Abstract
Significance: Parkinson's disease (PD) is characterized by the selective loss of dopaminergic neurons of the substantia nigra pars compacta, which has been widely associated with oxidative stress. However, the mechanisms by which redox signaling regulates cell death progression remain elusive. Recent Advances: Early studies demonstrated that depletion of glutathione (GSH), the most abundant low-molecular-weight thiol and major antioxidant defense in cells, is one of the earliest biochemical events associated with PD, prompting researchers to determine the role of oxidative stress in dopaminergic cell death. Since then, the concept of oxidative stress has evolved into redox signaling, and its complexity is highlighted by the discovery of a variety of thiol-based redox-dependent processes regulating not only oxidative damage, but also the activation of a myriad of signaling/enzymatic mechanisms. Critical Issues: GSH and GSH-based antioxidant systems are important regulators of neurodegeneration associated with PD. In addition, thiol-based redox systems, such as peroxiredoxins, thioredoxins, metallothioneins, methionine sulfoxide reductases, transcription factors, as well as oxidative modifications in protein thiols (cysteines), including cysteine hydroxylation, glutathionylation, and nitrosylation, have been demonstrated to regulate dopaminergic cell loss. Future Directions: In this review, we summarize major advances in the understanding of the role of thiol-redox signaling in dopaminergic cell death in experimental PD. Future research is still required to clearly understand how integrated thiol-redox signaling regulates the activation of the cell death machinery, and the knowledge generated should open new avenues for the design of novel therapeutic approaches against PD. Antioxid. Redox Signal. 17, 1764–1784.
Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disorder affecting ∼6 million people worldwide. Afflicted PD patients cost the U.S. economy billions of dollars each year in direct health care costs and lost work force. PD is a neurodegenerative disorder characterized by the selective loss of dopaminergic neurons of the substantia nigra pars compacta (SNpc). The accumulation of α-synuclein is a crucial step in pathogenesis and a key constituent of intraneuronal proteinaceous inclusions known as Lewy bodies. There is no current treatment to stop neuronal cell death and/or cure PD. Current therapies available only delay the onset of PD and/or ameliorate motor symptoms by addressing dopamine deficit. Thus, in order to find neuroprotective strategies, a clear understanding of the mechanism(s) involved in dopaminergic cell death in PD is needed. Although neuronal cell death is a cardinal feature of PD, the mechanisms and pathways involved remain unclear, mainly because in the majority of cases the cause of PD is unknown. Current evidence supports a role for mitochondrial dysfunction, oxidative stress, and abnormal protein accumulation as early triggers of neuronal death in PD pathogenesis (105, 193).
Oxidative stress can be defined as an imbalance between the production of reactive species (RS, including both reactive oxygen species [ROS] and reactive nitrogen species [RNS]) and the ability to detoxify them and their intermediates/byproducts. Oxidative damage to lipids, proteins, and DNA has been detected in autopsies from individuals with PD (5, 6, 51). Interestingly, a decrease in the levels of the low-molecular-weight thiol antioxidant glutathione (GSH) is one of the earliest biochemical alterations detected in association with PD (84, 139). Since then, a number of research studies have addressed the role of GSH homeostasis in PD progression. GSH, a major antioxidant defense and the most abundant nonprotein thiol in mammalian cells, contains a free thiol group that mediates its redox functions (59, 60). Thiols are organosulfur compounds that contain a carbon-bonded sulfhydryl group. As part of the functional group of the amino acid cysteine, protein cysteines (protein thiols) are now thought to mediate redox signaling processes in response to oxidative stress. This has significantly changed researchers' interest from characterizing oxidative stress or damage to identifying specific redox signaling events involved in human disease progression. Cysteine residues serve numerous functions. Catalytic redox-active cysteines in the active sites of thiol oxidoreductases are involved in their catalytic activity and participate in oxidation, reduction, and isomerization of disulfide bonds, as well as in reactions that modulate their redox states. Regulatory cysteines present in proteins, such as transcription factors and phosphatases, alter protein activity by changing their redox state, without being catalytic themselves, and can be regulated by reversible intramolecular and intermolecular oxidative modifications. Structural cysteines participate in protein structure and folding via the formation of stable intramolecular and intermolecular disulfide bonds. Metal-coordinating cysteines coordinate metal ions and occur in the form of CxxC motifs. Catalytic non-redox cysteines participate in catalysis without alteration in their redox state and are present in various proteases. And finally, cysteines can act as sites of post-translational modifications, which are utilized for targeting proteins to membranes and as sites of post-translational modifications that influence protein activity, localization, and/or protein–protein interactions (57). An increasingly complex scenario of distinct thiol-dependent redox signaling pathways and enzymatic processes has emerged since GSH was found to participate in cellular redox homeostasis. In PD, a variety of thiol-based redox signaling events have been recently described as important triggers/regulators of dopaminergic neurodegeneration, and in this review, we aim to highlight the most important discoveries in this area.
PD Versus Experimental PD
A fraction of PD occurrence is related to mutations in genes such as those encoding α-synuclein (SNCA), DJ-1 (PARK7), PTEN-induced putative kinase 1 (PINK1), leucine-rich repeat kinase 2 (LRRK2), and Parkin (PARK2). Over 90% of PD occurs most commonly in a sporadic (idiopathic) form without a clearly defined genetic basis, and only a vaguely delineated pathogenesis likely linked to environmental causes. The major risk factor identified for PD is aging as its prevalence and incidence increase exponentially from ages 65 to 90. Thus, it is thought that PD arises from the convergence of genetic susceptibility, environmental exposures, and aging. However, the etiology of PD has yet to be clearly established. Cellular and animal disease models based on both genetic- and toxin-induced neurodegeneration have been used to understand PD pathogenesis. However, as reviewed by Horowitz and Greenamyre and Vance et al. (77, 177), not all experimental models recapitulate dopaminergic cell death, Lewy body formation, and/or symptoms observed in PD. Mitochondrial/environmental toxins, such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) or its active metabolite 1-methyl-4-phenylpyridine (MPP+), and pesticides rotenone and paraquat induce dopaminergic cell death in vitro and in vivo (24). The support for the use of MPTP as an experimental PD model originates in 1982 from the discovery of a group of young drug addicts, which developed a clinical picture almost indistinguishable from PD after self-administration of a synthetic heroin analog accidentally contaminated with MPTP. Epidemiological evidence shows an increase in the risk of developing PD upon exposure to environmental toxicants, such as the pesticides rotenone and paraquat (167). Several other toxicants, such as metals, maneb, organochlorine/organophosphorus pesticides, polychlorinated biphenyls, as well as early life inflammatory processes and occupational manganese exposure, have been implied to increase PD risk (62). Genetically engineered PD animal models have been developed for the overexpression of mutant autosomal-dominant genes (SNCA and LRRK2) and knockout/knockdown of autosomal-recessive genes (PARK2, PARK7, and PINK1). However, only marginal or null dopaminergic cell death has been observed in genetic-based animal models (144, 170), which suggests that genetic risk factors are not sufficient and that environmental exposures may also be required for nigral dopamine neuronal degeneration (gene–environment interactions). For example, high occupational pesticide exposure to paraquat and maneb in individuals carrying dopamine transporter (DAT) genetic variants increases the risk of developing PD (93, 146). New models studying gene–environment interactions have emerged since then. As reviewed by Gao and Hong (62), neurotoxins, such as paraquat and MPTP, as well as lipopolysaccharide (LPS)-induced inflammation exacerbate α-synuclein pathology. It is important to state that although many of the features of PD are observed in the current existing models of PD, none of them recapitulate the full spectrum of the disease. However, the use of both genetic- and toxin-based experimental PD models has helped us to identify important mechanisms regulating dopaminergic cell death and survival, and should keep helping us understand PD.
Oxidative Stress in Dopaminergic Cell Death and PD
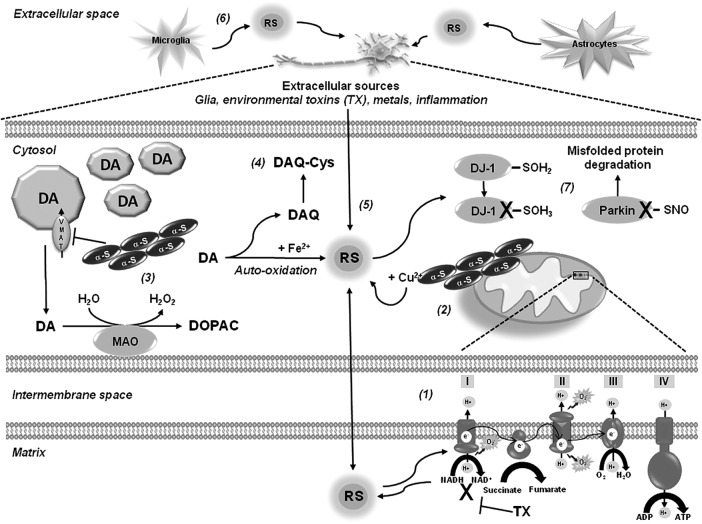
Oxidative stress in PD has been primarily linked to mitochondrial dysfunction, as decreased activity of the mitochondrial electron transport chain (ETC) was evidenced in the substantia nigra of patients with PD (75, 157) (Fig. 1). Accordingly, toxins, such as MPTP, rotenone, and paraquat, act primarily by inhibition of complex I, a major component of the ETC, leading to an increased generation of RS (20, 159). Interestingly, recent reports suggest that complex I inhibition might not be the only mechanism involved in dopaminergic neuron death induced by rotenone, MPP+, or paraquat (34, 129, 132, 143). Additional sources for RS generation have been described in the mitochondria besides complex I (2) and differential contribution of mitochondrial respiratory chain complexes seen to mediate RS production in response to paraquat (25, 53).
FIG. 1.
Oxidative stress and thiol-redox signaling in Parkinson's disease (PD). Oxidative stress in PD is linked primarily to mitochondrial dysfunction. Decreased activity of the mitochondrial complex I in the substantia nigra pars compacta (SNpc) of patients with PD has been reported, and mitochondrial toxins, such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and rotenone, used in experimental PD, act primarily by inhibition of complex I leading to an increased generation of reactive species (RS) (1). α-Synuclein and α-synuclein-metal complexes induce mitochondrial dysfunction and oxidative damage, and metallothioneins (MTs) have been shown to exert a protective effect (2). Mutant α-synuclein and environmental toxins impair vesicular dopamine transporter 2 (VMAT2) augmenting cytosolic dopamine levels, where dopamine is metabolized by monoamine oxidase (MAO) or auto-oxidized generating RS and dopamine-quinone species (DAQ) (3). DAQ are highly reactive products that can inactivate proteins by reacting with protein thiols (4). Environmental toxins have been shown to induce oxidative damage through a variety of mechanisms, including impairment of mitochondrial electron transport chain (ETC) (1), auto-catalytic generation of RS (5), increased intracellular dopamine catabolism, activation of plasma membrane nicotinamide adenine dinucleotide phosphate (NADPH)-oxidases, and extracellular sources through the activation of glial cells and induction of inflammatory processes (6). An important cellular target of RS is the thiol group of protein cysteine residues. Protein sulfonic acid (PSO3H) and protein nitrosylation (PSNO) of the PD-associated genes DJ-1 and Parkin have been shown to impair their redox sensing and ubiquitin E3 ligase activity, respectively (7). Broken arrows depict the magnification of the indicated region.
Accumulation of α-synuclein has been suggested to produce mitochondrial oxidative damage (49), while α-synuclein-copper (Cu2+) complexes have been shown to induce RS accumulation and dopamine oxidation (180). Metal-induced RS generation also contributes to oxidative damage in PD. Increased iron (Fe) deposition and increased free Fe levels were found in the substantia nigra of PD brains (162). Oxidative stress in PD is also associated with the pro-oxidant properties of dopamine (131). Mutant α-synuclein downregulates the vesicular dopamine transporter (VMAT2) augmenting cytosolic dopamine (111), which is either metabolized by monoamine oxidase (MAO) to generate hydrogen peroxide (H2O2), or auto-oxidized in the presence of Fe generating superoxide anion (•O2−), H2O2, and dopamine-quinone species (DAQ) (1). Increased Fe concentrations have been found in the SNpc of PD brains, which might promote dopamine oxidation and generation of RS (194). Further, the neurotoxins rotenone, MPTP, and paraquat increase intracellular dopamine oxidation (89, 110, 112). Plasma membrane nicotinamide adenine dinucleotide phosphate (NADPH) oxidases have also been proposed as sources of intracellular (dopaminergic cells) and extracellular (microglial cells) ROS generation upon MPP+, rotenone, and paraquat treatment (43, 63, 187, 188).
GSH in Dopaminergic Cell Death and PD
As mentioned previously, a decrease in the GSH levels is one of the earliest biochemical alterations detected in association with PD as demonstrated by the observation that GSH loss occurs in incidental Lewy body disease, considered an asymptomatic precursor to PD (84, 139, 163). GSH itself regulates dopaminergic cell death through a wide variety of homeostatic processes. Because of space restrictions we will only highlight some major findings regarding the role of GSH in dopaminergic cell death and PD. However, recent reviews have covered this topic extensively (115, 196).
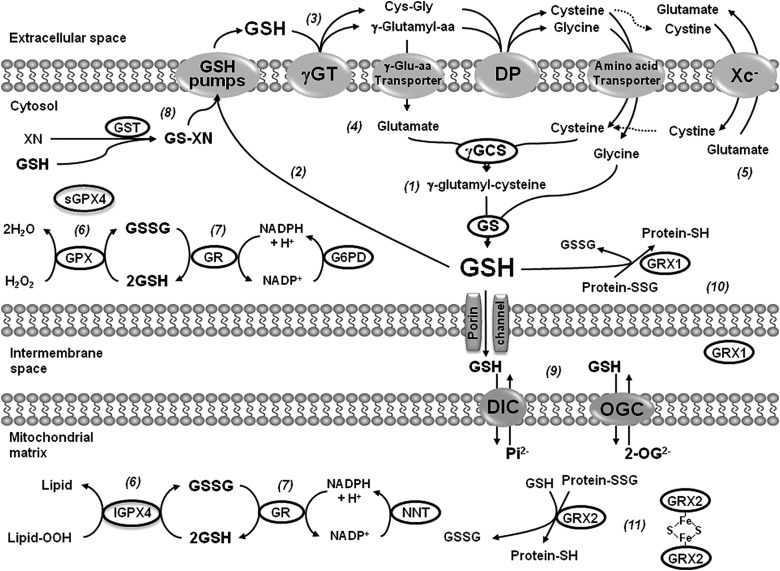
In experimental PD models, GSH depletion is linked to its oxidation in association with oxidative stress (52). Interestingly, GSH depletion induced by MPTP toxicity has also been associated with the impairment of neuronal cysteine uptake via the excitatory amino acid carrier-1 (EAAC1) (10) (Fig. 2). Intracellular GSH has a short half-life; thus, cells require the constant renewal of the GSH pool. Cysteine availability is rate limiting for the synthesis of GSH. However, because cysteine is unstable, the main extracellular source of cysteine is the dipeptide cystine (two conjugated cysteines via auto-oxidation). Blood plasma contains relatively high concentrations of cystine (100–200 μM), but only 10–20 μM of cysteine. Cystine uptake by a Na+-independent heteroexchange mechanism denominated that xc− operates as a 1:1 exchanger with intracellular glutamate, where cystine is transported inward in exchange for glutamate. By providing cystine to cells, system xc− has been shown to be an important regulator of intracellular GSH levels and antioxidant capacity of the brain (7). Interestingly, xc− deletion does not decrease GSH content in the striatum, nor it increases oxidative stress in striatum or substantia nigra. In contrast, it results in increased resistance to 6-hydroxydopamine (6-OHDA)-induced neurodegeneration, an effect connected to a decrease in the extracellular glutamate levels (116).
FIG. 2.
Glutathione (GSH) homeostasis and GSH-dependent antioxidant systems. GSH (l-γ-glutamyl-l-cysteinyl-glycine) synthesis occurs in the cytosol from the combination of glutamate and cysteine by the γ-glutamylcysteine synthetase (γ-GCS), also known as γ-glutamylcysteine ligase. Glycine is subsequently added to the γ-glutamylcysteine by the activity of glutathione synthetase (GS) (1). Export of GSH, GSSG, and GSH adducts is an important step in its catabolism (2), which is mediated extracellularly through the removal of the γ-glutamyl moiety from GSH and GSH-conjugated compounds, producing cysteinylglycine or cysteinylglycine conjugates by the activity of the γ-glutamyl transpeptidase (γ-GT). These products are hydrolyzed by dipeptidases (DPs) and then both cysteine and γ-glutamyl amino acids formed are taken up by the activity of specific transporters (3). γ-Glutamyl derivatives are substrates converted to glutamate by the activity of the 5-oxoprolinase (4). Cysteine availability is rate limiting for the synthesis of GSH, and cystine is the prevailing form of cysteine in the extracellular space due to cysteine auto-oxidation. Cystine is taken up by the xc− system, which exchanges cystine for glutamate (5). GSH can catalytically detoxify cells from peroxides in both the cytosol and the mitochondrial compartments by the action of glutathione peroxidase (GPX) and phospholipid hydroperoxide GPXs (GPX4) leading to the formation of glutathione disulfide (GSSG). A long and a short form of GPX4 have been described, which are distinguishable by the presence or lack of a mitochondrial signal peptide at the N-terminus (6). GSSG is reduced by the action of glutathione reductase (GR), which requires reduced NADPH as the electron donor. In the cytosol, glucose-6-phosphate dehydrogenase (G6PD) is indispensable for the regeneration of NADPH from NADP+, while in the mitochondria the nicotinamide nucleotide transhydrogenase (NNT)-catalyzed reduction of NADP+ accounts for more than half of the mitochondrial NADPH pool (7). Glutathione-S-transferases (GSTs) catalyze the conjugation of GSH electrophilic centers facilitating the detoxification and excretion of different compounds (8). A significant pool of GSH is compartmentalized in the mitochondria by the dicarboxylate carrier (DIC) or 2-oxoglutarate transporter (OGC) (9). Glutaredoxins (GRXs) are oxidoreductases that under reducing conditions utilize the reducing power of GSH to catalyze protein deglutathionylation. GRX1 has been found in both cytosolic and intermembrane space compartments (10). Mitochondrial matrix GRX2 contains a Fe-S cluster that bridges two GRX2 monomers and serves as a redox sensor (11).
GSH depletion inhibits mitochondrial complex I activity in vitro and in vivo (28, 47, 86), and this has been associated with cysteine post-translational modifications (29, 47). Glutathione reductase (GR), which reduces glutathione disulfide (GSSG) back to GSH, requires reduced NADPH as the electron donor reductant, and glucose-6-phosphate dehydrogenase (G6PD) is indispensable for the regeneration of NADPH from NADP+. Transgenic mice overexpressing G6PD in dopaminergic cells are resistant to MPTP-induced toxicity (117). GSH-based antioxidant systems have also been reported to exert a protective effect against dopaminergic cell death, as mice deficient in glutathione peroxidase 1 (GPX1) exhibit an increased sensitivity to MPTP toxicity (98), while its overexpression protects against MPP+- and 6-OHDA-induced toxicity in vitro and in vivo (17, 88, 145, 169). GSH homeostasis is also modulated by PD-related genes. The protective effects of DJ-1 against oxidative stress have been associated with the upregulation of intracellular GSH content (109, 198). A recent randomized clinical trial failed to demonstrate a protective effect of intravenous GSH administration in PD patients (74). However, it is not clear how well GSH crosses the blood–brain barrier (BBB). Studies have demonstrated that GSH is transported across the BBB in the rat and guinea pig, while human cerebrovascular endothelial cells exhibit GSH uptake and efflux (90, 91, 201). In contrast, oral administration of N-acetyl-l-cysteine (NAC), which acts as donor of cysteine for the synthesis of GSH and has a good penetration through the BBB (56), was shown to attenuate dopaminergic cell loss induced by MPTP, 6-OHDA, and overexpression of α-synuclein in vivo (38, 128, 136, 137). Furthermore, central delivery of cell-permeable GSH, but not GSH, elevates brain intracellular GSH and reduces toxicity of MPP+ in vivo (195).
GSH is compartmentalized within the mitochondria, endoplasmic reticulum (ER), and nuclei, and it is known that distinct GSH pools, particularly that of mitochondria and ER, are key regulators of cell death pathways. However, to date, there is little evidence regarding the distinct role of cytosolic versus mitochondrial GSH in dopaminergic cell death in spite of the clear importance of mitochondria in PD.
Thiol-Based Oxidoreductases in Dopaminergic Cell Death and PD
Thioredoxins
Thioredoxins (TRXs) are dithiol-redox proteins with a conserved Cys-Gly-Pro-Cys catalytic site that reduces or binds to proteins modifying their activity. The TRX redox system depends on thiol-disulfide exchange reactions at the active site. Thioredoxin reductase (TRXR), a homodimeric selenium containing flavoprotein, transfers reducing equivalents from NADPH to TRXs reducing them. While the TRX1/TRXR1 system is localized in the cytoplasm, the TRX2/TRXR2 couple is mitochondrial. In addition, TRXs provide reducing equivalents for the peroxide scavenging activity of peroxiredoxins (PRDXs) (Fig. 3). TRX1 has also been shown to reduce and activate a number of transcription factors. In the substantia nigra both TRX1 and TRX2 mRNA have been detected (107, 150). At the protein level, recent immunohistochemical analyses have demonstrated the strong expression of both TRX1 and TRX2 in the substantia nigra of mouse and rat brain, while weaker but consistent detection of TRXR1 and TRXR2 was also found (9, 66). Overexpression and administration of TRX1 protect against MPP+- and paraquat-induced toxicity in dopaminergic cells (12, 190). However, oxidation of TRX2 but not TRX1 induced by rotenone and MPP+, was reported in a different study. Contradictory results exist regarding the ability of paraquat to induce either TRX1 or TRX2 oxidation (141, 147). TRX1 also functions as a chaperone, and this seems to be independent of its redox activity. Expression of the Parkin-associated endothelin receptor-like receptor (Pael-R), a substrate of the E3 ubiquitin ligase Parkin, causes selective age-dependent degeneration of Drosophila dopaminergic neurons. Interestingly, overexpression of redox-inactive mutants of Drosophila TRX homologs, which remained active as chaperones, prevented neuronal loss by Pael-R expression (174).
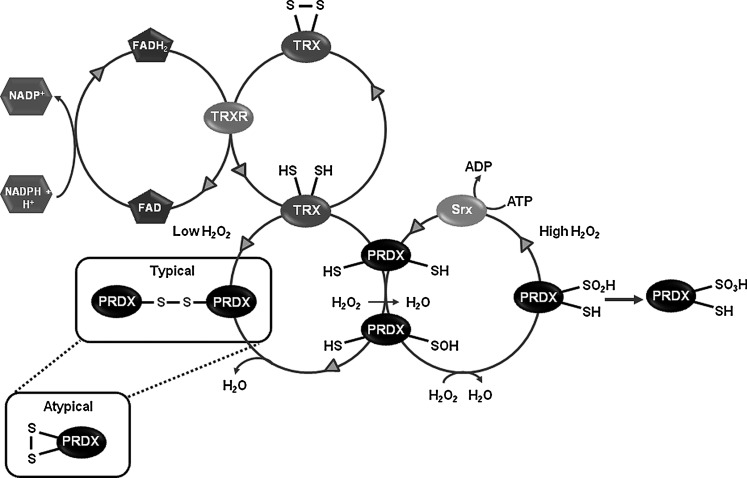
FIG. 3.
Thioredoxin–peroxiredoxin (TRX-PRDX) system. PRDXs catalyze the reduction of hydrogen peroxide (H2O2) to H2O. H2O2 oxidizes the peroxidatic cysteine of PRDXs to protein sulfenic acid (PSOH), which can react with the thiol (SH) group of the resolving Cys to yield the formation of an inter- (typical) or intramolecular (atypical) disulfide bond. TRX/thioredoxin reductase (TRXR) system mediates the reduction of the PRDX disulfide bond. TRX reduced state is maintained by the flavoenzyme TRXR in the presence of NADPH. When H2O2 exceeds the normal levels, PRDXs are overoxidized from PSOH to protein sulfinic acids (PSO2H). The latter can be reduced back to the native form of the enzyme by sulfiredoxin (SRX) in the presence of ATP. However, further oxidation of PRDXs to PSO3H is irreversible.
Peroxiredoxins
PRDXs are ubiquitous thiol peroxidases that scavenge peroxides in cells (Fig. 3). Catalysis of H2O2 is initiated by the reaction of their active site cysteine residue with H2O2, and recent studies have demonstrated that the reaction rate for PRDXs with H2O2 is in many cases comparable to that of catalase (42, 71). PRDXs are broadly distributed and highly abundant proteins comprising up to 1% of soluble cellular protein. Mammals have six PRDXs, with PRDX1, 2, and 6 found in the cytoplasm, PRDX4 in the ER, PRDX3 in the mitochondria, and PRDX5 in various compartments within the cell, including peroxisomes and mitochondria. In the substantia nigra, dopaminergic neurons express PRDX2–5 at very low levels, being PRDX5 the isoform with the lowest expression. Interestingly, non-dopaminergic neurons of the substantia nigra show higher expression levels of PRDX3 and PRDX5, while PRDX1 and PRDX6 are only expressed in glial cells (9, 67). An increase in PRDX2 and a decrease in PRDX3 and PRDX6 levels are observed in postmortem PD brains (99). PRDX2 oxidation has also been reported in PD brains (55). Paraquat induces oxidation of both PRDX3 and PRDX1 (147). Overexpression of PRDX1, PRDX2, and PRDX4 protects against 6-OHDA-induced dopaminergic cell death (79, 104), whereas silencing mitochondrial PRDX3 and PRDX5 increases sensitivity to MPP+ (48). Interestingly, PRDX2 protective effects seem to be mediated by modulation of TRX1 oxidation and apoptosis signal-regulating kinase-1 (ASK-1) (Fig. 4). In addition, mutations in LRRK2 are associated with autosomal dominant PD and a recent report shows that in vitro and in postmortem human samples of PD patients, mutant LRKK2 increases the inhibition of PRDX3 by phosphorylation (8).
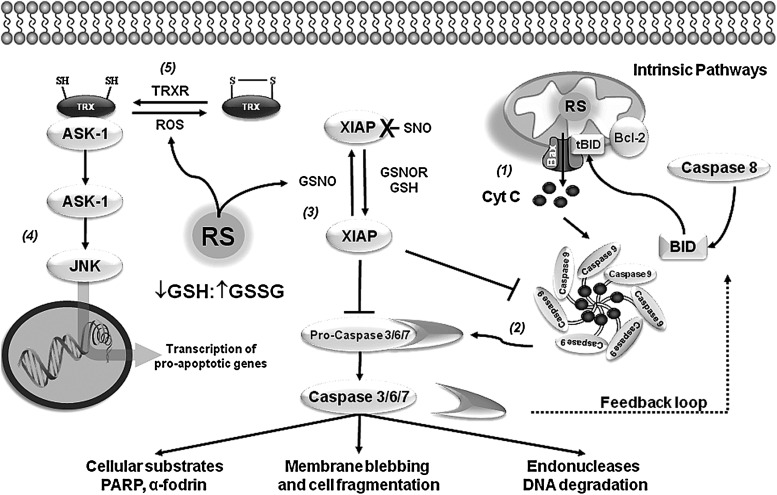
FIG. 4.
Thiol-redox signaling and apoptotic signaling cascades. Studies on postmortem brain tissue of PD patients have reported the presence of apoptotic markers in the substantia nigra supporting a role for apoptosis (programmed cell death) in neuronal cell loss. Accordingly, activation of caspases and pro-apoptotic members of the B-cell lymphoma 2 (Bcl-2) family of proteins was reported in the substantia nigra of PD patient autopsies, suggesting a role for the mitochondrial pathway of apoptosis in neuronal cell death. Oxidative stress and alterations in GSH/GSSG balance are known to regulate the apoptotic machinery. However, very little is known about the mechanisms by which thiol-redox signaling regulates the progression of apoptosis in PD. Mitochondrial dysfunction in PD conveys the formation of RS, leading to the activation of the intrinsic pathway (1). Activation of the mitochondrial pathway mediates the release of cytochrome C (Cyt C) that is regulated by the Bcl-2 protein family. The BH3-only members derepress Bcl-2 associated X protein (Bax) and Bcl-2 homologous antagonist/killer (Bak) by direct inhibition of the antiapoptotic Bcl-2 proteins to promote apoptosis. Bax and Bak induce the permeabilization of the outer mitochondrial membrane and the release of Cyt C, leading to the recruitment of apoptotic protease activating factor 1 (Apaf1) into an apoptosome and activation of caspase-9. Initiator caspases further activate/cleave executioner caspases (3, 6, and 7), which mediate cell demise (2). Proteins of the inhibitor of apoptosis family, including X-linked inhibitor of apoptosis protein (XIAP), inhibit executioner caspases through the occupation of their active site. In addition, XIAP can prevent caspase 9 dimerization that is required for its activation. XIAP-SNO is found in PD brains, and this oxidative modification impairs its ability to inhibit caspase activation (3). Apoptosis signal-regulating kinase-1 (ASK-1) is a member of the mitogen-activated protein kinase (MAPK) kinase family that activates c-Jun N-terminal kinase (JNK) and the p38 MAPK family members involved in apoptosis (4). TRX1 associates with the N-terminal portion of ASK-1 inhibiting its kinase activity. When oxidation of TRX1 cysteine residues occurs, the TRX1-ASK-1 complex dissociates leading to the phosphorylation and activation of ASK-1, which mediates the expression of pro-apoptotic genes (5).
Protein Cysteine Oxidation in Dopaminergic Cell Death and PD
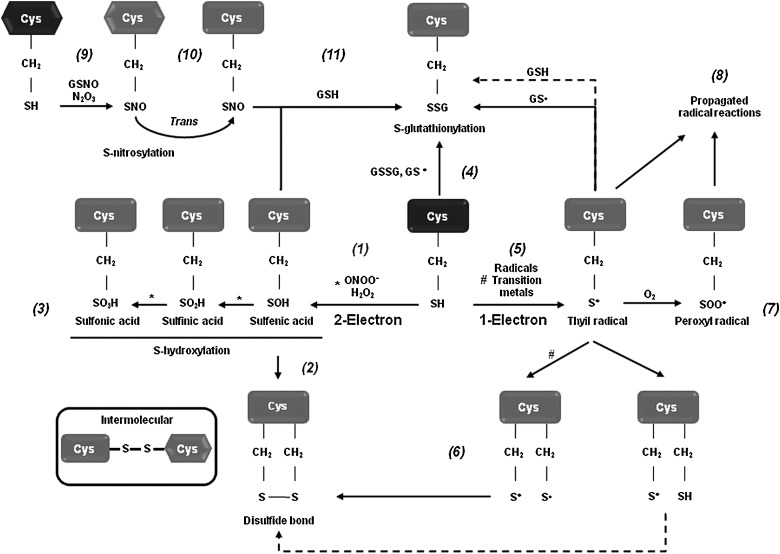
To understand the exact regulatory role of oxidative stress in the signal transduction events leading to dopaminergic cell death, we must determine the substrate specificity and biomolecular alterations caused by RS. Recent efforts have been undertaken to understand the molecular mechanisms by which oxidative stress regulates dopaminergic cell death associated with PD. Protein, lipid, and nucleic acid oxidation has been demonstrated to have an active role in dopaminergic cell death, but the exact mechanisms remain unclear (5, 6, 11, 46, 50). Specific amino acids, such as cysteine, methionine, tryptophan, and tyrosine residues, are prone to oxidative modification. By regulating protein structure and activity, oxidative post-translational modifications regulate a variety of physiological processes. An increase in protein oxidation has been reported in brains from PD patients as evidenced by the accumulation of oxidative and nitrative protein modifications (5, 46). An important cellular target or sensor of RS is the thiol group of the amino acid cysteine. Redox-sensitive cysteines undergo reversible and irreversible thiol modifications in response to ROS or RNS, thereby modulating protein function, activity, or localization. Oxidation and reduction of cellular thiols are thought to be one of the major mechanisms by which oxidative stress is integrated into cellular signal transduction pathways. Almost all physiological oxidants react with thiols (140, 186). Distinct RS, such as •O2− and peroxides (H2O2, and peroxynitrite [ONOO−]), mediate one- and two-electron oxidation of protein cysteines, respectively, leading to the formation of reactive intermediates, including protein sulfenic acids (PSOH) and protein thiyl radicals (PS•) (Fig. 5). Once formed, a PSOH can lead to the formation of additional oxidative modifications that act as signaling events regulating protein function. The reaction of PSOH with either a neighboring cysteine or GSH will generate a disulfide bond (PSSP) or a glutathionylated residue (PSSG). Both disulfide products can be reduced back to the thiol by the action of the TRX/TRXR and the glutaredoxin (GRX)/GSH systems, respectively. Additionally, PSOH can undergo further reaction with H2O2 to irreversibly generate protein sulfinic (PSO2H) and sulfonic (PSO3H) acids. To prevent overoxidation of critical cysteine residues, PSOH may be converted to disulfides or PSSG (138, 140, 186). A variety of oxidative cysteine modifications have been reported in PD and experimental PD models (Table 1).
FIG. 5.
Oxidative cysteine modifications. Redox-sensitive cysteines undergo reversible and irreversible thiol modifications in response to reactive oxygen species (ROS) or reactive nitrogen species (RNS), thereby modulating protein function, activity, or localization. Almost all physiological oxidants react with protein thiols (PSH). The two-electron oxidation (S-hydroxylation) involves the reaction between protein cysteine thiols (PSH) and ROS/RNS (H2O2, ONOO−) to generate the unstable PSOH (1), which subsequently reacts with another thiol to produce inter- or intramolecular mixed disulfides (2). ROS/RNS can further react with the PSOH to yield a hyperoxidized PSO2H and ultimately PSO3H (3). Likewise, PSOH can form mixed disulfides with GSH (PSSG) through reaction of PSH with GSSG (4). One-electron oxidation of PSH residues with free radicals and transition metal ions leads to the formation of protein thiyl radicals (PS•) (5). PS• can form inter- or intraprotein disulfide bonds (PSSP) by reaction with another PS• or a PSH (this last one through a disulfide radical anion intermediate, broken line) (6), or can generate protein peroxyl radicals (PSOO•) by reaction with O2 (7). Similar reactions can lead to PSSG formation by the reaction of a PS• with GS• or GSH. If either PSSP or PSSG bonds are not formed, protein radicals (PS• and PSOO•) can lead to the amplification of radical reactions (8). Covalent adduction of a nitroso (NO) group to a PSH residue is referred to PSNO, which is mediated by nitrosylating agents, such as dinitrogen trioxide (N2O3), transition-metal-catalyzed addition of NO (9), or transfer of the NO group between PSNO or nitrosoglutathione (GSNO) and a PSH residue (transnitrosation) (10). Interestingly, PSNO has also been shown to act as an intermediate for PSSG formation by reaction with GSH (11).
Table 1.
Protein Thiol Modifications in Parkinson's Disease and Experimental Parkinson's Disease Models
| Thiol modification | Protein | Reaction type | Reference | |
|---|---|---|---|---|
| Paraquat | Sulfinic acid | DJ-1 | Irreversible | (23) |
| Sulfinic/sulfonic acid | DJ-1 | Irreversible | (4, 23) | |
| Parkin | (120) | |||
| Sulfonic acid | Parkin | Irreversible | (120) | |
| S-nitrosylation | Parkin | Reversible | (35, 192) | |
| PRDX2 | Reversible | (55) | ||
| S-nitrosylation | XIAP | Reversible | (172) | |
| MPP+ | Glutathionylation | IDPM | Reversible | (97) |
| Rotenone | S-nitrosylation | Parkin | Reversible | (192) |
| PDI | Reversible | (173) | ||
| PRDX2 | Reversible | (55) | ||
| 6-OHDA | Sulfonic acid | DJ-1 | Irreversible | (4) |
| Sulfinic/sulfonic acid | PRDX1 | Irreversible | (104) | |
| PRDX2 | Irreversible | (79) | ||
| PD | S-nitrosylation | Parkin | Reversible | (35, 192) |
| Sulfonic acid | Irreversible | (120) | ||
| S-nitrosylation | PDI | Reversible | (173) | |
| Sulfonic acid | DJ-1 | Irreversible | (156) | |
| S-nitrosylation | PRDX2 | Reversible | (55) | |
| Sulfonic acid | SOD1 | Irreversible | (33) | |
| Sulfonic acid | UCH-L1 | Irreversible | (32) | |
| S-nitrosylation | XIAP | Reversible | (172) | |
| DLBD | S-nitrosylation | Parkin | Reversible | (35) |
DLBD, diffuse Lewy body disease; MPP+, 1-methyl-4-phenylpyridine; 6-OHDA, 6-hydroxydopamine; PD, Parkinson's disease.
Recent studies have demonstrated the importance of protein cysteine oxidation in the regulation of dopaminergic neurodegeneration and mitochondrial activity in PD (Table 1). DJ-1 mutations are associated with early onset PD. DJ-1 protects against oxidative stress and dopaminergic cell death induced by mitochondrial/environmental toxins. PSO2H formation at Cys106 in DJ-1 has been detected upon treatment with MPP+ and paraquat, as well as in erythrocytes of PD patients (4, 23, 156), and this oxidation has been proposed to regulate ROS scavenging properties and chaperone function of DJ-1 (185, 200) (Fig. 1). Cysteine oxidation to PSO2H/PSO3H inhibits the E3 ligase activity of the autosomal recessive PD gene Parkin, which regulates ubiquitination-mediated degradation of misfolded proteins (120). Cysteine sulfonation of the deubiquitinating enzyme ubiquitin carboxyl-terminal hydrolase L1 (UCH-L1), whose mutation is linked to early onset PD, was also found in PD brains (Table 1) (32).
DAQ byproducts from dopamine oxidation have been reported to bind to nucleophilic sulfhydryl groups on protein cysteines (73, 131) (Fig. 1). DAQ binding to protein thiols is thought to inactivate proteins, and thus replenishment of intracellular GSH pools prevents dopamine-induced toxicity by binding to DAQ (72). Inactivation of the Na+-K+-ATPase has been observed in the presence of dopamine (96). A recent proteomic analysis demonstrates the susceptibility of a variety of proteins to be covalently modified by dopamine, including the PD-associated proteins UCH-L1 and DJ-1 (176), but the exact amino acid residues involved were not identified.
Protein glutathionylation and GRXs in PD
Protein glutathionylation (PSSG) is defined as the reversible formation of mixed disulfides between GSH and protein thiols involved in the redox regulation of protein function. PSSG has been demonstrated to occur by mechanisms other than the reaction of GSH with PSOH (122) (Fig. 5). For example, PSSG formation might occur by thiol exchange reactions between protein cysteines and GSSG, which requires an unusually high redox potential only reported for a limited number of protein targets (122). Nitrosylation of protein cysteines and GSH generates protein nitrosylation (PSNO) and nitrosoglutathione (GSNO), and biochemical studies demonstrate the potential of GSNO to promote PSSG (122). Previous studies have demonstrated the occurrence of PSSG/deglutathionylation in dopaminergic cells and experimental PD models. However, very few protein targets susceptible to PSSG have been identified in dopaminergic cells, which are restricted to the mitochondrial compartment. Mitochondria PSSG has been reported in response to oxidative stress in dopaminergic cells (130). In contrast, a recent report demonstrates that mitochondrial NADP(+)-dependent isocitrate dehydrogenase (IDPm), which supplies NADPH for GSH production, is inactivated by glutathionylation in response to oxidative stress. Interestingly, mice treated with MPTP exhibit an increased level of IDPm-SSG (Table 1) (97). On the other hand, deglutathionylation of mitochondrial proteins and impairment of complex I activity have also been associated with cell death in dopaminergic neurons (130).
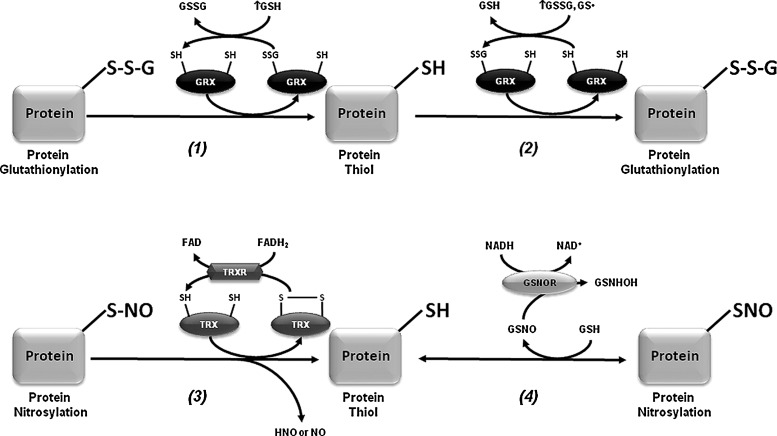
PSSG linkages are removed by changes in the intracellular GSH/GSSG balance and/or the activity of GRXs (Fig. 6). GRXs are oxidoreductases that utilize the reducing power of GSH to catalyze protein deglutathionylation. However, GRXs can induce both PSSG and deglutathionylation depending on the GSH/GSSG ratio and the presence of thiyl radicals (GS•) (15, 122). GRX1 is expressed at lower levels in the substantia nigra and striatum compared with hippocampus and cerebral cortex (9, 13). GRX1 is localized primarily in the cytosol although recent reports also demonstrate its presence in the intermembrane space (134). GRX1 exerts a protective effect against dopamine and MPTP toxicity in neuronal cells (44, 45, 95). Interestingly, incidence of PD is lower in women than in men, and it has been demonstrated that GRX1 is highly expressed in female compared with male mouse midbrain and striatum regions, which correlates with a decreased sensitivity to MPTP toxicity (94). Downregulation of GRX1 induces the loss of complex I activity, mitochondrial membrane potential, and DJ-1, as well as translocation of the death-domain-associated protein (DAXX), which is associated with dopaminergic cell death (95, 153, 154). The dopamine precursor levodopa (l-DOPA) is widely used in the clinical treatment for PD. However, l-DOPA induces dopaminergic cell death by direct inactivation of GRX1 and concomitant activation of ASK-1 (151, 152).
FIG. 6.
Enzymatic regulation of glutathionylation and nitrosylation. GRXs are oxidoreductases that under reducing conditions utilize the reducing power of GSH to remove PSSG residues (1). However, GRXs can also catalyze the formation of PSSG residues in the presence of high levels of oxidized GSH (GSSG or GS•) (2). Then, GRX1 activity depends upon the redox environment of the cell, with the potential to act as a glutathionylating enzyme under oxidative stress, and as a deglutathionylase under reducing conditions (high GSH availability). Removal of PSNO residues is catalyzed by the TRX system that involves the release of nitroxyl (HNO) and the formation of oxidized TRX that is further reduced by TRXR. This mechanism might involve the intermediate formation of either TRX-SNO or the intermolecular disulfide between TRX and the protein target (TRX-SS-P) (3). Transfer of NO groups by GSNO has been reported as one of the major mechanisms mediating PSNO. PSNO residues can also be denitrosylated by GSH leading to GSNO formation. GSNO reductase (GSNOR) prevents GSNO-mediated nitrosylation by its metabolism to N-hydroxysulphenamide (GSNHOH) (4).
Mitochondrial GRX2 is primarily localized in the matrix and is abundantly expressed in dopaminergic cells of the substantia nigra (9). GRX2 contains a Fe-S cluster that bridges two GRX2 monomers and serves as a redox sensor for its activation during conditions of oxidative stress (Fig. 2). Downregulation of GRX2 disrupts Fe-S center biogenesis (decreased Fe incorporation into mitochondrial proteins) and complex I activity in dopaminergic cells (92, 102), while its overexpression protects against MPTP-induced toxicity (92).
PSNO in dopaminergic cell death and PD
PSNO (also named cysteine nitrosation) refers to the reversible covalent adduction of a nitroso (NO) group to a protein cysteine resulting in the formation of PSNO. PSNO formation is regulated by nitric oxide synthase (NOS) expression and activity, and by endogenous NO-mediated nitrosylating agents, such as dinitrogen trioxide (N2O3), or by transition-metal-catalyzed addition of NO. Transfer of NO groups between PSNO and GSNO (transnitrosation) has been reported as one of the major mechanisms mediating PSNO. GSNO is formed during the oxygen-dependent oxidation of nitric oxide (•NO) in the presence of GSH, and as a minor byproduct from the oxidation of GSH by ONOO− (58) (Figs. 5 and 6). Increased PSNO levels of distinct substrates have been reported in PD and, in most cases, this modification is linked to the impairment of enzymatic function. Parkin–SNO has been shown in PD brains and in mice treated with MPTP, which inhibits its ubiquitin E3 ligase activity (35, 192) (Fig. 1 and Table 1). Similarly, protein disulfide isomerase (PDI), an ER enzyme that catalyses thiol-disulfide exchange reactions facilitating disulfide bond formation and protein folding, is PSNO in PD brains, which also inhibits its enzymatic activity leading to the increased accumulation of misfolded proteins and concomitant activation of the unfolded protein response (173). In addition, increased PSNO of PRDX2 and X-linked inhibitor of apoptosis protein (XIAP) is also found in PD brains (55, 172). PRDX2-SNO is associated with loss of its peroxide scavenging activity (55), while XIAP-SNO impairs its ability to inhibit caspase 3 (172). Interestingly, XIAP-SNO formation was shown to involve the transfer of the NO group from the executioner caspase 3 to XIAP (transnitrosation) (Fig. 4 and Table 1) (129). Progressive dopaminergic degeneration by LPS-induced neuroinflammation was recently shown to be preceded by PSNO of mitochondrial complex I, which was prevented by NOS inhibitors (31). Protein denitrosylation can be regulated by TRXs and also through the metabolism of GSNO via GSH-dependent formaldehyde dehydrogenase or class III alcohol dehydrogenase, also known as GSNO reductase (GSNOR) (Fig. 6) (16). However, neither a role for TRXs nor for GSNOR has been reported in PSNO formation in association with PD.
Glutathione-S-Transferases in PD
Although polymorphisms in glutathione-S-transferases (GSTs) have been associated with PD (119, 184), the exact role that these enzymes play in dopaminergic degeneration is far from understood. Loss-of-function mutations of GST-S1 enhance dopaminergic degeneration induced by Parkin mutants in Drosophila, while overexpression of GST-S1 prevents neuronal cell loss (183). Interestingly, inhibition or downregulation of GST-pi, which is found highly expressed in PD brains compared with controls (160), increases sensitivity to MPTP and dopamine toxicity (81, 165). However, there is no clear evidence of whether the protective mechanism of GST-pi against dopaminergic cell death is associated with its catalytic function in GSH conjugation to electrophilic substrates (Fig. 2), its ability to directly regulate signal transduction pathways through protein–protein interactions, or its recently proposed role in protein glutathionylation, reviewed in this Forum and in the article by Tew et al. (168).
Antioxidant Gene Transcription
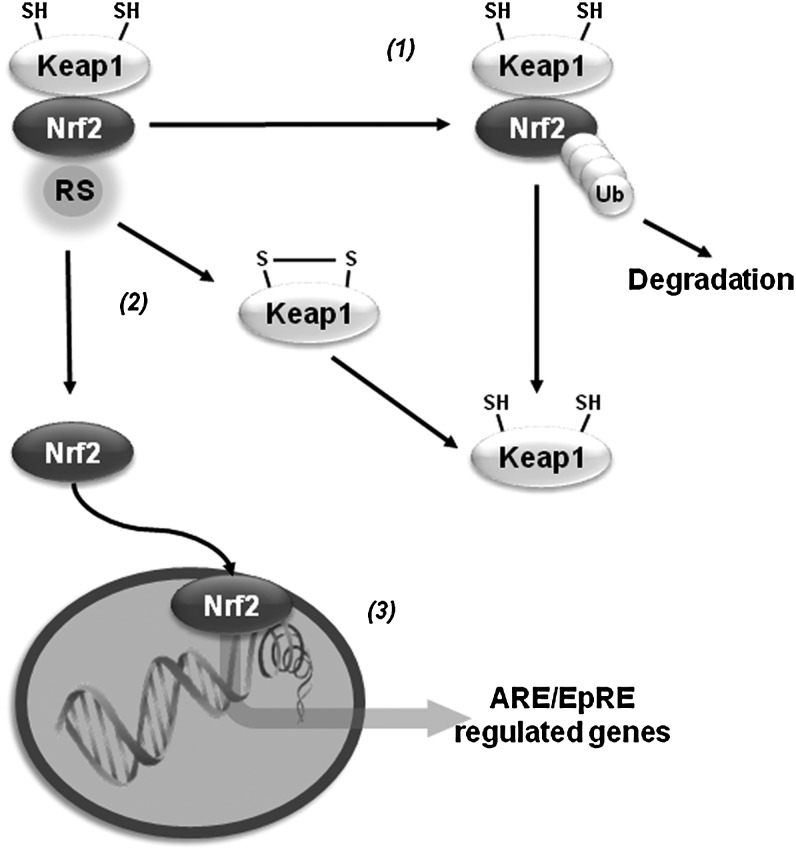
Oxidative stress activates the transcription of a variety of antioxidant genes through cis-acting sequences known as antioxidant response elements (AREs) or electrophile-responsive elements (EpREs). Members of the Cap-N-Collar family of transcription factors, including the nuclear factor (erythroid-derived 2)-like 1 (Nrf1) and 2 (Nrf2), have been identified to bind ARE. Nrf2 regulates the expression of proteins that directly or indirectly exert antioxidant functions and that also enhance GSH synthesis and regeneration. Under resting conditions, Nrf2 is kept in a cytosolic complex with the kelch-like ECH-associated protein 1 (Keap1, also called inhibitor of Nrf2 or INrf2), where it is subjected to proteasomal degradation. Oxidants or electrophiles induce a conformational change in Keap1 that allows the translocation of Nrf2 to the nucleus for transduction. Keap1 contains zinc (Zn)-coordinated Cys273 and Cys288 that are sensitive to alkylation. Initial thiylation of cysteines is thought to be followed by thiol-disulfide exchange to form internal disulfides within Keap1. However, recent evidence suggests that distinct activators act on different sets of cysteines in Keap1, which translates into specific biological effects (cysteine code). Transcription of γ-glutamylcysteine synthetase (γ-GCS), G6PD, GPXs, PRDXs, TRX/TRXR, sulfiredoxin (SRX), and GSTs by Nrf2 synergizes to improve the capacity of the GSH/thiol-based antioxidant systems described previously to counteract oxidative damage (21). Similar to Nrf2, Nrf1 has also been shown to regulate the expression of γ-GCS (18). A protective Nrf2 haplotype associated with increased transcriptional activity was linked to decreased risk of PD (179). Initial reports indicated that DJ-1 affects the transcriptional function and stability of Nrf2 by preventing association of Nrf2 with Keap1 (40). However, a separate study was not able to confirm these results (61). Overexpression of Nrf2 protects against α-synuclein toxicity in Drosophila (14). Paraquat induces a decrease in the expression of Nrf2, which is paralleled by a decrease in γ-GCS and intracellular GSH levels (191), while 6-OHDA and maneb induce Nrf2 activation (113, 147). Nrf2 activators protect against MPTP and 6-OHDA (83, 164), while Nrf2 knockout mice show exacerbated neurodegeneration and inflammation induced by MPTP (22, 27, 149) (Fig. 7).
FIG. 7.
Antioxidant gene regulation by nuclear factor (erythroid-derived 2)-like 2 (Nrf2). Under resting conditions kelch-like ECH-associated protein 1 or inhibitor of Nrf2 (INrf2) (Keap1) targets Nrf2 for proteosomal degradation through ubiquitylation of Nrf2 Lys residues that are located at its N terminus (1). Oxidation/alkylation of Keap1 disrupts the association of Nrf2 with Keap1, leading to its nuclear translocation (2). Nrf2 activates the transcription of a variety of antioxidant genes through antioxidant response elements (AREs) also known as electrophile-responsive elements (EpREs) (3).
Metallothioneins in PD
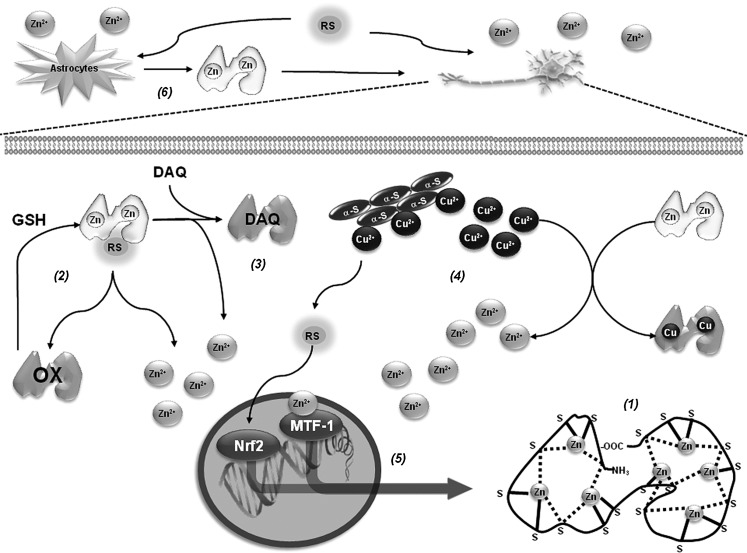
Alterations in metal homeostasis are associated with PD. More specifically, Zn and Fe are found increased, while Cu levels are decreased in the substantia nigra of PD brains. In the cerebrospinal fluid and blood of PD patients, an increased level of Cu has been reported (19, 78, 135). Interestingly, higher intake of Zn reduces the risk of PD (123). In contrast, occupational exposure to Cu increases the risk to develop PD (68). Metallothioneins (MTs) are a family of small cysteine-rich proteins with free radical scavenging and heavy metal binding properties (Fig. 8). GSH acts as a reducing agent for free-radical-induced MT oxidation (171). GSH alone stabilizes Zn binding to MTs and the presence of GSSG (or any other oxidizing agent) results in a release of Zn that is synergistically increased by GSH (87). There are four mammalian MT isoforms, MT-I to MT-IV. MT-I (encoded by seven genes) appears to be a superior scavenger for free radicals compared with MT-II (100). Within the brain, the most abundant isoforms are MT-I/II produced primarily by astrocytes (36). Brain MTs are single polypeptides of 61–68 amino acids, including 20 highly conserved cysteines and no disulfide bonds. MTs usually bind 7 divalent metal ions (Zn2+) and up to 12 monovalent Cu+ ions, partitioned into 12 metal-thiolate clusters located in separate protein domains (182). In PD brains and in vivo PD models, expression levels of MTs are reduced (126, 148). In addition, increased MT-I/II expression in reactive astrocytes was found in PD brains (121). MT gene expression is regulated by AREs. Dopamine exposure increases MT-I/II expression, which is associated to Nrf2 binding to ARE of MT genes (125). In addition, MT-I and -II have been reported to protect against DAQ-induced toxicity (124). MT-I/II transgenic mice are resistant to MPTP-induced toxicity, which is linked to increased levels of complex I activity and coenzyme Q10 (158), while their knockout increases susceptibility to MPTP (54). MT-III is highly expressed in the normal human brain, where it is present in neurons and astrocytes as well as the extracellular space. 6-OHDA downregulates MT-III mRNA levels in the basal ganglia (126), and those of MT-I in the striatum (161). Cu2+ binds to α-synuclein and accelerates its aggregation (103, 142, 175). MT-III prevents cell death induced by 6-OHDA (80) and hydroxyl radical (•OH) generation from α-synuclein-Cu2+ complexes through removal of Cu2+ (69, 118). The ability of MTs to protect against oxidized dopamine products has been reported to involve direct scavenging by the formation of covalent adducts (64).
FIG. 8.
Metallothioneins regulate metal homeostasis and redox signaling in PD. MTs contain two metal-thiolate clusters located in separate protein domains that bind seven divalent metal ions (Zn2+). There are three bridging (broken lines) and six terminal cysteine ligands (continuous lines) in the N-terminal β-domain (Zn3 cluster) and four bridging and six terminal ligands in the C-terminal α-domain (Zn4 cluster) (1). Scavenging of RS by MTs induces the release of Zn2+. GSH reduces and stabilizes Zn2+ binding to MTs (2). MTs protect against oxidized dopamine products, such as DAQ, by direct scavenging and formation of covalent adducts (3). Cu2+ has the ability to displace Zn2+ binding to MTs and MT-III removes Cu2+ from α-synuclein-Cu2+ preventing the generation of RS (4). MT gene expression is regulated by AREs and metal-responsive elements (MREs) within their promoter region (5). Once Zn2+ is liberated from MTs it binds to the zinc (Zn) fingers of metal-responsive transcription factor-1 (MTF-1) formation, while oxidative stress promotes Nrf2 binding to ARE and activation of MT genes. Once MTs are synthesized they immediately bind Zn2+ (1). MT-I and -II are highly inducible in response to metals, oxidative stress, and inflammation, and increased MT-I/II levels have been found in reactive astrocytes in PD brains. Release of MT-I/II from astrocytes has been reported to exert neuroprotective effects associated to their ability to scavenge metals and RS (6).
MT-I and -II are highly inducible in response to metals, oxidative agents, inflammation, and stress. Metal-induced synthesis of MTs is mediated through cis-acting DNA sequences known as metal-responsive elements (MREs), which are present in the promoter region (Fig. 8). The metal-responsive transcription factor-1 (MTF-1), also termed MRE-binding transcription factor-1 or metal regulatory transcription factor-1, is a pluripotent transcriptional regulator involved in cellular adaptation to heavy metal exposure and oxidative stress, and is the main inducer of MT expression (70). Double-mutant flies for Parkin and MTF-1 genes are not viable, while overexpression of MTF-1 of Drosophila MT dramatically augments life span and protects mitochondrial structure (155). People who abused methamphetamine or other amphetamine-like stimulants have been found to be more likely to develop PD. Methamphetamine induces dopamine release and oxidative damage in dopaminergic cells. Overexpression of MT-II protects against methamphetamine-induced cell death (3). (+/-)3,4-Methylenedioxymethamphetamine (MDMA or “ecstasy”) also increases MT-I and MT-II mRNA levels and double knockout mice were more vulnerable to MDMA toxicity (189).
Methionine Sulfoxide Reductases in PD
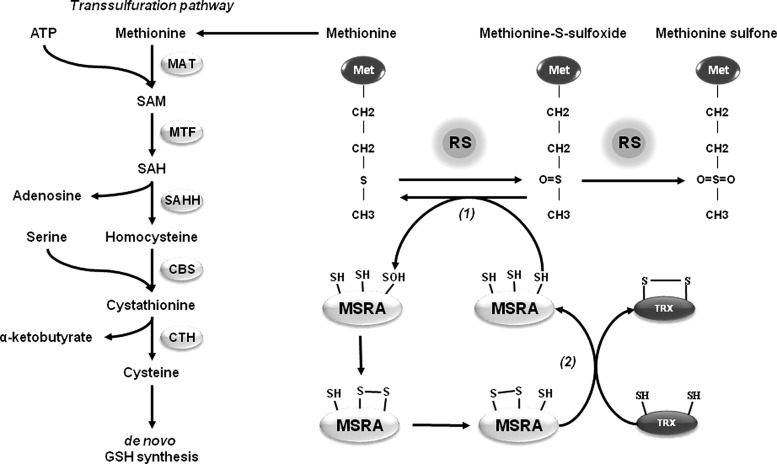
Methionine is one of the most vulnerable amino acid residues to oxidation by RS. Addition of an extra oxygen atom oxidizes methionine to methionine sulfoxide (MetSO), and a strong oxidant might further oxidize MetSO to methionine sulfone (MetSO2) (Fig. 9). Two different stereoisomers of methionine sulfoxide, l-Met-S-SO and l-Met-R-SO, can be found at physiological conditions and they are reverted by MetSO reductases (MSRs), which are thiol oxidoreductases. In humans, there are two different MSRs, MSRA and MSRB. MSRA is specific to the S-stereoisomer of MetSO, whereas MSRB reduces the R-stereoisomer of MetSO. Except for mouse and rat, mammalian MSRA is localized in the mitochondria. Mammals have three MSRB proteins. MSRB1 is a selenoprotein that contains selenocysteine (Se) in the place of the catalytic cysteine residue. MSRB1 is primarily located in the cytosol and nucleus, while MSRB2 is located in the mitochondria. MSRB3 presents two isoforms where MSRB3A is located in the ER and MSRB3B is targeted to the mitochondria (101). Methionine oxidation of α-synuclein inhibits fibrillation of this protein (65, 199). In contrast, MSRA and supplementation with S-methyl-l-cysteine reduce α-synuclein toxicity in Drosophila (181). Overexpression of MSRA also protects against rotenone and α-synuclein toxicity in dopaminergic cells in culture (108), while the yeast MSRA-null mutant leads to an increased accumulation of fibrillated α-synuclein (133). Methionine itself has a central role in maintaining cellular redox homeostasis as it is a precursor for other sulfur-containing amino acids and compounds. Cysteine can be generated from methionine through the transsulfuration pathway to support de novo GSH synthesis under oxidative stress conditions (Fig. 9) (101). Elevated homocysteine and diminished GSH levels are reported in PD patients, suggesting impairment of the transsulfuration pathway, which is functionally active in the brain (82, 178). Because MSRA reduces both free and protein-based MetSO, it can potentially contribute to GSH synthesis to counteract oxidative stress in PD.
FIG. 9.
Methionine sulfoxide and methionine sulfoxide reductase (MSR) redox cycle. Methionine oxidation by RS generates methionine sulfoxide (MetSO), which can be further hyperoxidized to MetSO2. MSRs share similar catalytic mechanism involving the initial formation of PSOH or protein selenocysteine sulfenic acid (PSeOH) (MSRB1) on the catalytic cysteine or selenocysteine, with consequent methionine release (1). In MSRA and MSRB1 the catalytic PSOH or PSeOH subsequently forms an intradisulfide bond with the resolving cysteine, which is ultimately reduced by TRX. In MSRA, a third cysteine mediates a thiol-exchange reaction to transfer the disulfide bond to the protein surface prior to its reduction by TRX (2). In contrast, MSRB2 and MSRB3 have only one cysteine, which is reduced directly by TRX. Because MSRA reduces both free and protein-based Met-S-SO, its protective effects can be associated to enhanced GSH synthesis through the transsulfuration pathway (3). In the transsulfuration pathway methionine is converted to S-adenosylmethionine (SAM) in an ATP-dependent reaction catalyzed by methionine adenosyltransferase (MAT). Subsequently, S-adenosylhomocysteine (SAH) is generated via methyltransferase (MTF) activity. SAH is hydrolyzed by SAH hydrolase (SAHH) to adenosine and homocysteine. Homocysteine is conjugated with serine to provide cystathionine by the action of cystathionine beta synthase (CBS). Finally, cystathionine-γ-lyase (CTH, or γ-cystathionase) catalyses the conversion of cystathionine to cysteine.
Potential Therapeutic Approaches
As described above, distinct thiol-based redox systems have been reported to protect against experimental PD. Thus, it could be envisioned that GSH replenishment or gene transfer of genes involved in de novo GSH synthesis and GSH-dependent antioxidant systems, such as GPXs, could represent a potential therapeutic approach against oxidative stress associated with PD. Accordingly, cell-permeable GSH analogs or NAC (30, 38, 128, 136, 137, 169), and overexpression of G6PD and GPX1 protect dopaminergic cells from experimental PD in vivo (17, 117, 145). The main goals of gene therapy in PD are to restore the ability of the brain to produce dopamine and to stop progressive neurodegeneration. Gene delivery using adenoviral vectors has been the most commonly utilized approach for gene therapy and a number of phase I and II clinical trials using viral vectors as means to transport enzymes to the striatum of PD patients are currently being tested. Unfortunately, no available treatment has yet proven to have a definitive neuroprotective effect for patients with PD (76). The knowledge generated in the last years supports a protective role of thiol-redox systems against PD. Recently, lentiviral delivery of PRDX2 has been shown to protect against 6-OHDA-induced dopaminergic cell death in vivo (79). Thiol-based antioxidants function as integrated coupled systems, where the activity of enzymes, such as GPX, GRX, and PRDX, depends on the reducing capacity of their reducing counterparts GSH/GR and TRX/TRXR. Thus, it is important to consider that simultaneous gene transfer of thiol-redox systems might have an increased protective effect compared with the overexpression of single enzymes.
Oxidative stress in PD is associated with mitochondrial dysfunction. Thus, mitochondrial-targeted antioxidants also represent a preemptive approach to counteract oxidative damage. Overexpression of mitochondrial manganese superoxide dismutase (MnSOD or SOD2) has been shown to protect against MPTP-induced toxicity (106). Mitoquinone or mitochondria-targeted ubiquinone (MitoQ)—which comprises the lipophilic triphenylphosphonium cation covalently linked to ubiquinone, the active antioxidant moiety of CoQ10, enabling MitoQ to cross membranes and to accumulate several-hundred fold within mitochondria—has been recently described as a potent mitochondrial antioxidant. However, in a double-blind placebo-controlled study, MitoQ did not slow PD progression (166). Other sources of RS formation that have been suggested to participate in PD are metal complexes and dopamine metabolism. In this regard, MT-III prevents ROS formation induced by α-synuclein-Cu2+ complexes in vitro, but this needs to be translated into experimental PD models in vivo (118). The monoamine oxidase inhibitors (MAO-Is) are used clinically as PD therapeutic agents (85). By inhibition of dopamine metabolism, MAO-Is decrease the formation of H2O2 (41, 114). Furthermore, MAO-Is also protect against α-synuclein toxicity (26).
Transcriptional regulation of antioxidant genes by Nrf2 might also represent an approach to prevent dopaminergic cell death as demonstrated by its protective effect against α-synuclein toxicity in vivo (14). However, mitochondrial dysfunction in PD is not only expected to generate oxidative stress but also energy deficiency. The proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α) is a transcription co-activator for nuclear receptors that coordinates a number of gene expression programs to promote mitochondrial biogenesis and oxidative phosphorylation, as well as antioxidant gene expression (39). Overexpression of PGC-1α protects against neuronal cell death induced by MPTP, rotenone, and α-synuclein (127, 197). However, viral-mediated overexpression in vivo of PGC-1α induces dopaminergic cell loss, suggesting that PGC-1α levels cannot be increased for therapeutic purposes above physiological levels (37).
Conclusions and Perspectives
Thiol-redox signaling has emerged as a central mechanism by which the generation of RS and oxidative stress are integrated into the activation of distinct signaling cascades regulating diverse homeostatic processes and pathological outcomes. Dopaminergic cell death associated with PD is largely linked to oxidative stress, and recent studies demonstrate the importance of thiol-redox signaling regulating neuronal loss. GSH depletion is one of the earliest biochemical alterations described during the progression of PD, and GSH-based antioxidants, such as GPX1, have been demonstrated to exert protective effects against dopaminergic cell death in experimental PD. Thiol-based oxidoreductases, such as PRDXs and TRXs, have also been demonstrated to mediate protective effects by scavenging peroxides and regulating the activation of pro-apoptotic signaling cascades, respectively. On the other hand, oxidative post-translational protein modifications in cysteines, including cysteine hydroxylation, glutathionylation, and nitrosylation, have been shown to regulate the function of PD genes (DJ-1 and Parkin), mitochondrial activity (complex I), and anti-apoptotic proteins (XIAP). The results summarized here demonstrate the relevance of thiol-redox signaling cascades in PD and guarantee further interest into elucidating novel therapeutic approaches against PD by regulation of thiol-redox homeostasis. In this regard, potential therapeutic approaches against oxidative stress and redox imbalance in PD include: (i) replenishment of the intracellular GSH pool and/or gene transfer of thiol-based antioxidants systems, such as GPX(GRX)/GR/NADPH and PRDX/TRX/TRXR; (ii) the use of mitochondrial-targeted antioxidants, MTs and/or MAO-Is to counteract oxidative stress in PD, which is mainly associated with impairment of mitochondrial function, RS formation by metal complexes, and dopamine oxidation; and (iii) transcriptional regulation of both antioxidant and gene expression programs to promote mitochondrial biogenesis and oxidative phosphorylation by Nrf2 and PGC-1α, in order to target both oxidative stress and energy failure due to mitochondrial dysfunction in PD.
Abbreviations Used
- 6-OHDA
6-hydroxydopamine
- γ-GCS
γ-glutamylcysteine synthetase
- γ-GT
γ-glutamyl transpeptidase
- Apaf1
apoptotic protease activating factor 1
- AREs
antioxidant response elements
- ASK-1
apoptosis signal-regulating kinase-1
- Bak
Bcl-2 homologous antagonist/killer
- Bax
Bcl-2 associated X protein
- BBB
blood–brain barrier
- Bcl-2
B-cell lymphoma 2
- CBS
cystathionine beta synthase
- CTH
cystathionine-γ-lyase or γ-cystathionase
- Cu
copper
- Cyt C
cytochrome C
- DAQ
dopamine-quinone species
- DAT
dopamine transporter
- DAXX
death-domain-associated protein
- DIC
dicarboxylate carrier
- DLBD
diffuse Lewy body disease
- DPs
dipeptidases
- EAAC1
excitatory amino acid carrier-1
- EpREs
electrophile-responsive elements
- ER
endoplasmic reticulum
- ETC
electron transport chain
- Fe
iron
- G6PD
glucose-6-phosphate dehydrogenase
- GPX
glutathione peroxidase
- GR
glutathione reductase
- GRX
glutaredoxin
- GS
glutathione synthetase
- GS•
glutathionyl radical
- GSH
glutathione
- GSNHOH
N-hydroxysulphenamide
- GSNO
nitrosoglutathione
- GSNOR
GSH-dependent formaldehyde dehydrogenase, class III alcohol dehydrogenase or GSNO reductase
- GSSG
glutathione disulfide
- GSTs
glutathione-S-transferases
- HNO
nitroxyl
- H2O2
hydrogen peroxide
- IDPm
NADP(+)-dependent isocitrate dehydrogenase
- JNK
c-jun N-terminal kinase
- Keap1
kelch-like ECH-associated protein 1 or inhibitor of Nrf2 (INrf2)
- LPSs
lipopolysaccharides
- LRRK2
leucine-rich repeat kinase 2
- MAO
monoamine oxidase
- MAO-Is
monoamine oxidase inhibitors
- MAPK
mitogen-activated protein kinase
- MAT
methionine adenosyltransferase
- MDMA
(+/-)3,4-methylenedioxymethamphetamine or “ecstasy”
- MetSO
methionine sulfoxide
- MetSO2
methionine sulfone
- MitoQ
mitoquinone or mitochondria-targeted ubiquinone
- MnSOD
manganese superoxide dismutase
- MPP+
1-methyl-4-phenylpyridine
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MREs
metal-responsive elements
- MSR
methionine sulfoxide reductase
- MT
metallothionein
- MTF
methyltransferase
- MTF-1
metal-responsive transcription factor-1
- NAC
N-acetyl-l-cysteine
- NADPH
nicotinamide adenine dinucleotide phosphate
- NNT
nicotinamide nucleotide transhydrogenase
- •NO
nitric oxide
- NO
nitroso group
- N2O3
dinitrogen trioxide
- NOS
nitric oxide synthase
- Nrf
nuclear factor (erythroid-derived 2)-like
- •O2−
superoxide anion
- OGC
oxoglutarate transporters
- •OH
hydroxyl radical
- ONOO−
peroxynitrite
- PD
Parkinson's disease
- PDI
protein disulfide isomerase
- PGC-1α
proliferator-activated receptor gamma coactivator 1 alpha
- PINK1
PTEN-induced putative kinase 1
- PRDXs
peroxiredoxins
- PS•
protein thiyl radical
- PSeOH
protein selenocysteine sulfenic acid
- PSH
protein thiols
- PSNO
protein (P) nitrosylation (SNO)
- PSOH
protein sulfenic acid
- PSO2H
protein sulfinic acid
- PSO3H
protein sulfonic acid
- PSOO•
protein peroxyl radical
- PSSG
protein glutathionylation
- PSSP
inter- or intramolecular disulfide bond
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RS
reactive species including both ROS and RNS
- SAH
S-adenosylhomocysteine
- SAHH
SAH hydrolase
- SAM
S-adenosylmethionine
- Se
selenocysteine
- SH
thiol group
- SNCA
synuclein, alpha gene
- SNpc
substantia nigra pars compacta
- SRX
sulfiredoxin
- TRX
thioredoxin
- TRXR
thioredoxin reductase
- UCH-L1
ubiquitin carboxyl-terminal hydrolase L1
- VMAT
vesicular dopamine transporter
- XIAP
X-linked inhibitor of apoptosis protein
- Zn
zinc
Acknowledgments
This work was supported by the National Institutes of Health Grant P20RR17675 Centers of Biomedical Research Excellence (COBRE), and Interdisciplinary Grant from the Research Council and the Life Sciences Grant Program of the University of Nebraska-Lincoln. LZF received a Post-doctoral Fellowship from the National Council of Science and Technology (CONACYT 211456) of Mexico.
References
- 1.Abou-Sleiman PM. Muqit MM. Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat Rev Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- 2.Adam-Vizi V. Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal. 2005;7:1140–1149. doi: 10.1089/ars.2005.7.1140. [DOI] [PubMed] [Google Scholar]
- 3.Aizenman E. McCord MC. Saadi RA. Hartnett KA. He K. Complex role of zinc in methamphetamine toxicity in vitro. Neuroscience. 2010;171:31–39. doi: 10.1016/j.neuroscience.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akazawa YO. Saito Y. Hamakubo T. Masuo Y. Yoshida Y. Nishio K. Shichiri M. Miyasaka T. Iwanari H. Mochizuki Y. Kodama T. Noguchi N. Niki E. Elevation of oxidized DJ-1 in the brain and erythrocytes of Parkinson disease model animals. Neurosci Lett. 2010;483:201–205. doi: 10.1016/j.neulet.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Alam ZI. Daniel SE. Lees AJ. Marsden DC. Jenner P. Halliwell B. A generalised increase in protein carbonyls in the brain in Parkinson's but not incidental Lewy body disease. J Neurochem. 1997;69:1326–1329. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- 6.Alam ZI. Jenner A. Daniel SE. Lees AJ. Cairns N. Marsden CD. Jenner P. Halliwell B. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem. 1997;69:1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- 7.Albrecht P. Lewerenz J. Dittmer S. Noack R. Maher P. Methner A. Mechanisms of oxidative glutamate toxicity: the glutamate/cystine antiporter system xc − as a neuroprotective drug target. CNS Neurol Disord Drug Targets. 2010;9:373–382. doi: 10.2174/187152710791292567. [DOI] [PubMed] [Google Scholar]
- 8.Angeles DC. Gan BH. Onstead L. Zhao Y. Lim KL. Dachsel J. Melrose H. Farrer M. Wszolek ZK. Dickson DW. Tan EK. Mutations in LRRK2 increase phosphorylation of peroxiredoxin 3 exacerbating oxidative stress-induced neuronal death. Hum Mutat. 2011;32:1390–1397. doi: 10.1002/humu.21582. [DOI] [PubMed] [Google Scholar]
- 9.Aon-Bertolino ML. Romero JI. Galeano P. Holubiec M. Badorrey MS. Saraceno GE. Hanschmann EM. Lillig CH. Capani F. Thioredoxin and glutaredoxin system proteins-immunolocalization in the rat central nervous system. Biochim Biophys Acta. 2011;1810:93–110. doi: 10.1016/j.bbagen.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Aoyama K. Matsumura N. Watabe M. Nakaki T. Oxidative stress on EAAC1 is involved in MPTP-induced glutathione depletion and motor dysfunction. Eur J Neurosci. 2008;27:20–30. doi: 10.1111/j.1460-9568.2007.05979.x. [DOI] [PubMed] [Google Scholar]
- 11.Ara J. Przedborski S. Naini AB. Jackson-Lewis V. Trifiletti RR. Horwitz J. Ischiropoulos H. Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Proc Natl Acad Sci U S A. 1998;95:7659–7663. doi: 10.1073/pnas.95.13.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai J. Nakamura H. Hattori I. Tanito M. Yodoi J. Thioredoxin suppresses 1-methyl-4-phenylpyridinium-induced neurotoxicity in rat PC12 cells. Neurosci Lett. 2002;321:81–84. doi: 10.1016/s0304-3940(02)00058-7. [DOI] [PubMed] [Google Scholar]
- 13.Balijepalli S. Tirumalai PS. Swamy KV. Boyd MR. Mieyal JJ. Ravindranath V. Rat brain thioltransferase: regional distribution, immunological characterization, and localization by fluorescent in situ hybridization. J Neurochem. 1999;72:1170–1178. doi: 10.1046/j.1471-4159.1999.0721170.x. [DOI] [PubMed] [Google Scholar]
- 14.Barone MC. Sykiotis GP. Bohmann D. Genetic activation of Nrf2 signaling is sufficient to ameliorate neurodegenerative phenotypes in a Drosophila model of Parkinson's disease. Dis Model Mech. 2011;4:701–707. doi: 10.1242/dmm.007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beer SM. Taylor ER. Brown SE. Dahm CC. Costa NJ. Runswick MJ. Murphy MP. Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: implications for mitochondrial redox regulation and antioxidant defense. J Biol Chem. 2004;279:47939–47951. doi: 10.1074/jbc.M408011200. [DOI] [PubMed] [Google Scholar]
- 16.Benhar M. Forrester MT. Stamler JS. Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat Rev Mol Cell Biol. 2009;10:721–732. doi: 10.1038/nrm2764. [DOI] [PubMed] [Google Scholar]
- 17.Bensadoun JC. Mirochnitchenko O. Inouye M. Aebischer P. Zurn AD. Attenuation of 6-OHDA-induced neurotoxicity in glutathione peroxidase transgenic mice. Eur J Neurosci. 1998;10:3231–3236. doi: 10.1046/j.1460-9568.1998.00345.x. [DOI] [PubMed] [Google Scholar]
- 18.Biswas M. Chan JY. Role of Nrf1 in antioxidant response element-mediated gene expression and beyond. Toxicol Appl Pharmacol. 2010;244:16–20. doi: 10.1016/j.taap.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bocca B. Alimonti A. Senofonte O. Pino A. Violante N. Petrucci F. Sancesario G. Forte G. Metal changes in CSF and peripheral compartments of parkinsonian patients. J Neurol Sci. 2006;248:23–30. doi: 10.1016/j.jns.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Bove J. Prou D. Perier C. Przedborski S. Toxin-induced models of Parkinson's disease. NeuroRx. 2005;2:484–494. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brigelius-Flohe R. Flohe L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid Redox Signal. 2011;15:2335–2381. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burton NC. Kensler TW. Guilarte TR. In vivo modulation of the Parkinsonian phenotype by Nrf2. Neurotoxicology. 2006;27:1094–1100. doi: 10.1016/j.neuro.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Canet-Aviles RM. Wilson MA. Miller DW. Ahmad R. McLendon C. Bandyopadhyay S. Baptista MJ. Ringe D. Petsko GA. Cookson MR. The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci U S A. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cannon JR. Greenamyre JT. Neurotoxic in vivo models of Parkinson's disease recent advances. Prog Brain Res. 2010;184:17–33. doi: 10.1016/S0079-6123(10)84002-6. [DOI] [PubMed] [Google Scholar]
- 25.Castello PR. Drechsel DA. Patel M. Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain. J Biol Chem. 2007;282:14186–14193. doi: 10.1074/jbc.M700827200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chau KY. Cooper JM. Schapira AH. Rasagiline protects against alpha-synuclein induced sensitivity to oxidative stress in dopaminergic cells. Neurochem Int. 2010;57:525–529. doi: 10.1016/j.neuint.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen PC. Vargas MR. Pani AK. Smeyne RJ. Johnson DA. Kan YW. Johnson JA. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson's disease: critical role for the astrocyte. Proc Natl Acad Sci U S A. 2009;106:2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chinta SJ. Andersen JK. Reversible inhibition of mitochondrial complex I activity following chronic dopaminergic glutathione depletion in vitro: implications for Parkinson's disease. Free Radic Biol Med. 2006;41:1442–1448. doi: 10.1016/j.freeradbiomed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Chinta SJ. Kumar MJ. Hsu M. Rajagopalan S. Kaur D. Rane A. Nicholls DG. Choi J. Andersen JK. Inducible alterations of glutathione levels in adult dopaminergic midbrain neurons result in nigrostriatal degeneration. J Neurosci. 2007;27:13997–14006. doi: 10.1523/JNEUROSCI.3885-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chinta SJ. Rajagopalan S. Butterfield DA. Andersen JK. In vitro and in vivo neuroprotection by gamma-glutamylcysteine ethyl ester against MPTP: relevance to the role of glutathione in Parkinson's disease. Neurosci Lett. 2006;402:137–141. doi: 10.1016/j.neulet.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 31.Choi DY. Liu M. Hunter RL. Cass WA. Pandya JD. Sullivan PG. Shin EJ. Kim HC. Gash DM. Bing G. Striatal neuroinflammation promotes Parkinsonism in rats. PLoS One. 2009;4:e5482. doi: 10.1371/journal.pone.0005482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi J. Levey AI. Weintraub ST. Rees HD. Gearing M. Chin LS. Li L. Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson's and Alzheimer's diseases. J Biol Chem. 2004;279:13256–13264. doi: 10.1074/jbc.M314124200. [DOI] [PubMed] [Google Scholar]
- 33.Choi J. Rees HD. Weintraub ST. Levey AI. Chin LS. Li L. Oxidative modifications and aggregation of Cu, Zn-superoxide dismutase associated with Alzheimer and Parkinson diseases. J Biol Chem. 2005;280:11648–11655. doi: 10.1074/jbc.M414327200. [DOI] [PubMed] [Google Scholar]
- 34.Choi WS. Kruse SE. Palmiter RD. Xia Z. Mitochondrial complex I inhibition is not required for dopaminergic neuron death induced by rotenone, MPP+, or paraquat. Proc Natl Acad Sci U S A. 2008;105:15136–15141. doi: 10.1073/pnas.0807581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung KK. Thomas B. Li X. Pletnikova O. Troncoso JC. Marsh L. Dawson VL. Dawson TM. S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science. 2004;304:1328–1331. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- 36.Chung RS. Hidalgo J. West AK. New insight into the molecular pathways of metallothionein-mediated neuroprotection and regeneration. J Neurochem. 2008;104:14–20. doi: 10.1111/j.1471-4159.2007.05026.x. [DOI] [PubMed] [Google Scholar]
- 37.Ciron C. Lengacher S. Dusonchet J. Aebischer P. Schneider BL. Sustained expression of PGC-1alpha in the rat nigrostriatal system selectively impairs dopaminergic function. Hum Mol Genet. 2012 doi: 10.1093/hmg/ddr618. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark J. Clore EL. Zheng K. Adame A. Masliah E. Simon DK. Oral N-acetyl-cysteine attenuates loss of dopaminergic terminals in alpha-synuclein overexpressing mice. PLoS One. 2010;5:e12333. doi: 10.1371/journal.pone.0012333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark J. Simon DK. Transcribe to survive: transcriptional control of antioxidant defense programs for neuroprotection in Parkinson's disease. Antioxid Redox Signal. 2009;11:509–528. doi: 10.1089/ars.2008.2241. [DOI] [PubMed] [Google Scholar]
- 40.Clements CM. McNally RS. Conti BJ. Mak TW. Ting JP. DJ-1, a cancer- and Parkinson's disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci U S A. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen G. Farooqui R. Kesler N. Parkinson disease: a new link between monoamine oxidase and mitochondrial electron flow. Proc Natl Acad Sci U S A. 1997;94:4890–4894. doi: 10.1073/pnas.94.10.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cox AG. Winterbourn CC. Hampton MB. Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem J. 2010;425:313–325. doi: 10.1042/BJ20091541. [DOI] [PubMed] [Google Scholar]
- 43.Cristovao AC. Choi DH. Baltazar G. Beal MF. Kim YS. The role of NADPH oxidase 1-derived reactive oxygen species in paraquat-mediated dopaminergic cell death. Antioxid Redox Signal. 2009;11:2105–2118. doi: 10.1089/ars.2009.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daily D. Vlamis-Gardikas A. Offen D. Mittelman L. Melamed E. Holmgren A. Barzilai A. Glutaredoxin protects cerebellar granule neurons from dopamine-induced apoptosis by activating NF-kappa B via Ref-1. J Biol Chem. 2001;276:1335–1344. doi: 10.1074/jbc.M008121200. [DOI] [PubMed] [Google Scholar]
- 45.Daily D. Vlamis-Gardikas A. Offen D. Mittelman L. Melamed E. Holmgren A. Barzilai A. Glutaredoxin protects cerebellar granule neurons from dopamine-induced apoptosis by dual activation of the ras-phosphoinositide 3-kinase and jun n-terminal kinase pathways. J Biol Chem. 2001;276:21618–21626. doi: 10.1074/jbc.M101400200. [DOI] [PubMed] [Google Scholar]
- 46.Danielson SR. Andersen JK. Oxidative and nitrative protein modifications in Parkinson's disease. Free Radic Biol Med. 2008;44:1787–1794. doi: 10.1016/j.freeradbiomed.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danielson SR. Held JM. Oo M. Riley R. Gibson BW. Andersen JK. Quantitative mapping of reversible mitochondrial Complex I cysteine oxidation in a Parkinson disease mouse model. J Biol Chem. 2011;286:7601–7608. doi: 10.1074/jbc.M110.190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Simoni S. Goemaere J. Knoops B. Silencing of peroxiredoxin 3 and peroxiredoxin 5 reveals the role of mitochondrial peroxiredoxins in the protection of human neuroblastoma SH-SY5Y cells toward MPP+ Neurosci Lett. 2008;433:219–224. doi: 10.1016/j.neulet.2007.12.068. [DOI] [PubMed] [Google Scholar]
- 49.Devi L. Raghavendran V. Prabhu BM. Avadhani NG. Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dexter D. Carter C. Agid F. Agid Y. Lees AJ. Jenner P. Marsden CD. Lipid peroxidation as cause of nigral cell death in Parkinson's disease. Lancet. 1986;2:639–640. doi: 10.1016/s0140-6736(86)92471-2. [DOI] [PubMed] [Google Scholar]
- 51.Dexter DT. Carter CJ. Wells FR. Javoy-Agid F. Agid Y. Lees A. Jenner P. Marsden CD. Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J Neurochem. 1989;52:381–389. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 52.Drechsel DA. Liang LP. Patel M. 1-methyl-4-phenylpyridinium-induced alterations of glutathione status in immortalized rat dopaminergic neurons. Toxicol Appl Pharmacol. 2007;220:341–348. doi: 10.1016/j.taap.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drechsel DA. Patel M. Differential contribution of the mitochondrial respiratory chain complexes to reactive oxygen species production by redox cycling agents implicated in parkinsonism. Toxicol Sci. 2009;112:427–434. doi: 10.1093/toxsci/kfp223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ebadi M. Brown-Borg H. El Refaey H. Singh BB. Garrett S. Shavali S. Sharma SK. Metallothionein-mediated neuroprotection in genetically engineered mouse models of Parkinson's disease. Brain Res Mol Brain Res. 2005;134:67–75. doi: 10.1016/j.molbrainres.2004.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fang J. Nakamura T. Cho DH. Gu Z. Lipton SA. S-nitrosylation of peroxiredoxin 2 promotes oxidative stress-induced neuronal cell death in Parkinson's disease. Proc Natl Acad Sci U S A. 2007;104:18742–18747. doi: 10.1073/pnas.0705904104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farr SA. Poon HF. Dogrukol-Ak D. Drake J. Banks WA. Eyerman E. Butterfield DA. Morley JE. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003;84:1173–1183. doi: 10.1046/j.1471-4159.2003.01580.x. [DOI] [PubMed] [Google Scholar]
- 57.Fomenko DE. Marino SM. Gladyshev VN. Functional diversity of cysteine residues in proteins and unique features of catalytic redox-active cysteines in thiol oxidoreductases. Mol Cells. 2008;26:228–235. [PMC free article] [PubMed] [Google Scholar]
- 58.Foster MW. Hess DT. Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franco R. Cidlowski JA. Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ. 2009;16:1303–1314. doi: 10.1038/cdd.2009.107. [DOI] [PubMed] [Google Scholar]
- 60.Franco R. Schoneveld OJ. Pappa A. Panayiotidis MI. The central role of glutathione in the pathophysiology of human diseases. Arch Physiol Biochem. 2007;113:234–258. doi: 10.1080/13813450701661198. [DOI] [PubMed] [Google Scholar]
- 61.Gan L. Johnson DA. Johnson JA. Keap1-Nrf2 activation in the presence and absence of DJ-1. Eur J Neurosci. 2010;31:967–977. doi: 10.1111/j.1460-9568.2010.07138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao HM. Hong JS. Gene-environment interactions: key to unraveling the mystery of Parkinson's disease. Prog Neurobiol. 2011;94:1–19. doi: 10.1016/j.pneurobio.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao HM. Liu B. Hong JS. Critical role for microglial NADPH oxidase in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2003;23:6181–6187. doi: 10.1523/JNEUROSCI.23-15-06181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gauthier MA. Eibl JK. Crispo JA. Ross GM. Covalent arylation of metallothionein by oxidized dopamine products: a possible mechanism for zinc-mediated enhancement of dopaminergic neuron survival. Neurotox Res. 2008;14:317–328. doi: 10.1007/BF03033856. [DOI] [PubMed] [Google Scholar]
- 65.Glaser CB. Yamin G. Uversky VN. Fink AL. Methionine oxidation, alpha-synuclein and Parkinson's disease. Biochim Biophys Acta. 2005;1703:157–169. doi: 10.1016/j.bbapap.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 66.Godoy JR. Funke M. Ackermann W. Haunhorst P. Oesteritz S. Capani F. Elsasser HP. Lillig CH. Redox atlas of the mouse. Immunohistochemical detection of glutaredoxin-, peroxiredoxin-, and thioredoxin-family proteins in various tissues of the laboratory mouse. Biochim Biophys Acta. 2011;1810:2–92. doi: 10.1016/j.bbagen.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 67.Goemaere J. Knoops B. Peroxiredoxin distribution in the mouse brain with emphasis on neuronal populations affected in neurodegenerative disorders. J Comp Neurol. 2011;520:258–280. doi: 10.1002/cne.22689. [DOI] [PubMed] [Google Scholar]
- 68.Gorell JM. Johnson CC. Rybicki BA. Peterson EL. Kortsha GX. Brown GG. Richardson RJ. Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson's disease. Neurotoxicology. 1999;20:239–247. [PubMed] [Google Scholar]
- 69.Greisen P. Jespersen JB. Kepp KP. Metallothionein Zn(2+)- and Cu(2+)-clusters from first-principles calculations. Dalton Trans. 2012;41:2247–2256. doi: 10.1039/c1dt11785h. [DOI] [PubMed] [Google Scholar]
- 70.Gunther V. Lindert U. Schaffner W. The taste of heavy metals: gene regulation by MTF-1. Biochim Biophys Acta 2012. http://dx.doi.org/10.1016/j.bbamcr.2012.01.005. http://dx.doi.org/10.1016/j.bbamcr.2012.01.005 [DOI] [PubMed]
- 71.Hall A. Karplus PA. Poole LB. Typical 2-Cys peroxiredoxins—structures, mechanisms and functions. FEBS J. 2009;276:2469–2477. doi: 10.1111/j.1742-4658.2009.06985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hastings TG. The role of dopamine oxidation in mitochondrial dysfunction: implications for Parkinson's disease. J Bioenerg Biomembr. 2009;41:469–472. doi: 10.1007/s10863-009-9257-z. [DOI] [PubMed] [Google Scholar]
- 73.Hastings TG. Lewis DA. Zigmond MJ. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc Natl Acad Sci U S A. 1996;93:1956–1961. doi: 10.1073/pnas.93.5.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hauser RA. Lyons KE. McClain T. Carter S. Perlmutter D. Randomized, double-blind, pilot evaluation of intravenous glutathione in Parkinson's disease. Mov Disord. 2009;24:979–983. doi: 10.1002/mds.22401. [DOI] [PubMed] [Google Scholar]
- 75.Henchcliffe C. Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. 2008;4:600–609. doi: 10.1038/ncpneuro0924. [DOI] [PubMed] [Google Scholar]
- 76.Hickey P. Stacy M. Available and emerging treatments for Parkinson's disease: a review. Drug Des Devel Ther. 2011;5:241–254. doi: 10.2147/DDDT.S11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Horowitz MP. Greenamyre JT. Gene-environment interactions in Parkinson's disease: the importance of animal modeling. Clin Pharmacol Ther. 2010;88:467–474. doi: 10.1038/clpt.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hozumi I. Hasegawa T. Honda A. Ozawa K. Hayashi Y. Hashimoto K. Yamada M. Koumura A. Sakurai T. Kimura A. Tanaka Y. Satoh M. Inuzuka T. Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. J Neurol Sci. 2011;303:95–99. doi: 10.1016/j.jns.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 79.Hu X. Weng Z. Chu CT. Zhang L. Cao G. Gao Y. Signore A. Zhu J. Hastings T. Greenamyre JT. Chen J. Peroxiredoxin-2 protects against 6-hydroxydopamine-induced dopaminergic neurodegeneration via attenuation of the apoptosis signal-regulating kinase (ASK1) signaling cascade. J Neurosci. 2011;31:247–261. doi: 10.1523/JNEUROSCI.4589-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hwang YP. Kim HG. Han EH. Jeong HG. Metallothionein-III protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a PI3K and ERK/Nrf2-dependent manner. Toxicol Appl Pharmacol. 2008;231:318–327. doi: 10.1016/j.taap.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 81.Ishisaki A. Hayashi H. Suzuki S. Ozawa K. Mizukoshi E. Miyakawa K. Suzuki M. Imamura T. Glutathione S-transferase Pi is a dopamine-inducible suppressor of dopamine-induced apoptosis in PC12 cells. J Neurochem. 2001;77:1362–1371. doi: 10.1046/j.1471-4159.2001.00351.x. [DOI] [PubMed] [Google Scholar]
- 82.Isobe C. Murata T. Sato C. Terayama Y. Increase of total homocysteine concentration in cerebrospinal fluid in patients with Alzheimer's disease and Parkinson's disease. Life Sci. 2005;77:1836–1843. doi: 10.1016/j.lfs.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 83.Jazwa A. Rojo AI. Innamorato NG. Hesse M. Fernandez-Ruiz J. Cuadrado A. Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid Redox Signal. 2011;14:2347–2360. doi: 10.1089/ars.2010.3731. [DOI] [PubMed] [Google Scholar]
- 84.Jenner P. Altered mitochondrial function, iron metabolism and glutathione levels in Parkinson's disease. Acta Neurol Scand Suppl. 1993;146:6–13. [PubMed] [Google Scholar]
- 85.Jenner P. Preclinical evidence for neuroprotection with monoamine oxidase-B inhibitors in Parkinson's disease. Neurology. 2004;63:S13–S22. doi: 10.1212/wnl.63.7_suppl_2.s13. [DOI] [PubMed] [Google Scholar]
- 86.Jha N. Jurma O. Lalli G. Liu Y. Pettus EH. Greenamyre JT. Liu RM. Forman HJ. Andersen JK. Glutathione depletion in PC12 results in selective inhibition of mitochondrial complex I activity. Implications for Parkinson's disease. J Biol Chem. 2000;275:26096–26101. doi: 10.1074/jbc.M000120200. [DOI] [PubMed] [Google Scholar]
- 87.Jiang LJ. Maret W. Vallee BL. The glutathione redox couple modulates zinc transfer from metallothionein to zinc-depleted sorbitol dehydrogenase. Proc Natl Acad Sci U S A. 1998;95:3483–3488. doi: 10.1073/pnas.95.7.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kalivendi SV. Kotamraju S. Cunningham S. Shang T. Hillard CJ. Kalyanaraman B. 1-Methyl-4-phenylpyridinium (MPP+)-induced apoptosis and mitochondrial oxidant generation: role of transferrin-receptor-dependent iron and hydrogen peroxide. Biochem J. 2003;371:151–164. doi: 10.1042/BJ20021525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kang MJ. Gil SJ. Koh HC. Paraquat induces alternation of the dopamine catabolic pathways and glutathione levels in the substantia nigra of mice. Toxicol Lett. 2009;188:148–152. doi: 10.1016/j.toxlet.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 90.Kannan R. Chakrabarti R. Tang D. Kim KJ. Kaplowitz N. GSH transport in human cerebrovascular endothelial cells and human astrocytes: evidence for luminal localization of Na+-dependent GSH transport in HCEC. Brain Res. 2000;852:374–382. doi: 10.1016/s0006-8993(99)02184-8. [DOI] [PubMed] [Google Scholar]
- 91.Kannan R. Mittur A. Bao Y. Tsuruo T. Kaplowitz N. GSH transport in immortalized mouse brain endothelial cells: evidence for apical localization of a sodium-dependent GSH transporter. J Neurochem. 1999;73:390–399. doi: 10.1046/j.1471-4159.1999.0730390.x. [DOI] [PubMed] [Google Scholar]
- 92.Karunakaran S. Saeed U. Ramakrishnan S. Koumar RC. Ravindranath V. Constitutive expression and functional characterization of mitochondrial glutaredoxin (Grx2) in mouse and human brain. Brain Res. 2007;1185:8–17. doi: 10.1016/j.brainres.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 93.Kelada SN. Checkoway H. Kardia SL. Carlson CS. Costa-Mallen P. Eaton DL. Firestone J. Powers KM. Swanson PD. Franklin GM. Longstreth WT., Jr. Weller TS. Afsharinejad Z. Costa LG. 5′ and 3′ region variability in the dopamine transporter gene (SLC6A3), pesticide exposure and Parkinson's disease risk: a hypothesis-generating study. Hum Mol Genet. 2006;15:3055–3062. doi: 10.1093/hmg/ddl247. [DOI] [PubMed] [Google Scholar]
- 94.Kenchappa RS. Diwakar L. Annepu J. Ravindranath V. Estrogen and neuroprotection: higher constitutive expression of glutaredoxin in female mice offers protection against MPTP-mediated neurodegeneration. FASEB J. 2004;18:1102–1104. doi: 10.1096/fj.03-1075fje. [DOI] [PubMed] [Google Scholar]
- 95.Kenchappa RS. Ravindranath V. Glutaredoxin is essential for maintenance of brain mitochondrial complex I: studies with MPTP. FASEB J. 2003;17:717–719. doi: 10.1096/fj.02-0771fje. [DOI] [PubMed] [Google Scholar]
- 96.Khan FH. Sen T. Chakrabarti S. Dopamine oxidation products inhibit Na+, K+-ATPase activity in crude synaptosomal-mitochondrial fraction from rat brain. Free Radic Res. 2003;37:597–601. doi: 10.1080/1071576031000115651. [DOI] [PubMed] [Google Scholar]
- 97.Kil IS. Park JW. Regulation of mitochondrial NADP+-dependent isocitrate dehydrogenase activity by glutathionylation. J Biol Chem. 2005;280:10846–10854. doi: 10.1074/jbc.M411306200. [DOI] [PubMed] [Google Scholar]
- 98.Klivenyi P. Andreassen OA. Ferrante RJ. Dedeoglu A. Mueller G. Lancelot E. Bogdanov M. Andersen JK. Jiang D. Beal MF. Mice deficient in cellular glutathione peroxidase show increased vulnerability to malonate, 3-nitropropionic acid, and 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. J Neurosci. 2000;20:1–7. doi: 10.1523/JNEUROSCI.20-01-00001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Krapfenbauer K. Engidawork E. Cairns N. Fountoulakis M. Lubec G. Aberrant expression of peroxiredoxin subtypes in neurodegenerative disorders. Brain Res. 2003;967:152–160. doi: 10.1016/s0006-8993(02)04243-9. [DOI] [PubMed] [Google Scholar]
- 100.Kumari MV. Hiramatsu M. Ebadi M. Free radical scavenging actions of metallothionein isoforms I and II. Free Radic Res. 1998;29:93–101. doi: 10.1080/10715769800300111. [DOI] [PubMed] [Google Scholar]
- 101.Lee BC. Gladyshev VN. The biological significance of methionine sulfoxide stereochemistry. Free Radic Biol Med. 2011;50:221–227. doi: 10.1016/j.freeradbiomed.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee DW. Kaur D. Chinta SJ. Rajagopalan S. Andersen JK. A disruption in iron-sulfur center biogenesis via inhibition of mitochondrial dithiol glutaredoxin 2 may contribute to mitochondrial and cellular iron dysregulation in mammalian glutathione-depleted dopaminergic cells: implications for Parkinson's disease. Antioxid Redox Signal. 2009;11:2083–2094. doi: 10.1089/ars.2009.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee JC. Gray HB. Winkler JR. Copper(II) binding to alpha-synuclein, the Parkinson's protein. J Am Chem Soc. 2008;130:6898–6899. doi: 10.1021/ja711415b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee YM. Park SH. Shin DI. Hwang JY. Park B. Park YJ. Lee TH. Chae HZ. Jin BK. Oh TH. Oh YJ. Oxidative modification of peroxiredoxin is associated with drug-induced apoptotic signaling in experimental models of Parkinson disease. J Biol Chem. 2008;283:9986–9998. doi: 10.1074/jbc.M800426200. [DOI] [PubMed] [Google Scholar]
- 105.Levy OA. Malagelada C. Greene LA. Cell death pathways in Parkinson's disease: proximal triggers, distal effectors, and final steps. Apoptosis. 2009;14:478–500. doi: 10.1007/s10495-008-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liang LP. Patel M. Iron-sulfur enzyme mediated mitochondrial superoxide toxicity in experimental Parkinson's disease. J Neurochem. 2004;90:1076–1084. doi: 10.1111/j.1471-4159.2004.02567.x. [DOI] [PubMed] [Google Scholar]
- 107.Lippoldt A. Padilla CA. Gerst H. Andbjer B. Richter E. Holmgren A. Fuxe K. Localization of thioredoxin in the rat brain and functional implications. J Neurosci. 1995;15:6747–6756. doi: 10.1523/JNEUROSCI.15-10-06747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu F. Hindupur J. Nguyen JL. Ruf KJ. Zhu J. Schieler JL. Bonham CC. Wood KV. Davisson VJ. Rochet JC. Methionine sulfoxide reductase A protects dopaminergic cells from Parkinson's disease-related insults. Free Radic Biol Med. 2008;45:242–255. doi: 10.1016/j.freeradbiomed.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu F. Nguyen JL. Hulleman JD. Li L. Rochet JC. Mechanisms of DJ-1 neuroprotection in a cellular model of Parkinson's disease. J Neurochem. 2008;105:2435–2453. doi: 10.1111/j.1471-4159.2008.05333.x. [DOI] [PubMed] [Google Scholar]
- 110.Liu HQ. Zhu XZ. Weng EQ. Intracellular dopamine oxidation mediates rotenone-induced apoptosis in PC12 cells. Acta Pharmacol Sin. 2005;26:17–26. doi: 10.1111/j.1745-7254.2005.00003.x. [DOI] [PubMed] [Google Scholar]
- 111.Lotharius J. Barg S. Wiekop P. Lundberg C. Raymon HK. Brundin P. Effect of mutant alpha-synuclein on dopamine homeostasis in a new human mesencephalic cell line. J Biol Chem. 2002;277:38884–38894. doi: 10.1074/jbc.M205518200. [DOI] [PubMed] [Google Scholar]
- 112.Lotharius J. O'Malley KL. The parkinsonism-inducing drug 1-methyl-4-phenylpyridinium triggers intracellular dopamine oxidation. A novel mechanism of toxicity. J Biol Chem. 2000;275:38581–38588. doi: 10.1074/jbc.M005385200. [DOI] [PubMed] [Google Scholar]
- 113.Ma L. Cao TT. Kandpal G. Warren L. Fred Hess J. Seabrook GR. Ray WJ. Genome-wide microarray analysis of the differential neuroprotective effects of antioxidants in neuroblastoma cells overexpressing the familial Parkinson's disease alpha-synuclein A53T mutation. Neurochem Res. 2010;35:130–142. doi: 10.1007/s11064-009-0038-1. [DOI] [PubMed] [Google Scholar]
- 114.Magyar K. Szende B. Jenei V. Tabi T. Palfi M. Szoko E. R-deprenyl: pharmacological spectrum of its activity. Neurochem Res. 2010;35:1922–1932. doi: 10.1007/s11064-010-0238-8. [DOI] [PubMed] [Google Scholar]
- 115.Martin HL. Teismann P. Glutathione—a review on its role and significance in Parkinson's disease. FASEB J. 2009;23:3263–3272. doi: 10.1096/fj.08-125443. [DOI] [PubMed] [Google Scholar]
- 116.Massie A. Schallier A. Kim SW. Fernando R. Kobayashi S. Beck H. De Bundel D. Vermoesen K. Bannai S. Smolders I. Conrad M. Plesnila N. Sato H. Michotte Y. Dopaminergic neurons of system x(c)-deficient mice are highly protected against 6-hydroxydopamine-induced toxicity. FASEB J. 2011;25:1359–1369. doi: 10.1096/fj.10-177212. [DOI] [PubMed] [Google Scholar]
- 117.Mejias R. Villadiego J. Pintado CO. Vime PJ. Gao L. Toledo-Aral JJ. Echevarria M. Lopez-Barneo J. Neuroprotection by transgenic expression of glucose-6-phosphate dehydrogenase in dopaminergic nigrostriatal neurons of mice. J Neurosci. 2006;26:4500–4508. doi: 10.1523/JNEUROSCI.0122-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Meloni G. Vasak M. Redox activity of alpha-synuclein-Cu is silenced by Zn-metallothionein-3. Free Radic Biol Med. 2011;50:1471–1479. doi: 10.1016/j.freeradbiomed.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 119.Menegon A. Board PG. Blackburn AC. Mellick GD. Le Couteur DG. Parkinson's disease, pesticides, and glutathione transferase polymorphisms. Lancet. 1998;352:1344–1346. doi: 10.1016/s0140-6736(98)03453-9. [DOI] [PubMed] [Google Scholar]
- 120.Meng F. Yao D. Shi Y. Kabakoff J. Wu W. Reicher J. Ma Y. Moosmann B. Masliah E. Lipton SA. Gu Z. Oxidation of the cysteine-rich regions of parkin perturbs its E3 ligase activity and contributes to protein aggregation. Mol Neurodegener. 2011;6:34. doi: 10.1186/1750-1326-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Michael GJ. Esmailzadeh S. Moran LB. Christian L. Pearce RK. Graeber MB. Up-regulation of metallothionein gene expression in Parkinsonian astrocytes. Neurogenetics. 2011;12:295–305. doi: 10.1007/s10048-011-0294-5. [DOI] [PubMed] [Google Scholar]
- 122.Mieyal JJ. Gallogly MM. Qanungo S. Sabens EA. Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miyake Y. Tanaka K. Fukushima W. Sasaki S. Kiyohara C. Tsuboi Y. Yamada T. Oeda T. Miki T. Kawamura N. Sakae N. Fukuyama H. Hirota Y. Nagai M. Dietary intake of metals and risk of Parkinson's disease: a case-control study in Japan. J Neurol Sci. 2011;306:98–102. doi: 10.1016/j.jns.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 124.Miyazaki I. Asanuma M. Hozumi H. Miyoshi K. Sogawa N. Protective effects of metallothionein against dopamine quinone-induced dopaminergic neurotoxicity. FEBS Lett. 2007;581:5003–5008. doi: 10.1016/j.febslet.2007.09.046. [DOI] [PubMed] [Google Scholar]
- 125.Miyazaki I. Asanuma M. Kikkawa Y. Takeshima M. Murakami S. Miyoshi K. Sogawa N. Kita T. Astrocyte-derived metallothionein protects dopaminergic neurons from dopamine quinone toxicity. Glia. 2011;59:435–451. doi: 10.1002/glia.21112. [DOI] [PubMed] [Google Scholar]
- 126.Miyazaki I. Sogawa CA. Asanuma M. Higashi Y. Tanaka KI. Nakanishi T. Ogawa N. Expression of metallothionein-III mRNA and its regulation by levodopa in the basal ganglia of hemi-parkinsonian rats. Neurosci Lett. 2000;293:65–68. doi: 10.1016/s0304-3940(00)01488-9. [DOI] [PubMed] [Google Scholar]
- 127.Mudo G. Makela J. Liberto VD. Tselykh TV. Olivieri M. Piepponen P. Eriksson O. Malkia A. Bonomo A. Kairisalo M. Aguirre JA. Korhonen L. Belluardo N. Lindholm D. Transgenic expression and activation of PGC-1alpha protect dopaminergic neurons in the MPTP mouse model of Parkinson's disease. Cell Mol Life Sci. 2011;69:1153–1165. doi: 10.1007/s00018-011-0850-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Munoz AM. Rey P. Soto-Otero R. Guerra MJ. Labandeira-Garcia JL. Systemic administration of N-acetylcysteine protects dopaminergic neurons against 6-hydroxydopamine-induced degeneration. J Neurosci Res. 2004;76:551–562. doi: 10.1002/jnr.20107. [DOI] [PubMed] [Google Scholar]
- 129.Nakamura K. Bindokas VP. Marks JD. Wright DA. Frim DM. Miller RJ. Kang UJ. The selective toxicity of 1-methyl-4-phenylpyridinium to dopaminergic neurons: the role of mitochondrial complex I and reactive oxygen species revisited. Mol Pharmacol. 2000;58:271–278. doi: 10.1124/mol.58.2.271. [DOI] [PubMed] [Google Scholar]
- 130.Naoi M. Maruyama W. Yi H. Yamaoka Y. Shamoto-Nagai M. Akao Y. Gerlach M. Tanaka M. Riederer P. Neuromelanin selectively induces apoptosis in dopaminergic SH-SY5Y cells by deglutathionylation in mitochondria: involvement of the protein and melanin component. J Neurochem. 2008;105:2489–2500. doi: 10.1111/j.1471-4159.2008.05329.x. [DOI] [PubMed] [Google Scholar]
- 131.Napolitano A. Manini P. d'Ischia M. Oxidative chemistry of catecholamines and neuronal degeneration: an update. Cur Med Chem. 2011;18:1832–1845. doi: 10.2174/092986711795496863. [DOI] [PubMed] [Google Scholar]
- 132.Obata T. Dopamine efflux by MPTP and hydroxyl radical generation. J Neural Transm. 2002;109:1159–1180. doi: 10.1007/s00702-001-0683-2. [DOI] [PubMed] [Google Scholar]
- 133.Oien DB. Shinogle HE. Moore DS. Moskovitz J. Clearance and phosphorylation of alpha-synuclein are inhibited in methionine sulfoxide reductase a null yeast cells. J Mol Neurosci. 2009;39:323–332. doi: 10.1007/s12031-009-9274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pai HV. Starke DW. Lesnefsky EJ. Hoppel CL. Mieyal JJ. What is the functional significance of the unique location of glutaredoxin 1 (GRx1) in the intermembrane space of mitochondria? Antioxid Redox Signal. 2007;9:2027–2033. doi: 10.1089/ars.2007.1642. [DOI] [PubMed] [Google Scholar]
- 135.Pall HS. Williams AC. Blake DR. Lunec J. Gutteridge JM. Hall M. Taylor A. Raised cerebrospinal-fluid copper concentration in Parkinson's disease. Lancet. 1987;2:238–241. doi: 10.1016/s0140-6736(87)90827-0. [DOI] [PubMed] [Google Scholar]
- 136.Pan J. Xiao Q. Sheng CY. Hong Z. Yang HQ. Wang G. Ding JQ. Chen SD. Blockade of the translocation and activation of c-Jun N-terminal kinase 3 (JNK3) attenuates dopaminergic neuronal damage in mouse model of Parkinson's disease. Neurochem Int. 2009;54:418–425. doi: 10.1016/j.neuint.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 137.Park SW. Kim SH. Park KH. Kim SD. Kim JY. Baek SY. Chung BS. Kang CD. Preventive effect of antioxidants in MPTP-induced mouse model of Parkinson's disease. Neurosci Lett. 2004;363:243–246. doi: 10.1016/j.neulet.2004.03.072. [DOI] [PubMed] [Google Scholar]
- 138.Paulsen CE. Carroll KS. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem Biol. 2010;5:47–62. doi: 10.1021/cb900258z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Perry TL. Yong VW. Idiopathic Parkinson's disease, progressive supranuclear palsy and glutathione metabolism in the substantia nigra of patients. Neurosci Lett. 1986;67:269–274. doi: 10.1016/0304-3940(86)90320-4. [DOI] [PubMed] [Google Scholar]
- 140.Poole LB. Nelson KJ. Discovering mechanisms of signaling-mediated cysteine oxidation. Curr Opin Chem Biol. 2008;12:18–24. doi: 10.1016/j.cbpa.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ramachandiran S. Hansen JM. Jones DP. Richardson JR. Miller GW. Divergent mechanisms of paraquat, MPP+, and rotenone toxicity: oxidation of thioredoxin and caspase-3 activation. Toxicol Sci. 2007;95:163–171. doi: 10.1093/toxsci/kfl125. [DOI] [PubMed] [Google Scholar]
- 142.Rasia RM. Bertoncini CW. Marsh D. Hoyer W. Cherny D. Zweckstetter M. Griesinger C. Jovin TM. Fernandez CO. Structural characterization of copper(II) binding to alpha-synuclein: insights into the bioinorganic chemistry of Parkinson's disease. Proc Natl Acad Sci U S A. 2005;102:4294–4299. doi: 10.1073/pnas.0407881102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Richardson JR. Quan Y. Sherer TB. Greenamyre JT. Miller GW. Paraquat neurotoxicity is distinct from that of MPTP and rotenone. Toxicol Sci. 2005;88:193–201. doi: 10.1093/toxsci/kfi304. [DOI] [PubMed] [Google Scholar]
- 144.Richfield EK. Thiruchelvam MJ. Cory-Slechta DA. Wuertzer C. Gainetdinov RR. Caron MG. Di Monte DA. Federoff HJ. Behavioral and neurochemical effects of wild-type and mutated human alpha-synuclein in transgenic mice. Exp Neurol. 2002;175:35–48. doi: 10.1006/exnr.2002.7882. [DOI] [PubMed] [Google Scholar]
- 145.Ridet JL. Bensadoun JC. Deglon N. Aebischer P. Zurn AD. Lentivirus-mediated expression of glutathione peroxidase: neuroprotection in murine models of Parkinson's disease. Neurobiol Dis. 2006;21:29–34. doi: 10.1016/j.nbd.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 146.Ritz BR. Manthripragada AD. Costello S. Lincoln SJ. Farrer MJ. Cockburn M. Bronstein J. Dopamine transporter genetic variants and pesticides in Parkinson's disease. Environ Health Perspect. 2009;117:964–969. doi: 10.1289/ehp.0800277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Roede JR. Hansen JM. Go YM. Jones DP. Maneb and paraquat-mediated neurotoxicity: involvement of peroxiredoxin/thioredoxin system. Toxicol Sci. 2011;121:368–375. doi: 10.1093/toxsci/kfr058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rojas P. Rojas-Castaneda J. Vigueras RM. Habeebu SS. Rojas C. Rios C. Ebadi M. MPTP decreases MT-I mRNA in mouse striatum. Neurochem Res. 2000;25:503–509. doi: 10.1023/a:1007564126478. [DOI] [PubMed] [Google Scholar]
- 149.Rojo AI. Innamorato NG. Martin-Moreno AM. De Ceballos ML. Yamamoto M. Cuadrado A. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson's disease. Glia. 2010;58:588–598. doi: 10.1002/glia.20947. [DOI] [PubMed] [Google Scholar]
- 150.Rybnikova E. Damdimopoulos AE. Gustafsson JA. Spyrou G. Pelto-Huikko M. Expression of novel antioxidant thioredoxin-2 in the rat brain. Eur J Neurosci. 2000;12:1669–1678. doi: 10.1046/j.1460-9568.2000.00059.x. [DOI] [PubMed] [Google Scholar]
- 151.Sabens EA. Distler AM. Mieyal JJ. Levodopa deactivates enzymes that regulate thiol-disulfide homeostasis and promotes neuronal cell death: implications for therapy of Parkinson's disease. Biochemistry. 2010;49:2715–2724. doi: 10.1021/bi9018658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sabens Liedhegner EA. Steller KM. Mieyal JJ. Levodopa activates apoptosis signaling kinase 1 (ASK1) and promotes apoptosis in a neuronal model: implications for the treatment of Parkinson's disease. Chem Res Toxicol. 2011;24:1644–1652. doi: 10.1021/tx200082h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Saeed U. Durgadoss L. Valli RK. Joshi DC. Joshi PG. Ravindranath V. Knockdown of cytosolic glutaredoxin 1 leads to loss of mitochondrial membrane potential: implication in neurodegenerative diseases. PLoS One. 2008;3:e2459. doi: 10.1371/journal.pone.0002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Saeed U. Ray A. Valli RK. Kumar AM. Ravindranath V. DJ-1 loss by glutaredoxin but not glutathione depletion triggers Daxx translocation and cell death. Antioxid Redox Signal. 2010;13:127–144. doi: 10.1089/ars.2009.2832. [DOI] [PubMed] [Google Scholar]
- 155.Saini N. Georgiev O. Schaffner W. The parkin mutant phenotype in the fly is largely rescued by metal-responsive transcription factor (MTF-1) Mol Cell Biol. 2011;31:2151–2161. doi: 10.1128/MCB.05207-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Saito Y. Hamakubo T. Yoshida Y. Ogawa Y. Hara Y. Fujimura H. Imai Y. Iwanari H. Mochizuki Y. Shichiri M. Nishio K. Kinumi T. Noguchi N. Kodama T. Niki E. Preparation and application of monoclonal antibodies against oxidized DJ-1. Significant elevation of oxidized DJ-1 in erythrocytes of early-stage Parkinson disease patients. Neurosci Lett. 2009;465:1–5. doi: 10.1016/j.neulet.2009.08.074. [DOI] [PubMed] [Google Scholar]
- 157.Schapira AH. Mitochondria in the aetiology and pathogenesis of Parkinson's disease. Lancet Neurol. 2008;7:97–109. doi: 10.1016/S1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- 158.Sharma SK. El Refaey H. Ebadi M. Complex-1 activity and 18F-DOPA uptake in genetically engineered mouse model of Parkinson's disease and the neuroprotective role of coenzyme Q10. Brain Res Bull. 2006;70:22–32. doi: 10.1016/j.brainresbull.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 159.Sherer TB. Richardson JR. Testa CM. Seo BB. Panov AV. Yagi T. Matsuno-Yagi A. Miller GW. Greenamyre JT. Mechanism of toxicity of pesticides acting at complex I: relevance to environmental etiologies of Parkinson's disease. J Neurochem. 2007;100:1469–1479. doi: 10.1111/j.1471-4159.2006.04333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shi M. Bradner J. Bammler TK. Eaton DL. Zhang J. Ye Z. Wilson AM. Montine TJ. Pan C. Identification of glutathione S-transferase pi as a protein involved in Parkinson disease progression. Am J Pathol. 2009;175:54–65. doi: 10.2353/ajpath.2009.081019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shiraga H. Pfeiffer RF. Ebadi M. The effects of 6-hydroxydopamine and oxidative stress on the level of brain metallothionein. Neurochem Int. 1993;23:561–566. doi: 10.1016/0197-0186(93)90104-d. [DOI] [PubMed] [Google Scholar]
- 162.Sian-Hulsmann J. Mandel S. Youdim MB. Riederer P. The relevance of iron in the pathogenesis of Parkinson's disease. J Neurochem. 2011;118:939–957. doi: 10.1111/j.1471-4159.2010.07132.x. [DOI] [PubMed] [Google Scholar]
- 163.Sian J. Dexter DT. Lees AJ. Daniel S. Agid Y. Javoy-Agid F. Jenner P. Marsden CD. Alterations in glutathione levels in Parkinson's disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- 164.Siebert A. Desai V. Chandrasekaran K. Fiskum G. Jafri MS. Nrf2 activators provide neuroprotection against 6-hydroxydopamine toxicity in rat organotypic nigrostriatal cocultures. J Neurosci Res. 2009;87:1659–1669. doi: 10.1002/jnr.21975. [DOI] [PubMed] [Google Scholar]
- 165.Smeyne M. Boyd J. Raviie Shepherd K. Jiao Y. Pond BB. Hatler M. Wolf R. Henderson C. Smeyne RJ. GSTpi expression mediates dopaminergic neuron sensitivity in experimental parkinsonism. Proc Natl Acad Sci U S A. 2007;104:1977–1982. doi: 10.1073/pnas.0610978104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Snow BJ. Rolfe FL. Lockhart MM. Frampton CM. O'Sullivan JD. Fung V. Smith RA. Murphy MP. Taylor KM. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson's disease. Mov Disord. 2010;25:1670–1674. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- 167.Tanner CM. Kamel F. Ross GW. Hoppin JA. Goldman SM. Korell M. Marras C. Bhudhikanok GS. Kasten M. Chade AR. Comyns K. Richards MB. Meng C. Priestley B. Fernandez HH. Cambi F. Umbach DM. Blair A. Sandler DP. Langston JW. Rotenone, paraquat and Parkinson's disease. Environ Health Perspect. 2011;119:866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tew KD. Manevich Y. Grek C. Xiong Y. Uys J. Townsend DM. The role of glutathione S-transferase P in signaling pathways and S-glutathionylation in cancer. Free Radic Biol Med. 2011;51:299–313. doi: 10.1016/j.freeradbiomed.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Thiruchelvam M. Prokopenko O. Cory-Slechta DA. Buckley B. Mirochnitchenko O. Overexpression of superoxide dismutase or glutathione peroxidase protects against the paraquat + maneb-induced Parkinson disease phenotype. J Biol Chem. 2005;280:22530–22539. doi: 10.1074/jbc.M500417200. [DOI] [PubMed] [Google Scholar]
- 170.Thiruchelvam MJ. Powers JM. Cory-Slechta DA. Richfield EK. Risk factors for dopaminergic neuron loss in human alpha-synuclein transgenic mice. Eur J Neurosci. 2004;19:845–854. doi: 10.1111/j.0953-816x.2004.03139.x. [DOI] [PubMed] [Google Scholar]
- 171.Thornalley PJ. Vasak M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim Biophys Acta. 1985;827:36–44. doi: 10.1016/0167-4838(85)90098-6. [DOI] [PubMed] [Google Scholar]
- 172.Tsang AH. Lee YI. Ko HS. Savitt JM. Pletnikova O. Troncoso JC. Dawson VL. Dawson TM. Chung KK. S-nitrosylation of XIAP compromises neuronal survival in Parkinson's disease. Proc Natl Acad Sci U S A. 2009;106:4900–4905. doi: 10.1073/pnas.0810595106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Uehara T. Nakamura T. Yao D. Shi ZQ. Gu Z. Ma Y. Masliah E. Nomura Y. Lipton SA. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 174.Umeda-Kameyama Y. Tsuda M. Ohkura C. Matsuo T. Namba Y. Ohuchi Y. Aigaki T. Thioredoxin suppresses Parkin-associated endothelin receptor-like receptor-induced neurotoxicity and extends longevity in Drosophila. J Biol Chem. 2007;282:11180–11187. doi: 10.1074/jbc.M700937200. [DOI] [PubMed] [Google Scholar]
- 175.Uversky VN. Li J. Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson's disease and heavy metal exposure. J Biol Chem. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- 176.Van Laar VS. Mishizen AJ. Cascio M. Hastings TG. Proteomic identification of dopamine-conjugated proteins from isolated rat brain mitochondria and SH-SY5Y cells. Neurobiol Dis. 2009;34:487–500. doi: 10.1016/j.nbd.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vance JM. Ali S. Bradley WG. Singer C. Di Monte DA. Gene-environment interactions in Parkinson's disease and other forms of parkinsonism. Neurotoxicology. 2010;31:598–602. doi: 10.1016/j.neuro.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 178.Vitvitsky V. Thomas M. Ghorpade A. Gendelman HE. Banerjee R. A functional transsulfuration pathway in the brain links to glutathione homeostasis. J Biol Chem. 2006;281:35785–35793. doi: 10.1074/jbc.M602799200. [DOI] [PubMed] [Google Scholar]
- 179.von Otter M. Landgren S. Nilsson S. Celojevic D. Bergstrom P. Hakansson A. Nissbrandt H. Drozdzik M. Bialecka M. Kurzawski M. Blennow K. Nilsson M. Hammarsten O. Zetterberg H. Association of Nrf2-encoding NFE2L2 haplotypes with Parkinson's disease. BMC Med Genet. 2010;11:36. doi: 10.1186/1471-2350-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang C. Liu L. Zhang L. Peng Y. Zhou F. Redox reactions of the alpha-synuclein-Cu(2+) complex and their effects on neuronal cell viability. Biochemistry. 2010;49:8134–8142. doi: 10.1021/bi1010909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wassef R. Haenold R. Hansel A. Brot N. Heinemann SH. Hoshi T. Methionine sulfoxide reductase A and a dietary supplement S-methyl-L-cysteine prevent Parkinson's-like symptoms. J Neurosci. 2007;27:12808–12816. doi: 10.1523/JNEUROSCI.0322-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.West AK. Hidalgo J. Eddins D. Levin ED. Aschner M. Metallothionein in the central nervous system: roles in protection, regeneration and cognition. Neurotoxicology. 2008;29:489–503. doi: 10.1016/j.neuro.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Whitworth AJ. Theodore DA. Greene JC. Benes H. Wes PD. Pallanck LJ. Increased glutathione S-transferase activity rescues dopaminergic neuron loss in a Drosophila model of Parkinson's disease. Proc Natl Acad Sci U S A. 2005;102:8024–8029. doi: 10.1073/pnas.0501078102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wilk JB. Tobin JE. Suchowersky O. Shill HA. Klein C. Wooten GF. Lew MF. Mark MH. Guttman M. Watts RL. Singer C. Growdon JH. Latourelle JC. Saint-Hilaire MH. DeStefano AL. Prakash R. Williamson S. Berg CJ. Sun M. Goldwurm S. Pezzoli G. Racette BA. Perlmutter JS. Parsian A. Baker KB. Giroux ML. Litvan I. Pramstaller PP. Nicholson G. Burn DJ. Chinnery PF. Vieregge P. Slevin JT. Cambi F. MacDonald ME. Gusella JF. Myers RH. Golbe LI. Herbicide exposure modifies GSTP1 haplotype association to Parkinson onset age: the GenePD Study. Neurology. 2006;67:2206–2210. doi: 10.1212/01.wnl.0000249149.22407.d1. [DOI] [PubMed] [Google Scholar]
- 185.Wilson MA. The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid Redox Signal. 2011;15:111–122. doi: 10.1089/ars.2010.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Winterbourn CC. Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 187.Wu DC. Teismann P. Tieu K. Vila M. Jackson-Lewis V. Ischiropoulos H. Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Proc Natl Acad Sci U S A. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wu XF. Block ML. Zhang W. Qin L. Wilson B. Zhang WQ. Veronesi B. Hong JS. The role of microglia in paraquat-induced dopaminergic neurotoxicity. Antioxid Redox Signal. 2005;7:654–661. doi: 10.1089/ars.2005.7.654. [DOI] [PubMed] [Google Scholar]
- 189.Xie T. Tong L. McCann UD. Yuan J. Becker KG. Mechan AO. Cheadle C. Donovan DM. Ricaurte GA. Identification and characterization of metallothionein-1 and -2 gene expression in the context of (+/-)3,4-methylenedioxymethamphetamine-induced toxicity to brain dopaminergic neurons. J Neurosci. 2004;24:7043–7050. doi: 10.1523/JNEUROSCI.1626-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yang W. Tiffany-Castiglioni E. Koh HC. Son IH. Paraquat activates the IRE1/ASK1/JNK cascade associated with apoptosis in human neuroblastoma SH-SY5Y cells. Toxicol Lett. 2009;191:203–210. doi: 10.1016/j.toxlet.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 191.Yang W. Tiffany-Castiglioni E. Lee MY. Son IH. Paraquat induces cyclooxygenase-2 (COX-2) implicated toxicity in human neuroblastoma SH-SY5Y cells. Toxicol Lett. 2010;199:239–246. doi: 10.1016/j.toxlet.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 192.Yao D. Gu Z. Nakamura T. Shi ZQ. Ma Y. Gaston B. Palmer LA. Rockenstein EM. Zhang Z. Masliah E. Uehara T. Lipton SA. Nitrosative stress linked to sporadic Parkinson's disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci U S A. 2004;101:10810–10814. doi: 10.1073/pnas.0404161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yao Z. Wood NW. Cell death pathways in Parkinson's disease: role of mitochondria. Antioxid Redox Signal. 2009;11:2135–2149. doi: 10.1089/ars.2009.2624. [DOI] [PubMed] [Google Scholar]
- 194.Zecca L. Youdim MB. Riederer P. Connor JR. Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- 195.Zeevalk GD. Manzino L. Sonsalla PK. Bernard LP. Characterization of intracellular elevation of glutathione (GSH) with glutathione monoethyl ester and GSH in brain and neuronal cultures: relevance to Parkinson's disease. Exp Neurol. 2007;203:512–520. doi: 10.1016/j.expneurol.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zeevalk GD. Razmpour R. Bernard LP. Glutathione and Parkinson's disease: is this the elephant in the room? Biomed Pharmacother. 2008;62:236–249. doi: 10.1016/j.biopha.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 197.Zheng B. Liao Z. Locascio JJ. Lesniak KA. Roderick SS. Watt ML. Eklund AC. Zhang-James Y. Kim PD. Hauser MA. Grunblatt E. Moran LB. Mandel SA. Riederer P. Miller RM. Federoff HJ. Wullner U. Papapetropoulos S. Youdim MB. Cantuti-Castelvetri I. Young AB. Vance JM. Davis RL. Hedreen JC. Adler CH. Beach TG. Graeber MB. Middleton FA. Rochet JC. Scherzer CR. PGC-1alpha, a potential therapeutic target for early intervention in Parkinson's disease. Sci Transl Med. 2010;2:52ra73. doi: 10.1126/scitranslmed.3001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhou W. Freed CR. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. J Biol Chem. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]
- 199.Zhou W. Long C. Reaney SH. Di Monte DA. Fink AL. Uversky VN. Methionine oxidation stabilizes non-toxic oligomers of alpha-synuclein through strengthening the auto-inhibitory intra-molecular long-range interactions. Biochim Biophys Acta. 2010;1802:322–330. doi: 10.1016/j.bbadis.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhou W. Zhu M. Wilson MA. Petsko GA. Fink AL. The oxidation state of DJ-1 regulates its chaperone activity toward alpha-synuclein. J Mol Biol. 2006;356:1036–1048. doi: 10.1016/j.jmb.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 201.Zlokovic BV. Mackic JB. McComb JG. Weiss MH. Kaplowitz N. Kannan R. Evidence for transcapillary transport of reduced glutathione in vascular perfused guinea-pig brain. Biochem Biophys Res Commun. 1994;201:402–408. doi: 10.1006/bbrc.1994.1715. [DOI] [PubMed] [Google Scholar]