inhibition by oxidation or alkylation of a critical Cys residue, Cys 179 (82). This inhibition has been observed with compounds, such as S-nitrosothiols, arsenite, and certain anti-inflammatory drugs, such as parthenolide (55, 82). In a more recent study, Cys179 was found to be S-glutathionylated upon treatment of lung epithelial cells with H2O2 (82). Glutathionylation of Cys179 of IKKβ in C10 cells resulted in inhibition of kinase activity and prevented TNFα-induced activation of NFκB. The IKKβ inhibition by H2O2 was reversible by DTT and diminished in cells overexpressing Grx1. Conversely, Grx1 knockdown caused an increased susceptibility of IKK to inhibition by H2O2. Presumably, this observation was due to an accumulation of IKK-SSG that would have otherwise been deglutathionylated by Grx1. The IKK inhibition was correlated with decreased TNFα-dependent changes in NFκB transcriptional activity (82). In another context, the presence of IKK-SSG was documented in retinal glial cells by mass spectrometry, and upregulation of Grx was implicated in activation of the pro-inflammatory role of NFκB signaling relevant to diabetic retinopathy (95). From the collective results it can be concluded that Grx tightly regulates signal transduction at multiple levels within the NFκB pathway, including PTEN, PKC, IKK, and NFκB. These proteins control apoptosis, cell growth, or pro-inflammatory responses, depending on the cell type and conditions. It is important to note also that there are many other potential sites of regulation of the NFκB pathway by reversible protein glutathionylation upstream and downstream of IKK, including the proteasome, as reviewed in (68). Along with these considerations, this previous review (68) emphasizes that further research is necessary to understand the network of control points in the NFκB pathway—a pathway that is implicated in many disease conditions involving a variety of cell types.
Mitochondrial proteins implicated in ROS generation and initiation of apoptosis
The mitochondrial permeability transition pore is strongly implicated in propagation of apoptosis, and both adenine nucleotide translocase and voltage-dependent anion channel are susceptible to modulation by oxidative stress (36, 58, 63, 65, 93). The activities of mammalian mitochondrial complex I (7, 46, 105), complex II (10, 11), and alpha-ketoglutarate dehydrogenase (5, 74) have been reported to be altered by S-glutathionylation under various conditions, affecting their propensities to generate ROS. In addition, Grx1 and Grx2 have been implicated in the regulation of the respective protein-SSG adducts. Remarkably, unlike Complex I, where glutathionylation deactivates electron transfer activity and increases ROS production, isolated Complex II-SSG displays increased activity and decreased ROS generation. Figure 6 depicts the separate localization of the mammalian Grx isozymes (75) and specific disulfide-prone targets in the mitochondria [outer mitochondrial membrane and inner mitochondrial membrane (IMM)]. With aging and oxidative stress associated with various diseases, the mitochondrial contents of Grx1 and Grx2 may be altered, leading to changes in the function of specific proteins and consequent changes in the threshold of initiation of apoptosis by the mitochondria. However, the impact of the potential changes in Grx1 and Grx2 activities on the glutathionylation status of specific oxidation-sensitive mitochondrial proteins is currently unknown and represents an important area of investigation. As depicted in Figure 6, the topology of Complex I straddles both sides of the IMM, and both Grx1 and Grx2 have been implicated in the potential regulation of Complex I (7, 26). However, the role of Grx2 as catalyst of thiol-disulfide regulation is uncertain because of its sequestration as an inactive (Grx2)2-Fe2S2 dimer (48, 60). Moreover, the Grx2 monomer displays lower activity (∼10%) relative to Grx1 (29). However, Grx1 and Grx2 contents in rat mitochondria (75), and estimates of volumes of the IMS and matrix, indicate Grx2 concentration in the matrix as ∼1 μM, that is, about 10-fold higher than Grx1 in the IMS (∼0.1 μM). Hence, if all of the Grx2 were available as active monomer, the deglutathionylase activity in the matrix would be similar to that in the IMS with Grx1. Distinguishing whether Grx2 serves as a deglutathionylase enzyme in mitochondria or primarily as a catalyst of iron-sulfur homeostasis (85) represents an important focus for future studies. Additional discussion of cysteine modifications of mitochondrial proteins and their impact on mitochondrial function in health and disease can be found in other recent reviews (45, 73, 91).
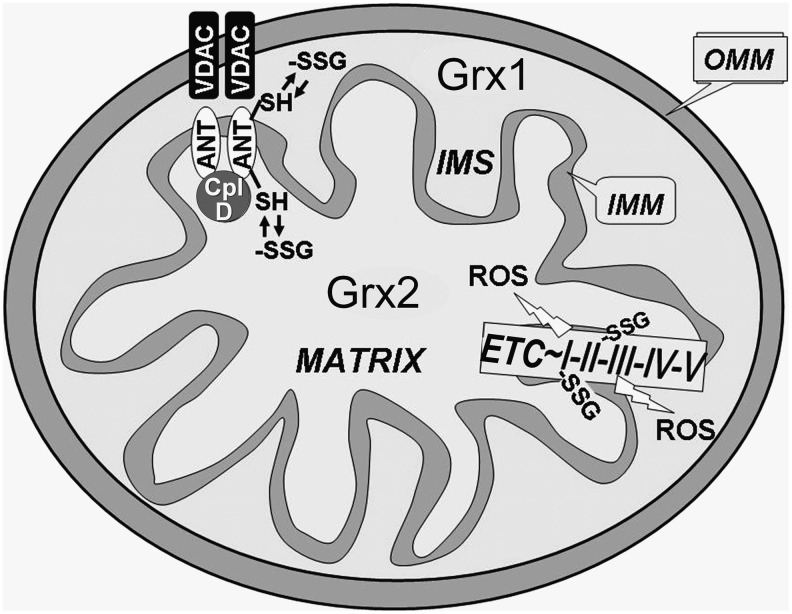
FIG. 6.
Potential regulation by Grx1 and Grx2 of mitochondrial proteins implicated in apoptotic initiation. The separate localization of the mammalian Grx isozymes is shown, along with specific glutathionylation-prone regulatory targets [outer mitochondrial membrane (OMM) and inner mitochondrial membrane (IMM)]. With oxidative stress associated with various diseases, the mitochondrial contents of Grx1 and Grx2 may be altered; however, the impact of such changes on specific oxidation-sensitive mitochondrial proteins and corresponding commitment to apoptosis represents an important avenue of investigation.
Regulation of Apoptosis by Grx in Nonmammalian Systems
Yeast Grxs and apoptosis
Given the broad range of apoptosis-related proteins whose function is regulated by Grxs in mammals, it is not surprising that Grxs are also important for regulating apoptosis in other species, such as yeast. Research has shown that a Grx2 deficiency in yeast prevents cadmium-induced apoptosis (33). Cadmium is a nonessential heavy metal that inhibits DNA mismatch repair and can lead to the accumulation of glutathionylated proteins and apoptosis in many organisms (14, 33). These research studies indicate that alterations in Grx activity may be a critical contributor to the initiation of apoptosis in both yeast and mammalian cells exposed to cadmium, although specific protein targets for deglutathionylation by Grx in yeast are yet to be determined.
In addition to cadmium, Grx has also been implicated in selenium toxicity in yeast. Selenium is a trace metal important in mammals for the functioning of enzymes, such as GSH peroxidase and thioredoxin reductase. At high concentrations, selenium can lead to oxidative stress and DNA damage. In contrast to mammals, yeast do not require selenium, and therefore have been used as a model system to investigate selenium toxicity. It has been determined that yeast with mutated Grx1p and Grx2p were hypersensitive to selenium, resulting in nonapoptotic cell death (47). While protein targets of Grxs in yeast have not yet been investigated, Grxs are known to be very important for iron homeostasis and the oxidative stress response in yeast (37, 49, 71, 79).
Summary of the Role of Impaired Regulation of S-Glutathionylation in Disease Susceptibility
There are many proteins important in the regulation of cellular signaling for growth and apoptosis that are regulated by Grx and glutathionylation (Figs. 3 and 4, and Table 1). It is important to note that this is not an exhaustive list; many other proteins have been identified as susceptible to Grx regulation, such as cAMP-dependent protein kinase, HIV protease, actin, and protein-tyrosine phosphatase. There are likely many more proteins that are regulated by protein glutathionylation/deglutathionylation yet to be determined. It will be very important to identify these proteins and to determine how the relative glutathionylation status of different proteins affects cell signaling and disease susceptibility.
Table 1.
Proteins Susceptible to Glutathionylation and Effects of This Modification on Apoptosis
| Protein | Challenge to induce glutathionylation | Effect on protein | Apoptosis or survival | References |
|---|---|---|---|---|
| Fas | Grx knockdown | Activation | Apoptosis | (3) |
| Pro-caspase-3 | Grx knockdown | Inhibition | Survival | (76) |
| Ras | Mechanical strain or angiotensin II | Activation | Apoptosis | (1,77) |
| PTEN | ATP-induced ROS | Inhibition | Survival | (20) |
| PKC | Diamide and GSH | Inhibition | Apoptosis | (15,108) |
| MEKK | H2O2 | Inhibition | Apoptosis | (19,41) |
| c-Jun | GSSG | Inhibition | Apoptosis | (52,53,109) |
| NFκB | ROS or N-acetyl Cys | Inhibition | Apoptosis | (28,80,81) |
| IKKβ | Grx knockdown or H2O2 | Inhibition | Apoptosis | (82) |
Oxidative stress and deactivation of Grx are main contributing factors to disruption of the sulfhydryl redox status of the cell, leading to activated thiols and an increased susceptibility to protein glutathionylation. Disruptions in Grx/glutathionylation regulation have been implicated in many different diseases, including diabetes, heart disease, cancer, pulmonary disease, and neurodegenerative disorders, reviewed in (22, 68, 69, 94, 96). A common risk factor for each of these diseases is age, a process that results in the accumulation of unrepaired cellular damage and the decreased function of cellular compensation mechanisms, such as the ability to repair DNA and proteins (16, 40, 51). A disruption in mitochondrial function is also common in diseases of the elderly, causing the accumulation of ROS, coupled with decreases in the antioxidant defenses of the cell (4, 13, 21, 22, 40, 44, 64, 68, 70, 103). These disruptions in redox regulation likely alter protein glutathionylation, leaving cells more susceptible to apoptosis and vulnerable to disease (Fig. 7).
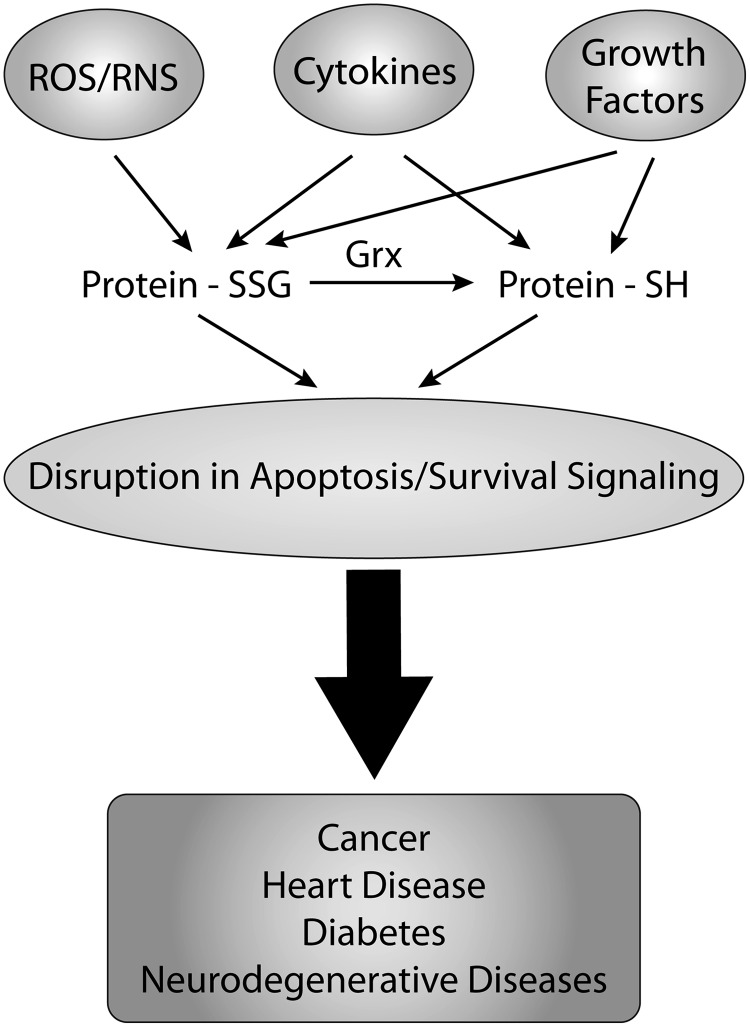
FIG. 7.
Various cellular signals [reactive oxygen species (ROS), cytokines, growth factors, etc.] can disrupt protein glutathionylation and deglutathionylation by Grx. The disruption in protein glutathionylation status may be caused by a number of different cellular insults, such as ROS, reactive nitrogen species (RNS), cytokines, or growth factors. Depending on the insult and the cell type, protein target, and cellular conditions, this can result in changes to the glutathionylation status of a protein from the normal, steady-state form. This disruption results in protein deglutathionylation by Grx or aberrant formation of protein-SSG—either of which can lead to dysregulated apoptosis/survival signaling, and contribute to diseases, such as cancer and diabetes.
Conclusions
The effect of Grx and glutathionylation has been investigated with respect to many different proteins within discrete pathways. It is important that future research focuses on investigating interrelationships among these pathways to better understand the crosstalk and the regulatory mechanisms of Grx in a much more global respect. Apoptosis signaling pathways are tightly regulated and highly intertwined, making it difficult to discern subtle regulatory differences and the effect of Grx/glutathionylation on each aspect of cellular homeostasis. Enhanced understanding of the role of Grx and glutathionylation as a regulatory mechanism in apoptosis/survival pathways will be important for developing more effective therapeutics for diseases where alterations in Grx activity and S-glutathionylation status of critical proteins play a prominent role, such as cardiovascular disease, diabetes, and neurodegenerative disorders.
Abbreviations Used
- Akt
protein kinase B
- ANT
adenine nucleotide translocase
- AP-1
activator factor 1
- ASK1
apoptosis signaling kinase
- Bcl-xL
B-cell lymphoma extra large
- c-FLIP
caspase-8-FADD-like interleukin-1β-converting enzyme inhibitory protein
- DD
death domain
- DISC
death-inducing signaling complex
- Erk
extracellular signal-regulated kinase
- FADD
Fas-associated death domain
- Fas
tumor necrosis factor superfamily receptor 6
- FasL
Fas ligand
- Grx
glutaredoxin
- GSH
glutathione
- GSSG
oxidized glutathione
- IKK
IκB kinase
- IMM
inner mitochondrial membrane
- IMS
intermembrane space
- JNK
jun N-terminal kinase
- L-DOPA
L-3,4-dihydroxyphenylalanine
- MAPK
mitogen-activated protein kinase
- Mdm2
murine double minute 2
- MEF
murine embryonic fibroblast
- MEKK
mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase
- MKK
MAPK-activated protein kinase
- NFκB
nuclear factor kappa-light chain enhancer of activated B cells
- OMM
outer mitochondrial membrane
- PIP3
phosphatidylinositol (3,4,5)-triphosphate
- PKC
protein kinase C
- Prot-SSG
glutathionylated protein
- PTEN
phosphatase and tensin homolog
- Ras
rat sarcoma
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- TNF
tumor necrosis factor
- TNFR
tumor necrosis factor receptor
- VDAC
voltage-dependent anion channel
Acknowledgments
This work was supported in part by a Merit Review research grant from the Department of Veteran's Affairs (J.J.M.), National Institutes of Health (NIH) research grant PO1 AG 15885 (J.J.M.), and NIH training grant T32-DK007319-32 (E.M.G.A.). The authors are grateful to Dr. Ruth Siegel for critical review of the article before submission.
References
- 1.Adachi T. Pimentel DR. Heibeck T. Hou X. Lee YJ. Jiang B. Ido Y. Cohen RA. S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J Biol Chem. 2004;279:29857–29862. doi: 10.1074/jbc.M313320200. [DOI] [PubMed] [Google Scholar]
- 2.Aesif SW. Kuipers I. van der Velden J. Tully JE. Guala AS. Anathy V. Sheely JI. Reynaert NL. Wouters EF. van der Vliet A. Janssen-Heininger YM. Activation of the glutaredoxin-1 gene by nuclear factor kappaB enhances signaling. Free Radic Biol Med. 2011;51:1249–1257. doi: 10.1016/j.freeradbiomed.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anathy V. Aesif SW. Guala AS. Havermans M. Reynaert NL. Ho YS. Budd RC. Janssen-Heininger YM. Redox amplification of apoptosis by caspase-dependent cleavage of glutaredoxin 1 and S-glutathionylation of Fas. J Cell Biol. 2009;184:241–252. doi: 10.1083/jcb.200807019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10(Suppl):S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 5.Applegate MA. Humphries KM. Szweda LI. Reversible inhibition of alpha-ketoglutarate dehydrogenase by hydrogen peroxide: glutathionylation and protection of lipoic acid. Biochemistry. 2008;47:473–8. doi: 10.1021/bi7017464. [DOI] [PubMed] [Google Scholar]
- 6.Askelof P. Axelsson K. Eriksson S. Mannervik B. Mechanism of action of enzymes catalyzing thiol-disulfide interchange. Thioltransferases rather than transhydrogenases. FEBS Lett. 1974;38:263–267. doi: 10.1016/0014-5793(74)80068-2. [DOI] [PubMed] [Google Scholar]
- 7.Beer SM. Taylor ER. Brown SE. Dahm CC. Costa NJ. Runswick MJ. Murphy MP. Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: implications for mitochondrial redox regulation and antioxidant defense. J Biol Chem. 2004;279:47939–47951. doi: 10.1074/jbc.M408011200. [DOI] [PubMed] [Google Scholar]
- 8.Berndt C. Lillig CH. Holmgren A. Thioredoxins and glutaredoxins as facilitators of protein folding. Biochim Biophys Acta. 2008;1783:641–650. doi: 10.1016/j.bbamcr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Castellano E. Downward J. RAS interaction with PI3K: more than just another effector pathway. Genes Cancer. 2011;2:261–274. doi: 10.1177/1947601911408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J. Chen CL. Rawale S. Chen CA. Zweier JL. Kaumaya PT. Chen YR. Peptide-based antibodies against glutathione-binding domains suppress superoxide production mediated by mitochondrial complex I. J Biol Chem. 2010;285:3168–3180. doi: 10.1074/jbc.M109.056846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YR. Chen CL. Pfeiffer DR. Zweier JL. Mitochondrial complex II in the post-ischemic heart: oxidative injury and the role of protein S-glutathionylation. J Biol Chem. 2007;282:32640–32654. doi: 10.1074/jbc.M702294200. [DOI] [PubMed] [Google Scholar]
- 12.Cheng NH. Liu JZ. Liu X. Wu Q. Thompson SM. Lin J. Chang J. Whitham SA. Park S. Cohen JD. Hirschi KD. Arabidopsis monothiol glutaredoxin, AtGRXS17, is critical for temperature-dependent postembryonic growth and development via modulating auxin response. J Biol Chem. 2011;286:20398–20406. doi: 10.1074/jbc.M110.201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cherra SJ. Chu CT. Autophagy in neuroprotection and neurodegeneration: a question of balance. Future Neurol. 2008;3:309–323. doi: 10.2217/14796708.3.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chrestensen CA. Starke DW. Mieyal JJ. Acute cadmium exposure inactivates thioltransferase (Glutaredoxin), inhibits intracellular reduction of protein-glutathionyl-mixed disulfides, and initiates apoptosis. J Biol Chem. 2000;275:26556–26565. doi: 10.1074/jbc.M004097200. [DOI] [PubMed] [Google Scholar]
- 15.Chu F. Ward NE. O'Brian CA. Potent inactivation of representative members of each PKC isozyme subfamily and PKD via S-thiolation by the tumor-promotion/progression antagonist glutathione but not by its precursor cysteine. Carcinogenesis. 2001;22:1221–1229. doi: 10.1093/carcin/22.8.1221. [DOI] [PubMed] [Google Scholar]
- 16.Collier TJ. Lipton J. Daley BF. Palfi S. Chu Y. Sortwell C. Bakay RA. Sladek JR., Jr. Kordower JH. Aging-related changes in the nigrostriatal dopamine system and the response to MPTP in nonhuman primates: diminished compensatory mechanisms as a prelude to parkinsonism. Neurobiol Dis. 2007;26:56–65. doi: 10.1016/j.nbd.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Couturier J. Jacquot JP. Rouhier N. Evolution and diversity of glutaredoxins in photosynthetic organisms. Cell Mol Life Sci. 2009;66:2539–2557. doi: 10.1007/s00018-009-0054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Couturier J. Stroher E. Albetel AN. Roret T. Muthuramalingam M. Tarrago L. Seidel T. Tsan P. Jacquot JP. Johnson MK. Dietz KJ. Didierjean C. Rouhier N. Arabidopsis chloroplastic glutaredoxin C5 as a model to explore molecular determinants for iron-sulfur cluster binding into glutaredoxins. J Biol Chem. 2011;286:27515–27527. doi: 10.1074/jbc.M111.228726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cross JV. Templeton DJ. Oxidative stress inhibits MEKK1 by site-specific glutathionylation in the ATP-binding domain. Biochem J. 2004;381:675–683. doi: 10.1042/BJ20040591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruz CM. Rinna A. Forman HJ. Ventura AL. Persechini PM. Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalle-Donne I. Colombo G. Gagliano N. Colombo R. Giustarini D. Rossi R. Milzani A. S-glutathiolation in life and death decisions of the cell. Free Radic Res. 2011;45:3–15. doi: 10.3109/10715762.2010.515217. [DOI] [PubMed] [Google Scholar]
- 22.Dalle-Donne I. Milzani A. Gagliano N. Colombo R. Giustarini D. Rossi R. Molecular mechanisms and potential clinical significance of S-glutathionylation. Antioxid Redox Signal. 2008;10:445–473. doi: 10.1089/ars.2007.1716. [DOI] [PubMed] [Google Scholar]
- 23.Dalle-Donne I. Rossi R. Colombo G. Giustarini D. Milzani A. Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem Sci. 2009;34:85–96. doi: 10.1016/j.tibs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Dalle-Donne I. Rossi R. Giustarini D. Colombo R. Milzani A. S-glutathionylation in protein redox regulation. Free Radic Biol Med. 2007;43:883–898. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Discola KF. de Oliveira MA. Rosa Cussiol JR. Monteiro G. Barcena JA. Porras P. Padilla CA. Guimaraes BG. Netto LE. Structural aspects of the distinct biochemical properties of glutaredoxin 1 and glutaredoxin 2 from Saccharomyces cerevisiae. J Mol Biol. 2009;385:889–901. doi: 10.1016/j.jmb.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 26.Diwakar L. Kenchappa RS. Annepu J. Saeed U. Sujanitha R. Ravindranath V. Down-regulation of glutaredoxin by estrogen receptor antagonist renders female mice susceptible to excitatory amino acid mediated complex I inhibition in CNS. Brain Res. 2006;1125:176–184. doi: 10.1016/j.brainres.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 27.Gallogly MM. Mieyal JJ. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr Opin Pharmacol. 2007;7:381–391. doi: 10.1016/j.coph.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Gallogly MM. Shelton MD. Qanungo S. Pai HV. Starke DW. Hoppel CL. Lesnefsky EJ. Mieyal JJ. Glutaredoxin regulates apoptosis in cardiomyocytes via NFkappaB targets Bcl-2 and Bcl-xL: implications for cardiac aging. Antioxid Redox Signal. 2010;12:1339–1353. doi: 10.1089/ars.2009.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallogly MM. Starke DW. Leonberg AK. Ospina SM. Mieyal JJ. Kinetic and mechanistic characterization and versatile catalytic properties of mammalian glutaredoxin 2: implications for intracellular roles. Biochemistry. 2008;47:11144–11157. doi: 10.1021/bi800966v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallogly MM. Starke DW. Mieyal JJ. Mechanistic and kinetic details of catalysis of thiol-disulfide exchange by glutaredoxins and potential mechanisms of regulation. Antioxid Redox Signal. 2009;11:1059–1081. doi: 10.1089/ars.2008.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao XH. Zaffagnini M. Bedhomme M. Michelet L. Cassier-Chauvat C. Decottignies P. Lemaire SD. Biochemical characterization of glutaredoxins from Chlamydomonas reinhardtii: kinetics and specificity in deglutathionylation reactions. FEBS Lett. 2010;584:2242–2248. doi: 10.1016/j.febslet.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 32.Gilbert HF. Molecular and cellular aspects of thiol-disulfide exchange. Adv Enzymol Relat Areas Mol Biol. 1990;63:69–172. doi: 10.1002/9780470123096.ch2. [DOI] [PubMed] [Google Scholar]
- 33.Gomes DS. Pereira MD. Panek AD. Andrade LR. Eleutherio EC. Apoptosis as a mechanism for removal of mutated cells of Saccharomyces cerevisiae: the role of Grx2 under cadmium exposure. Biochim Biophy Acta. 2008;1780:160–166. doi: 10.1016/j.bbagen.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Gopalakrishna R. Jaken S. Protein kinase C signaling and oxidative stress. Free Radic Biol Med. 2000;28:1349–1361. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 35.Gutscher M. Sobotta MC. Wabnitz GH. Ballikaya S. Meyer AJ. Samstag Y. Dick TP. Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. J Biol Chem. 2009;284:31532–31540. doi: 10.1074/jbc.M109.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halestrap AP. Brenner C. The adenine nucleotide translocase: a central component of the mitochondrial permeability transition pore and key player in cell death. Curr Med Chem. 2003;10:1507–1525. doi: 10.2174/0929867033457278. [DOI] [PubMed] [Google Scholar]
- 37.Herrero E. Belli G. Casa C. Structural and functional diversity of glutaredoxins in yeast. Curr Protein Pept Sci. 2010;11:659–668. doi: 10.2174/138920310794557637. [DOI] [PubMed] [Google Scholar]
- 38.Herrero E. de la Torre-Ruiz MA. Monothiol glutaredoxins: a common domain for multiple functions. Cell Mol Life Sci. 2007;64:1518–1530. doi: 10.1007/s00018-007-6554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higashi K. Takasawa R. Yoshimori A. Goh T. Tanuma S. Kuchitsu K. Identification of a novel gene family, paralogs of inhibitor of apoptosis proteins present in plants, fungi, and animals. Apoptosis. 2005;10:471–480. doi: 10.1007/s10495-005-1876-1. [DOI] [PubMed] [Google Scholar]
- 40.Hindle JV. Ageing, neurodegeneration and Parkinson's disease. Age Ageing. 2010;39:156–161. doi: 10.1093/ageing/afp223. [DOI] [PubMed] [Google Scholar]
- 41.Hirota K. Matsui M. Murata M. Takashima Y. Cheng FS. Itoh T. Fukuda K. Yodoi J. Nucleoredoxin, glutaredoxin, and thioredoxin differentially regulate NF-kappaB, AP-1, and CREB activation in HEK293 cells. Biochem Biophys Res Commun. 2000;274:177–182. doi: 10.1006/bbrc.2000.3106. [DOI] [PubMed] [Google Scholar]
- 42.Holmgren A. Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc Natl Acad Sci U S A. 1976;73:2275–2279. doi: 10.1073/pnas.73.7.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang Z. Pinto JT. Deng H. Richie JP., Jr Inhibition of caspase-3 activity and activation by protein glutathionylation. Biochem Pharmacol. 2008;75:2234–2244. doi: 10.1016/j.bcp.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hung CW. Chen YC. Hsieh WL. Chiou SH. Kao CL. Ageing and neurodegenerative diseases. Ageing Res Rev. 2010;9(Suppl 1):S36–S46. doi: 10.1016/j.arr.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Hurd TR. Costa NJ. Dahm CC. Beer SM. Brown SE. Filipovska A. Murphy MP. Glutathionylation of mitochondrial proteins. Antioxid Redox Signal. 2005;7:999–1010. doi: 10.1089/ars.2005.7.999. [DOI] [PubMed] [Google Scholar]
- 46.Hurd TR. Requejo R. Filipovska A. Brown S. Prime TA. Robinson AJ. Fearnley IM. Murphy MP. Complex I within oxidatively stressed bovine heart mitochondria is glutathionylated on Cys-531 and Cys-704 of the 75-kDa subunit: potential role of CYS residues in decreasing oxidative damage. J Biol Chem. 2008;283:24801–24815. doi: 10.1074/jbc.M803432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Izquierdo A. Casas C. Herrero E. Selenite-induced cell death in Saccharomyces cerevisiae: protective role of glutaredoxins. Microbiology. 2010;156:2608–2620. doi: 10.1099/mic.0.039719-0. [DOI] [PubMed] [Google Scholar]
- 48.Johansson C. Kavanagh KL. Gileadi O. Oppermann U. Reversible sequestration of active site cysteines in a 2Fe-2S-bridged dimer provides a mechanism for glutaredoxin 2 regulation in human mitochondria. J Biol Chem. 2007;282:3077–3082. doi: 10.1074/jbc.M608179200. [DOI] [PubMed] [Google Scholar]
- 49.Kim KD. Kim HJ. Lee KC. Roe JH. Multi-domain CGFS-type glutaredoxin Grx4 regulates iron homeostasis via direct interaction with a repressor Fep1 in fission yeast. Biochem Biophys Res Commun. 2011;408:609–614. doi: 10.1016/j.bbrc.2011.04.069. [DOI] [PubMed] [Google Scholar]
- 50.Kim Y. Chay KO. Kim I. Song YB. Kim TY. Han SJ. Ahn Y. Cho SH. Hoe KL. Ahn BW. Huh WK. Lee SR. Redox regulation of the tumor suppressor PTEN by glutaredoxin 5 and Ycp4. Biochem Biophys Res Commun. 2011;407:175–180. doi: 10.1016/j.bbrc.2011.02.133. [DOI] [PubMed] [Google Scholar]
- 51.Kirkwood TB. The most pressing problem of our age. BMJ. 2003;326:1297–9. doi: 10.1136/bmj.326.7402.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klatt P. Molina EP. De Lacoba MG. Padilla CA. Martinez-Galesteo E. Barcena JA. Lamas S. Redox regulation of c-Jun DNA binding by reversible S-glutathiolation. FASEB J. 1999;13:1481–1490. doi: 10.1096/fasebj.13.12.1481. [DOI] [PubMed] [Google Scholar]
- 53.Klatt P. Molina EP. Lamas S. Nitric oxide inhibits c-Jun DNA binding by specifically targeted S-glutathionylation. J Biol Chem. 1999;274:15857–15864. doi: 10.1074/jbc.274.22.15857. [DOI] [PubMed] [Google Scholar]
- 54.Kwak YD. Ma T. Diao S. Zhang X. Chen Y. Hsu J. Lipton SA. Masliah E. Xu H. Liao FF. NO signaling and S-nitrosylation regulate PTEN inhibition in neurodegeneration. Mol Neurodegeneration. 2010;5:49. doi: 10.1186/1750-1326-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwok BH. Koh B. Ndubuisi MI. Elofsson M. Crews CM. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem Biol. 2001;8:759–766. doi: 10.1016/s1074-5521(01)00049-7. [DOI] [PubMed] [Google Scholar]
- 56.Kwon SJ. Song JJ. Lee YJ. Signal pathway of hypoxia-inducible factor-1alpha phosphorylation and its interaction with von Hippel-Lindau tumor suppressor protein during ischemia in MiaPaCa-2 pancreatic cancer cells. Clin Cancer Res. 2005;11:7607–7613. doi: 10.1158/1078-0432.CCR-05-0981. [DOI] [PubMed] [Google Scholar]
- 57.Lavrik IN. Regulation of death receptor-induced apoptosis induced via CD95/FAS and other death receptors. Mol Biol. 2011;45:150–155. [PubMed] [Google Scholar]
- 58.Li Q. Sato EF. Kira Y. Nishikawa M. Utsumi K. Inoue M. A possible cooperation of SOD1 and cytochrome c in mitochondria-dependent apoptosis. Free Radic Biol Med. 2006;40:173–181. doi: 10.1016/j.freeradbiomed.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 59.Li S. Lauri A. Ziemann M. Busch A. Bhave M. Zachgo S. Nuclear activity of ROXY1, a glutaredoxin interacting with TGA factors, is required for petal development in Arabidopsis thaliana. Plant Cell. 2009;21:429–441. doi: 10.1105/tpc.108.064477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lillig CH. Berndt C. Vergnolle O. Lonn ME. Hudemann C. Bill E. Holmgren A. Characterization of human glutaredoxin 2 as iron-sulfur protein: a possible role as redox sensor. Proc Natl Acad Sci U S A. 2005;102:8168–8173. doi: 10.1073/pnas.0500735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lillig CH. Holmgren A. Thioredoxin and related molecules—from biology to health and disease. Antioxid Redox Signal. 2007;9:25–47. doi: 10.1089/ars.2007.9.25. [DOI] [PubMed] [Google Scholar]
- 62.Linares GR. Xing W. Govoni KE. Chen ST. Mohan S. Glutaredoxin 5 regulates osteoblast apoptosis by protecting against oxidative stress. Bone. 2009;44:795–804. doi: 10.1016/j.bone.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Majima E. Yamaguchi N. Chuman H. Shinohara Y. Ishida M. Goto S. Terada H. Binding of the fluorescein derivative eosin Y to the mitochondrial ADP/ATP carrier: characterization of the adenine nucleotide binding site. Biochemistry. 1998;37:424–432. doi: 10.1021/bi9710683. [DOI] [PubMed] [Google Scholar]
- 64.McNaught KS. Belizaire R. Isacson O. Jenner P. Olanow CW. Altered proteasomal function in sporadic Parkinson's disease. Exp Neurol. 2003;179:38–46. doi: 10.1006/exnr.2002.8050. [DOI] [PubMed] [Google Scholar]
- 65.McStay GP. Clarke SJ. Halestrap AP. Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. Biochem J. 2002;367:541–548. doi: 10.1042/BJ20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meyer M. Vilardell J. The quest for a message: budding yeast, a model organism to study the control of pre-mRNA splicing. Brief Funct Genomics Proteomics. 2009;8:60–67. doi: 10.1093/bfgp/elp002. [DOI] [PubMed] [Google Scholar]
- 67.Meyer Y. Siala W. Bashandy T. Riondet C. Vignols F. Reichheld JP. Glutaredoxins and thioredoxins in plants. Biochim Biophys Acta. 2008;1783:589–600. doi: 10.1016/j.bbamcr.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 68.Mieyal JJ. Gallogly MM. Qanungo S. Sabens EA. Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mieyal JJ. Srinivasan U. Starke DW. Gravina SA. Mieyal P. Glutathionyl specificity of thiotransferases: mechanistic and physiological implications. In: Packer L, editor; Cadenas E, editor. Biothiols in health and disease. New York, NY: M. Dekker; 1995. pp. 305–372. [Google Scholar]
- 70.Migliore L. Coppede F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat Res. 2009;674:73–84. doi: 10.1016/j.mrgentox.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 71.Muhlenhoff U. Molik S. Godoy JR. Uzarska MA. Richter N. Seubert A. Zhang Y. Stubbe J. Pierrel F. Herrero E. Lillig CH. Lill R. Cytosolic monothiol glutaredoxins function in intracellular iron sensing and trafficking via their bound iron-sulfur cluster. Cell Metab. 2010;12:373–385. doi: 10.1016/j.cmet.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murmu J. Bush MJ. DeLong C. Li S. Xu M. Khan M. Malcolmson C. Fobert PR. Zachgo S. Hepworth SR. Arabidopsis basic leucine-zipper transcription factors TGA9 and TGA10 interact with floral glutaredoxins ROXY1 and ROXY2 and are redundantly required for anther development. Plant Physiol. 2010;154:1492–1504. doi: 10.1104/pp.110.159111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murphy M. Mitochondrial Thiols in Antioxidant Protection and Redox Signalling: distinct roles for glutathionylation and other thiol modifications. Antioxid Redox Signal. 2012;16:476–495. doi: 10.1089/ars.2011.4289. [DOI] [PubMed] [Google Scholar]
- 74.Nulton-Persson AC. Starke DW. Mieyal JJ. Szweda LI. Reversible inactivation of alpha-ketoglutarate dehydrogenase in response to alterations in the mitochondrial glutathione status. Biochemistry. 2003;42:4235–4242. doi: 10.1021/bi027370f. [DOI] [PubMed] [Google Scholar]
- 75.Pai HV. Starke DW. Lesnefsky EJ. Hoppel CL. Mieyal JJ. What is the functional significance of the unique location of glutaredoxin 1 (GRx1) in the intermembrane space of mitochondria? Antioxid Redox Signal. 2007;9:2027–2033. doi: 10.1089/ars.2007.1642. [DOI] [PubMed] [Google Scholar]
- 76.Pan S. Berk BC. Glutathiolation regulates tumor necrosis factor-alpha-induced caspase-3 cleavage and apoptosis: key role for glutaredoxin in the death pathway. Circ Res. 2007;100:213–219. doi: 10.1161/01.RES.0000256089.30318.20. [DOI] [PubMed] [Google Scholar]
- 77.Pimentel DR. Adachi T. Ido Y. Heibeck T. Jiang B. Lee Y. Melendez JA. Cohen RA. Colucci WS. Strain-stimulated hypertrophy in cardiac myocytes is mediated by reactive oxygen species-dependent Ras S-glutathiolation. J Mol Cell Cardiol. 2006;41:613–622. doi: 10.1016/j.yjmcc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 78.Pineda-Molina E. Klatt P. Vazquez J. Marina A. Garcia de Lacoba M. Perez-Sala D. Lamas S. Glutathionylation of the p50 subunit of NF-kappaB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry. 2001;40:14134–14142. doi: 10.1021/bi011459o. [DOI] [PubMed] [Google Scholar]
- 79.Pujol-Carrion N. de la Torre-Ruiz MA. Glutaredoxins Grx4 and Grx3 of Saccharomyces cerevisiae play a role in actin dynamics through their Trx domains, which contributes to oxidative stress resistance. Appl Environ Microbiol. 2010;76:7826–7835. doi: 10.1128/AEM.01755-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qanungo S. Starke DW. Pai HV. Mieyal JJ. Nieminen AL. Glutathione supplementation potentiates hypoxic apoptosis by S-glutathionylation of p65-NFkappaB. J Biol Chem. 2007;282:18427–18436. doi: 10.1074/jbc.M610934200. [DOI] [PubMed] [Google Scholar]
- 81.Qanungo S. Wang M. Nieminen AL. N-Acetyl-L-cysteine enhances apoptosis through inhibition of nuclear factor-kappaB in hypoxic murine embryonic fibroblasts. J Biol Chem. 2004;279:50455–50464. doi: 10.1074/jbc.M406749200. [DOI] [PubMed] [Google Scholar]
- 82.Reynaert NL. van der Vliet A. Guala AS. McGovern T. Hristova M. Pantano C. Heintz NH. Heim J. Ho YS. Matthews DE. Wouters EF. Janssen-Heininger YM. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Acad Sci U S A. 2006;103:13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rodriguez-Manzaneque MT. Tamarit J. Belli G. Ros J. Herrero E. Grx5 is a mitochondrial glutaredoxin required for the activity of iron/sulfur enzymes. Mol Biol Cell. 2002;13:1109–1121. doi: 10.1091/mbc.01-10-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rouhier N. Plant glutaredoxins: pivotal players in redox biology and iron-sulphur centre assembly. New Phytologist. 2010;186:365–372. doi: 10.1111/j.1469-8137.2009.03146.x. [DOI] [PubMed] [Google Scholar]
- 85.Rouhier N. Couturier J. Johnson MK. Jacquot JP. Glutaredoxins: roles in iron homeostasis. Trends Biochem Sci. 2010;35:43–52. doi: 10.1016/j.tibs.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rouhier N. Gelhaye E. Jacquot JP. Plant glutaredoxins: still mysterious reducing systems. Cell Mol Life Sci. 2004;61:1266–1277. doi: 10.1007/s00018-004-3410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rouhier N. Lemaire SD. Jacquot JP. The role of glutathione in photosynthetic organisms: emerging functions for glutaredoxins and glutathionylation. Ann Rev Plant Biol. 2008;59:143–166. doi: 10.1146/annurev.arplant.59.032607.092811. [DOI] [PubMed] [Google Scholar]
- 88.Rouhier N. Villarejo A. Srivastava M. Gelhaye E. Keech O. Droux M. Finkemeier I. Samuelsson G. Dietz KJ. Jacquot JP. Wingsle G. Identification of plant glutaredoxin targets. Antioxid Redox Signal. 2005;7:919–929. doi: 10.1089/ars.2005.7.919. [DOI] [PubMed] [Google Scholar]
- 89.Saaranen MJ. Salo KE. Latva-Ranta MK. Kinnula VL. Ruddock LW. The C-terminal active site cysteine of Escherichia coli glutaredoxin 1 determines the glutathione specificity of the second step of peptide deglutathionylation. Antioxid Redox Signal. 2009;11:1819–1828. doi: 10.1089/ars.2008.2387. [DOI] [PubMed] [Google Scholar]
- 90.Sabens EA. Distler AM. Mieyal JJ. Levodopa deactivates enzymes that regulate thiol-disulfide homeostasis and promotes neuronal cell death: implications for therapy of Parkinson's disease. Biochemistry. 2010;49:2715–2724. doi: 10.1021/bi9018658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sabens EA. Gao XH. Mieyal JJ. Mechanisms of altered redox regulation in neurodegenerative diseases: focus on S-glutathionylation. Antioxid Redox Signal. 2012;16:543–566. doi: 10.1089/ars.2011.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sabens EA. Steller KM. Mieyal JJ. Levodopa activates apoptosis signaling kinase 1 (ask1) and promotes apoptosis in a neuronal model: implications for the treatment of parkinson's disease. Chem Res Toxicol. 2011;24:1644–1652. doi: 10.1021/tx200082h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saeed U. Durgadoss L. Valli RK. Joshi DC. Joshi PG. Ravindranath V. Knockdown of cytosolic glutaredoxin 1 leads to loss of mitochondrial membrane potential: implication in neurodegenerative diseases. PloS One. 2008;3:e2459. doi: 10.1371/journal.pone.0002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shelton MD. Chock PB. Mieyal JJ. Glutaredoxin: role in reversible protein s-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid Redox Signal. 2005;7:348–366. doi: 10.1089/ars.2005.7.348. [DOI] [PubMed] [Google Scholar]
- 95.Shelton MD. Distler AM. Kern TS. Mieyal JJ. Glutaredoxin regulates autocrine and paracrine proinflammatory responses in retinal glial (muller) cells. J Biol Chem. 2009;284:4760–4766. doi: 10.1074/jbc.M805464200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shelton MD. Mieyal JJ. Regulation by reversible S-glutathionylation: molecular targets implicated in inflammatory diseases. Mol Cells. 2008;25:332–346. [PMC free article] [PubMed] [Google Scholar]
- 97.Song JJ. Lee YJ. Differential role of glutaredoxin and thioredoxin in metabolic oxidative stress-induced activation of apoptosis signal-regulating kinase 1. Biochem J. 2003;373:845–853. doi: 10.1042/BJ20030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Song JJ. Lee YJ. Effect of glucose concentration on activation of the ASK1-SEK1-JNK1 signal transduction pathway. J Cell Biochem. 2003;89:653–662. doi: 10.1002/jcb.10541. [DOI] [PubMed] [Google Scholar]
- 99.Song JJ. Lee YJ. Role of the ASK1-SEK1-JNK1-HIPK1 signal in Daxx trafficking and ASK1 oligomerization. J Biol Chem. 2003;278:47245–47252. doi: 10.1074/jbc.M213201200. [DOI] [PubMed] [Google Scholar]
- 100.Song JJ. Rhee JG. Suntharalingam M. Walsh SA. Spitz DR. Lee YJ. Role of glutaredoxin in metabolic oxidative stress. Glutaredoxin as a sensor of oxidative stress mediated by H2O2. J Biol Chem. 2002;277:46566–46575. doi: 10.1074/jbc.M206826200. [DOI] [PubMed] [Google Scholar]
- 101.Sumbayev VV. S-nitrosylation of thioredoxin mediates activation of apoptosis signal-regulating kinase 1. Arch Biochem Biophys. 2003;415:133–136. doi: 10.1016/s0003-9861(03)00199-1. [DOI] [PubMed] [Google Scholar]
- 102.Sun QA. Su D. Novoselov SV. Carlson BA. Hatfield DL. Gladyshev VN. Reaction mechanism and regulation of mammalian thioredoxin/glutathione reductase. Biochemistry. 2005;44:14528–14537. doi: 10.1021/bi051321w. [DOI] [PubMed] [Google Scholar]
- 103.Tai HC. Schuman EM. Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nat Rev Neuroscience. 2008;9:826–838. doi: 10.1038/nrn2499. [DOI] [PubMed] [Google Scholar]
- 104.Tarrago L. Laugier E. Zaffagnini M. Marchand C. Le Marechal P. Rouhier N. Lemaire SD. Rey P. Regeneration mechanisms of Arabidopsis thaliana methionine sulfoxide reductases B by glutaredoxins and thioredoxins. J Biol Chem. 2009;284:18963–18971. doi: 10.1074/jbc.M109.015487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Taylor ER. Hurrell F. Shannon RJ. Lin TK. Hirst J. Murphy MP. Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J Biol Chem. 2003;278:19603–19610. doi: 10.1074/jbc.M209359200. [DOI] [PubMed] [Google Scholar]
- 106.Tew KD. Townsend DM. Regulatory functions of glutathione S-transferase P1-1 unrelated to detoxification. Drug Metab Rev. 2011;43:179–193. doi: 10.3109/03602532.2011.552912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wan X. Dennis AT. Obejero-Paz C. Overholt JL. Heredia-Moya J. Kirk KL. Ficker E. Oxidative inactivation of the lipid phosphatase phosphatase and tensin homolog on chromosome ten (PTEN) as a novel mechanism of acquired long QT syndrome. J Biol Chem. 2011;286:2843–2852. doi: 10.1074/jbc.M110.125526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ward NE. Stewart JR. Ioannides CG. O'Brian CA. Oxidant-induced S-glutathiolation inactivates protein kinase C-alpha (PKC-alpha): a potential mechanism of PKC isozyme regulation. Biochemistry. 2000;39:10319–10329. doi: 10.1021/bi000781g. [DOI] [PubMed] [Google Scholar]
- 109.Wilhelm D. Bender K. Knebel A. Angel P. The level of intracellular glutathione is a key regulator for the induction of stress-activated signal transduction pathways including Jun N-terminal protein kinases and p38 kinase by alkylating agents. Mol Cell Biol. 1997;17:4792–4800. doi: 10.1128/mcb.17.8.4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xing S. Salinas M. Huijser P. New players unveiled in early anther development. Plant Signal Behav. 2011;6:934–938. doi: 10.4161/psb.6.7.15668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang Y. Jao S. Nanduri S. Starke DW. Mieyal JJ. Qin J. Reactivity of the human thioltransferase (glutaredoxin) C7S, C25S, C78S, C82S mutant and NMR solution structure of its glutathionyl mixed disulfide intermediate reflect catalytic specificity. Biochemistry. 1998;37:17145–17156. doi: 10.1021/bi9806504. [DOI] [PubMed] [Google Scholar]
- 112.Yasinska IM. Kozhukhar AV. Sumbayev VV. S-nitrosation of thioredoxin in the nitrogen monoxide/superoxide system activates apoptosis signal-regulating kinase 1. Arch Biochem Biophy. 2004;428:198–203. doi: 10.1016/j.abb.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 113.Yu CX. Li S. Whorton AR. Redox regulation of PTEN by S-nitrosothiols. Mol Pharmacol. 2005;68:847–854. doi: 10.1124/mol.104.010504. [DOI] [PubMed] [Google Scholar]
- 114.Zaffagnini M. Bedhomme M. Marchand CH. Couturier J. Gao XH. Rouhier N. Trost P. Lemaire SD. Glutaredoxin s12: unique properties for redox signaling. Antioxid Redox Signal. 2012;16:17–32. doi: 10.1089/ars.2011.3933. [DOI] [PubMed] [Google Scholar]
- 115.Zaffagnini M. Bedhomme M. Marchand CH. Morisse S. Trost P. Lemaire SD. Redox regulation in photosynthetic organisms: focus on glutathionylation. Antioxid Redox Signal. 2012;16:567–586. doi: 10.1089/ars.2011.4255. [DOI] [PubMed] [Google Scholar]
- 116.Zaffagnini M. Michelet L. Massot V. Trost P. Lemaire SD. Biochemical characterization of glutaredoxins from Chlamydomonas reinhardtii reveals the unique properties of a chloroplastic CGFS-type glutaredoxin. J Biol Chem. 2008;283:8868–8876. doi: 10.1074/jbc.M709567200. [DOI] [PubMed] [Google Scholar]
- 117.Zhang R. Al-Lamki R. Bai L. Streb JW. Miano JM. Bradley J. Min W. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circ Res. 2004;94:1483–1491. doi: 10.1161/01.RES.0000130525.37646.a7. [DOI] [PubMed] [Google Scholar]