Abstract
Significance: Despite the recent decline in the prevalence of cardiovascular diseases, atherosclerosis remains the leading cause of death in industrialized countries. Monocyte recruitment into the vessel wall is a rate-limiting step in atherogenesis. Death of macrophage-derived foam cells promotes lesion progression and the majority of acute complications of atherosclerotic disease (e.g., myocardial infarction) occur in lesions that are intensely infiltrated with monocyte-derived macrophages, underlining the critical roles monocytes and macrophages play in this complex chronic inflammatory disease. Recent Advances: A rapidly growing body of literature supports a critical role for reactive oxygen species (ROS) in the regulation of monocyte and macrophage (dys)function associated with atherogenesis and macrophage death in atherosclerotic plaque. Critical Issues: In this review we highlight the important roles of NADHP oxidase 4 recently identified in monocytes and macrophages and the role of ROS and (thiol) redox signaling in different aspects of monocytes and macrophage biology associated with atherosclerosis. Future Directions: Studies aimed at identifying the intracellular targets of ROS involved in redox signaling in macrophages and at elucidating the redox signaling mechanisms that control differentiation, activation, polarization, and death of monocytes and macrophages may ultimately lead to the development of novel preventive and therapeutic strategies for atherosclerosis. Antioxid. Redox Signal. 17, 1785–1795.
Introduction
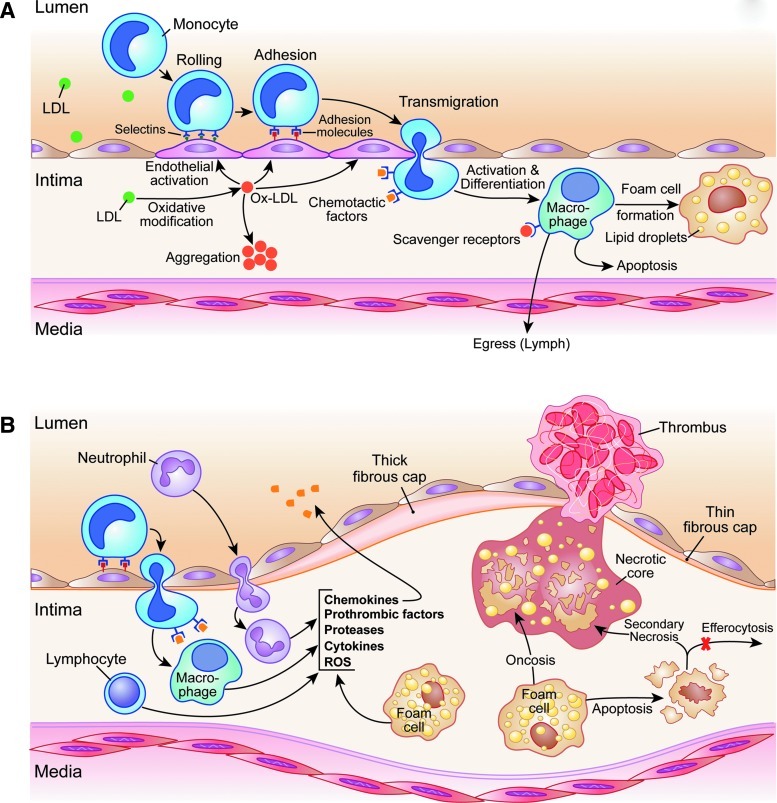
Focal infiltration and accumulation of low-density lipoproteins (LDLs) in the subendothelial space is one of the earliest events in the development of atherosclerosis (Fig. 1) (35, 72). The prolonged retention of LDLs in an increasingly pro-inflammatory environment results in physical and oxidative modifications of LDLs. The oxidation of LDLs generates cytotoxic products (70), which activate endothelial cells, promote the expression of adhesion molecules, and interrupt the normal barrier function of the endothelial layer (72). Activation and increased leakiness of endothelial vessel lining allow further continued infiltration and retention of macromolecules, including lipoproteins, in the subendothelial space, which sustains the injury to the vessel wall. Additionally, specific interactions of endothelial cell adhesion molecules, such as vascular cell adhesion molecule-1 and intercellular adhesion molecule-1, with their counterparts on inflammatory cells (e.g., very late antigen-4 and lymphocyte function-associated antigen 1) result in the rolling, adhesion, and subsequent transmigration of circulating immune cells, primarily monocytes, into the vessel wall (44, 72). Infiltrated monocytes subsequently differentiate into macrophages and overexpress a number of cell surface molecules, including scavenger receptors, such as scavenger receptor A, scavenger receptor BI, CD36, and CD68 (33).
FIG. 1.
An overview of the roles of monocytes and macrophages in atherogenesis. (A) The early atherosclerotic lesion. Oxidative modification of the low-density lipoproteins (LDLs) in the subendothelial space results in production of cytotoxic oxidatively modified LDL (OxLDL), which promotes endothelial dysfunction and the expression of adhesion molecules. Specific interactions of selectins and other adhesion molecules expressed by endothelial cells (e.g., vascular cell adhesion molecule-1 and intercellular adhesion molecule-1) with their counterparts on inflammatory cells (e.g., very late antigen-4 and lymphocyte function-associated antigen 1) result in the rolling, adhesion, and transmigration of circulating inflammatory cells, primarily monocytes, into the intima. Infiltrated monocytes differentiate into macrophages and express a number of scavenger receptors, for example, scavenger receptor A, scavenger receptor BI, CD36, and CD68. Scavenger receptor-mediated internalization of OxLDL and other LDL modifications result in the formation of lipid-laden foam cells. Monocyte cellularity in these early lesions is regulated by monocyte egress and apoptosis. (B) The advanced atherosclerotic plaques. The persistent production of inflammatory mediators, including chemokines (e.g., monocyte chemoattractant protein-1 [MCP-1] and RANTES), cytokines (e.g., interleukin [IL]-1, IL-6, tumor necrosis factor α, and interferon-γ), proteases (e.g., matrix metalloproteinases and cathepsins), and reactive oxygen species (ROS) formation by infiltrated immune cells, generates an inflammatory milieu, which promotes the recruitment, accumulation, and activation of additional inflammatory cells and smooth muscle cells, resulting in plaque expansion. In complex lesions, macrophage foam cells undergo cell death through apoptotic or nonapoptotic pathways (e.g., oncosis). Foam cell lysis and impaired clearance of apoptotic cells (efferocytosis) and subsequent secondary necrosis promote the formation of necrotic cores and further fuel inflammatory responses within advanced atherosclerotic plaques. In vulnerable plaques, these inflammatory mechanisms lead to the thinning of the protective fibrous cap and expansion of the necrotic core, predisposing these lesions to mechanical destabilization and plaque rupture. Exposure of the thrombogenic contents of the plaques to the coagulation cascade results in thrombus formation.
Oxidatively modified LDL (OxLDL), unlike native LDL, has a high affinity for scavenger receptors and is avidly internalized by macrophages through receptor-mediated endocytosis and phagocytosis (53, 55), resulting in the formation of lipid-laden foam cells, a hallmark of early atherosclerotic lesions, that is, fatty streak. OxLDL also activates vascular smooth muscle cells and macrophages to secrete a wide array of pro-inflammatory molecules, which further activate the endothelium, enhance the recruitment and differentiation of monocyte-derived macrophages, and promote the proliferation of vascular smooth muscle cells (72). Failure to counteract these inflammatory events results in the progression and expansion of the atherosclerotic plaque, and the development of luminal stenosis (70).
In addition to plaques' size, their composition is a critical determinant of clinical outcome. In fact, most culprit lesions in myocardial infarction produce only insignificant degrees of luminal stenosis (58). Mild stenosis produced by these lesions challenges their diagnosis by clinical tests (e.g., myocardial stress test) or imaging modalities (e.g., computed tomography or conventional coronary angiography and myocardial perfusion scan), which mostly rely on luminal stenosis or decreased perfusion reserve (72). Rupture or ulceration of these lesions, however, exposes the highly thrombogenic subendothelial contents of the plaques to the coagulation cascade, promoting thrombus formation, luminal occlusion, and acute ischemic syndromes, such as myocardial infarction, unstable angina, and stroke. These “vulnerable plaques” typically contain large necrotic cores, consisting primarily of dead macrophages and foam cells, and are covered by thin fibrous caps that are poor in supportive structures, such as collagen, and vascular smooth muscle cells (35, 72). Mechanical destabilization of these lesions, in particular in the region of the highest shear stress, that is, “shoulder,” is a critical factor in the vulnerability of plaques to rupture (58). Plaque destabilization may occur as a result of imbalanced degradation of the extracellular matrix or diminished support from the cellular component, in particular vascular smooth muscle cells.
Macrophages are a major source of extracellular proteases, including matrix metalloproteinases and cathepsins, as well as pro- and anti-inflammatory cytokines, all of which regulate extracellular matrix remodeling, inflammatory cell recruitment and activation, and vascular smooth muscle cell proliferation and apoptosis. Macrophages are also critical for the clearance of apoptotic cells before the dying cells lose membrane integrity through secondary or postapoptotic necrosis. The process of removal of apoptotic cells before secondary necrosis can occur is referred to as efferocytosis, and is critical in limiting inflammation induced by the leakage of intracellular contents (70). Thus, the fate of atherosclerotic plaques is highly dependent on the balance between recruitment and activation of monocyte-derived macrophages, and their clearance from the vessel wall either through efflux or by apoptosis and subsequent efferocytosis (70).
Reactive oxygen species (ROS), including hydrogen peroxide (H2O2), hydroxyl radical (OH•), and superoxide (O2−), are generated by a variety of both nonenzymatic processes and enzymatic sources (32). The respiratory burst induced via the activation of NADPH oxidase (Nox) 2 is one of the major mechanisms of ROS generation in macrophages (5). Although Nox2 is the major source of ROS in phagocytes, a second Nox isoform, Nox4, was recently identified in human and mouse monocytes and macrophages (32). Nox4 is expressed at lower level than Nox2 in macrophages but is inducible, for example, by OxLDL (32) or metabolic stress (76). Other sources of ROS in macrophages include myeloperoxidase (41), xanthine oxidase (71), lipoxygenase (8), mitochondrial cytochrome P450 (23), and the uncoupling of nitric oxide synthases (50). Produced in excess, for example, by ionizing radiation or inflammatory processes, ROS promote the modification of macromolecules (e.g., phospholipids, proteins, or nucleic acids), which often lead to the irreversible impairment of their function, and subsequently to cell injury. Physiologically, however, ROS perform important signaling functions as intracellular messengers and one of their key targets are protein thiols. ROS are involved in many aspects of atherogenesis, including OxLDL formation, endothelial activation, monocyte-derived macrophage recruitment, activation and death, vascular smooth muscle cell proliferation and death, and matrix remodeling (77). ROS also contribute to the pathogenesis of other vascular diseases, such as vascular remodeling and aneurysm formation (27, 28). Based on a large body of evidence that ROS play a fundamental role in cardiovascular diseases, significant efforts and resources were devoted to prevention and treatment strategies aimed at ROS “scavenging,” primarily involving dietary antioxidant vitamins. Despite promising data obtained in animal models, results of dietary antioxidant supplementation on prevention or treatment of human cardiovascular disease have been largely disappointing and, in some cases, controversial [recently reviewed in (66)]. In contrast, approaches directed at enhancing endogenous antioxidant systems of cells and tissues to prevent oxidative damage may prove to be more effective. For example, transgenic approaches to increase production of glutathione (GSH), a major intracellular antioxidant and electron donor for a variety of antioxidant enzymes, were recently shown to profoundly delay the development of atherosclerotic lesions in aortic root and innominate artery of apoE−/− mice (9). In hematopoietic cells, increasing the activity of GSH reductase, the enzyme that maintains the cellular thiol redox state by catalyzing the reduction of glutathione disulfide to GSH, was sufficient to suppress atherosclerotic lesion formation in LDL-receptor (LDL-R)−/− mice (56). In the following sections, we will focus the discussion on the roles of ROS, their sources, and their (thiol) targets, as they relate to monocytes and macrophages during atherogenesis.
Monocyte Subsets and Monocyte Recruitment
Clinical studies have demonstrated that the blood monocyte count is an independent risk factor of peripheral arterial disease (45) and a predictor of future atherosclerotic plaque formation in humans (26). Experiments in transgenic animals showed that reduction of blood monocytes or blocking their recruitment to atherosclerotic lesions significantly limits plaque expansion (11, 64, 65), illustrating the critical role circulating monocytes play in atherogenesis. Based on expression level of CD14 (lipopolysaccharide [LPS] receptor) and CD16 (Fcγ receptor-III), at least two major subsets of monocytes have been identified in humans, CD14+CD16lo and CD14loCD16hi (67, 80). Monocyte heterogeneity has also been recognized in other species. For example, two different subsets of mice monocytes have been identified based on the expression level of Ly6C antigen, that is, Ly-6Chi and Ly-6Clo monocytes. In the past, these subsets were considered analogous to the CD14+CD16lo and CD14loCD16hi monocyte subsets in humans, though molecular and functional differences have now been identified (25).
Recent evidence suggests that different monocyte subsets may play functionally distinct roles in inflammatory diseases, including atherosclerosis. Ly-6Chi monocytes appear to be pro-inflammatory and are efficiently recruited to inflammatory sites through interaction with inflammatory cytokines, such as monocyte chemoattractant protein-1 (MCP-1) (67). Ly-6Clo monocytes on the other hand are considered to be anti-inflammatory and are thought to contribute to healing and resolution of inflammation. Both monocyte subsets infiltrate atherosclerotic plaques, though Ly6-Chi monocytes are more efficient. Ly-6Clo monocytes have been proposed to contribute to resolution of inflammation leading to plaque stability (67); however, the exact roles of each of these subsets in the development and progression of atherosclerosis remain controversial. A recent study in patients with stable angina pectoris has demonstrated that increased circulating CD14+CD16hi monocytes is associated with radiographic features of plaque vulnerability (24). In mice, hypercholesterolemia causes Ly-6Chi monocytosis by enhancing cell proliferation and survival and possibly reducing conversion of Ly-6Chi to Ly-6Clo monocytes (67). Cystathionine β-synthase is a critical enzyme in homocysteine catabolism and its mutation has been identified in patients with homocystinuria and thrombosis. Cystathionine β-synthase knockout mice develop hyperhomocysteinemia and enhanced oxidative stress and have been used as an animal model to study cardiovascular diseases associated with hyperhomocysteinemia. These animals develop Ly-6Chi monocytosis and demonstrate increased recruitment of monocytes to atherosclerotic plaques, independent of their cholesterol levels. These effects are reversed by the Nox inhibitor apocyanin and the antioxidant catalase polyethylene glycerol, suggesting a role for Nox-derived ROS formation in these pathways (84).
Oxidative stress also affects monocyte responses to chemoattractants. We reported that metabolic stress induced by hypercholesterolemia alone or in association with hyperglycemia primes monocyte for activation by chemoattractants and increases in vivo their chemotactic activity. We went on to demonstrate in LDL-R−/− mice that the macrophage thiol redox state is a strong predictor of monocyte chemotaxis as well as atherosclerotic lesion size and macrophage content (57). These data are in good agreement with our previous studies demonstrating that overexpression of GSH reductase in macrophages of high-fat diet–fed LDL-R−/− mice protects against atherosclerosis (57). Our recent data demonstrate that metabolic stress primes monocytes for activation by chemokines, including MCP-1, platelet-derived growth factor-β, and chemokine ligand 5 (CCL5, also known as RANTES), converting the monocytes into a hyper-responsive phenotype (76). These effects are triggered by induction of Nox4, a novel Nox member we recently identified in monocytes and macrophages (32), and the increased production of intracellular H2O2. Primed monocytes also show increased levels of S-glutathionylated proteins, that is, formation of mixed disulfide between protein thiols and GSH, including actin. Our recent evidence suggests that the S-glutathionylation of actin may be directly responsible for the increased actin turnover and accelerated chemotaxis we observe in primed monocytes (76). Overexpression of cytosolic glutaredoxin-1, the enzyme that catalyzes the reduction of protein-GSH mixed disulfide, not only protected against protein-S-glutathionylation induced by metabolic stress, but also prevented the conversion of monocytes into this hyper-chemotactic, pro-atherogenic phenotype (76).
Macrophage Differentiation and Activation
There is growing evidence that plaque macrophages, like circulating blood monocytes, are a heterogeneous and plastic cell population, with different subsets potentially playing distinct roles in the pathogenesis of atherosclerosis. In an analogy with T1 and T2 lymphocyte activation, two main pathways of macrophage activation have been described: the M1 or classic activation state and M2 or alternatively activated phenotype.
The macrophage activation state appears to depends on the nature of cues the cells receive from the micro-environment, including cytokines, and interactions by adhesion molecules with the extracellular matrix (81). The pro-inflammatory, M1 or classic polarization pathway is induced by interferon-γ (IFN-γ), LPS, tumor necrosis factor α (TNF-α), and granulocyte-monocyte colony stimulating factor (39). These macrophages express inflammatory cytokines, such as TNF-α, interleukin (IL)-1β, IL-12, and IL-6, and are a major source of ROS in atherosclerotic lesions (54). Unlike M1 polarized macrophages, M2 or alternatively polarized macrophages are a heterogeneous group of cells that are activated by stimuli distinct from the known classic M1 inducers, such as IL-4, IL-10, IL-13, and monocyte colony stimulating factor (39). M2 polarized macrophages demonstrate an anti-inflammatory phenotype and appear to be critical for the resolution of inflammation, tissue repair, but also for tumor growth. Interestingly, ROS formation is significantly delayed and attenuated in M2 polarized macrophages (54). Nox2 is the major source of ROS formation in macrophages. Enhanced Nox2-generated ROS, which are primarily targeted to lysosomes and extracellular space, can impair the proteolytic capacity of the phagosomes by interfering with their ability to reduce disulfide bonds. Downregulation of Nox2, particularly in response to IL-4, plays an important role in the enhanced phagocytic activity of alternatively polarized macrophages, which is critical for their functions in tissue repair (5).
Studies on human coronary arteries demonstrated a predominance of M1 polarized macrophages in normal and M2 polarized macrophages in diseased arteries (78). On the other hand, studies on Apo-E-deficient mice have demonstrated the predominance of the M2 in early and M1 polarized macrophages in advanced atherosclerotic plaques (29). As elegantly demonstrated in the Reversa LDL-R−/− mouse model, plasma lipid reduction promotes the regression of aortic root atherosclerotic lesions, and the decrease in lesion size was associated with a decrease in macrophage content (51). Interestingly, plaque macrophages demonstrate a trend toward alternative polarization following reversal of hyperlipidemia, as detected by increased expression of multiple M2 polarization markers, for example, Arg-1, Fizz, and IL-10. Concomitant hyperglycemia in Reversa mice, induced by injection of streptozocin, interferes with plaque regression, with decreased macrophage content, and with alternative polarization. Similarly, in cell culture experiments, hyperglycemia blocks the capacity of bone-marrow-derived macrophages to express M2 polarization markers in response to IL-4 (51). The relevance of macrophage polarization in human atherogenesis is not well-understood, and the exact roles of these macrophage subsets during the development of atherosclerotic plaques and the importance of ROS in functions associated with these different phenotypes remain to be determined.
Macrophage Death
The consequence of macrophage death on atherosclerotic plaque expansion and vulnerability is another area of intense research. The role and impact of macrophages on the development of atherosclerotic lesions appears to depend on different stages of the plaque development. In early lesions, macrophage death occurs primarily through apoptosis, a mechanism that controls vessel wall cellularity and inhibits the early atherogenic process. Decreased macrophage apoptosis in transgenic CD68-hBcl-2 mice results in increased early plaque development but protects against progression to complex lesions (19). Induction of macrophage apoptosis in advanced plaques, using CD11c-DTR transgenic mice, results in increased lesion size, pro-inflammatory cytokine production, and accumulation of monocyte-derived macrophages in plaques (19). These data demonstrate the opposing, that is, atheroprotective versus atherogenic, functions of macrophage apoptosis in early and advanced atherosclerotic lesions (19, 70).
Accumulation of apoptotic cells, as a consequence of imbalanced apoptosis and efferocytosis, has been suggested as a potential mechanism underlying the detrimental effects of macrophage apoptosis in advanced lesions (19, 70). Ineffective efferocytosis leads to reduced clearance of apoptotic macrophages, which then undergo secondary (postapoptotic) necrosis, resulting in the spillage of the highly inflammatory cell content, including pro-inflammatory cytokines and proteolytic enzymes, which further activate the inflammatory cascade. These events will promote the recruitment of additional circulatory monocytes—the formation of new foam cells—feeding a vicious cycle that leads to the expansion of the necrotic core and weakening of the fibrous cap, predisposing the plaque to rupture, that is, converting the advanced lesion into a vulnerable plaque. This hypothetical sequence of events is supported by several lines of evidence. Disruption of efferocytotic pathways through targeted knockout of C1q or transglutaminase-2 genes leads not only to increased atherosclerotic plaque burden but also to more advanced and more inflammatory lesions in LDL-R−/− mice (6, 69), supporting a critical role of efferocytosis in the resolution of inflammation.
Inflammatory cytokines and oxidative stress are among the proposed causes of impaired efferocytosis in advanced plaques (42). Exogenously administered H2O2 or TNF-α impairs efferocytosis, but not phagocytosis, in a dose- and time-dependent manner in murine macrophages, an effect that can be blocked by exogenous antioxidants. ROS formation through cytosolic phospholipase A2, but not Nox or mitochondrial pathway, has been suggested to contribute to TNF-α-induced impairment of efferocytosis (42). Chronic granulomatous disease is an immunodeficiency syndrome caused by mutations in Nox genes, resulting in defective ROS formation and recurrent bacterial and fungal infections. Macrophages from gp91phox−/− mice, a genetic model of chronic granulomatous disease, demonstrated impaired efferocytosis both in cell culture and in animal studies. Interestingly, alternative activation of these macrophages using IL-4 restores macrophage efferocytosis, but classic activation of macrophages with LPS and IFN-γ does not. Additionally, blockade of IL-4 using a neutralizing antibody decreases efferocytosis in wild-type macrophages, confirming a critical role of IL-4-induced alternative polarization in macrophage efferocytosis and resolution of inflammation. IL-4-induced improvement of efferocytosis is mediated through 12/15 lipoxygenase and peroxisome-proliferator activated receptor γ pathways (16).
Mechanisms of Macrophage Apoptosis
Numerous mechanisms have been identified to mediate macrophage death in advanced lesions, including endoplasmic reticulum (ER) stress, OxLDL and free-cholesterol-induced cytotoxicity, oxidative stress, Fas-Fas ligand pathway, and inflammatory cytokines, such as TNF-α.
ER stress
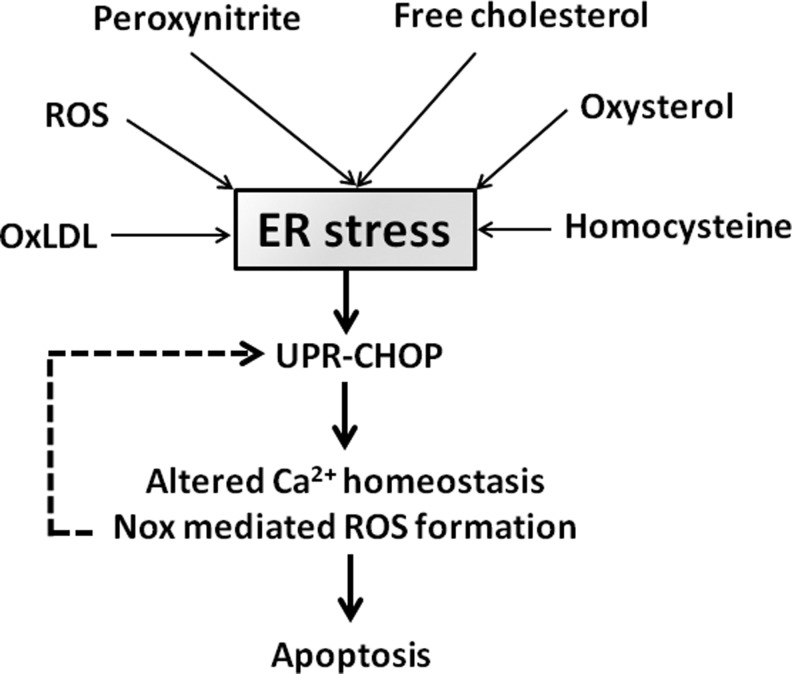
There is growing evidence that ER stress, mediated through the unfolded protein response (UPR) pathway, is a major cause of macrophage death in advanced atherosclerotic lesions. The UPR pathway is an evolutionary conserved adaptive response mechanism that is present in many cells and is activated in response to conditions deleterious to the ER, such as the accumulation of misfolded proteins (69), and alterations in ER lipid composition, calcium homeostasis (17), and cellular redox state (14, 20, 30, 36). The primary function of this pathway is to protect the cells from detrimental effects of misfolded proteins through the expression of chaperones and by diminishing protein translation. These effects are mediated through UPR downstream protein kinases, for example, inositol requiring kinase 1 and double-stranded RNA-activated protein kinase-like ER kinase (37). If the initial response fails to resolve the triggering stimulus and ER stress persists or becomes severe, UPR through its downstream effector, C/EBP homologous protein (CHOP), activates mitochondrial-dependent and -independent pathways of apoptosis (Fig. 2) (37).
FIG. 2.
Endoplasmic reticulum (ER) stress and macrophage apoptosis. ER stress is induced by numerous components of the atherosclerotic plaques, including OxLDL, free cholesterol, and ROS. Activation of the unfolded protein response–C/EBP homologous protein (UPR-CHOP) pathway by ER stress leads to altered calcium homeostasis and NADPH oxidase (Nox)–mediated ROS generation, promoting mitochondrial-dependent and -independent apoptosis. Nox-mediated ROS formation amplifies the CHOP pathway, creating a positive feedback loop.
There is strong evidence from mouse studies for the contribution of UPR/CHOP pathway to macrophage death and plaque vulnerability in advanced atherosclerotic lesions (86). UPR is highly expressed throughout different stages of atherosclerotic plaque development. In advanced lesions, UPR expression correlates with macrophage death (86). Ablation of the CHOP pathway in CHOP/ApoE and CHOP/LDL-R double-knockout mice results in smaller atherosclerotic lesions and reduced macrophage death and decreased plaque rupture (73). Decreased plaque rupture in CHOP/ApoE double-knockout mice is reversed by transplantation of bone marrow cells from CHOP+/+/ApoE−/− mice into CHOP−/−/ApoE−/− mice, suggesting a contribution for bone-marrow-derived cells to plaque rupture (75). Numerous agents have been identified that induce ER stress in macrophages and may contribute to atherosclerotic plaque development and vulnerability, including OxLDL (63), ROS (38), peroxynitrite (13), free cholesterol (12), oxysterols (43), and homocysteine (49).
Recent work by Tabas and colleagues suggests a multihit model in which ER stress-induced macrophage apoptosis is triggered through a combination of multiple activators, rather than a single robust ER stressor (63, 74). Seimon et al. have recently demonstrated that OxLDL, saturated fatty acids, and lipoprotein(a) promote macrophage apoptosis under thapsigargin-induced ER stress condition through CD36/Toll-like receptor 2 involving Nox-2-dependent ROS formation (63). The association between ER stress and cellular redox state is still not clear. Unlike the cytoplasm, the ER lumen is an oxidizing environment with a high ratio of oxidized-to-reduced GSH, which is required to ensure proper protein folding and disulfide formation (37). A number of mechanisms have been proposed by which ER stress may result in ROS formation, including excessive disulfide bond formation and cleavage leading to depletion of the reduced GSH, calcium leakage resulting in mitochondrial ROS generation, and stimulation of mitochondrial oxidative phosphorylation to replenish the depleted ATP store of the cells in response to excessive protein modification in ER (37). A recent report suggests that macrophage ER stress increases ROS generation by inducing Nox-2 expression. Interestingly, in this model, Nox-2 amplified the CHOP pathway, creating a positive feedback loop (34).
Free-cholesterol-induced cytotoxicity
Intracellular accumulation of free cholesterol triggers macrophage death (15). Macrophages have developed multiple protective mechanisms to prevent intracellular cholesterol accumulation, including (i) suppression of endogenous cholesterol biosynthesis, (ii) induction of cholesterol efflux, and (iii) intracellular storage as lipid droplets. The latter process involves the esterification of free cholesterol by the microsomal enzyme acyl-coenzyme A:cholesterol acyltransferase (ACAT) (15). Overwhelmingly, these adaptive mechanisms lead to accumulation of intracellular free cholesterol, which is one of the postulated mechanisms of macrophage cell death, particularly in advanced lesions (7). Transplantation of bone marrow cells from ACAT1-deficient mice into LDL-R knockout mice increases both macrophage free cholesterol content and macrophage death (69). Similarly, inhibitors of ACAT promote more advanced lesions in humans (69).
Multiple mechanisms have been suggested to explain free cholesterol cytotoxicity. Accumulation of intracellular free cholesterol may cause an increase in cell and ER membrane free cholesterol–to–phospholipid ratio, leading to membrane rigidity, which interferes with physiologic function of membrane and integral proteins. For example, dysfunction of ER-calcium-dependent ATPase was shown to result in the depletion of ER calcium stores, triggering macrophage apoptosis through the UPR/CHOP pathway (15, 68, 69). Physical disruption of the cellular integrity by formation of intracellular cholesterol crystals has also been postulated as a possible mechanism of free-cholesterol-induced cytotoxicity (68). Loading macrophages with free cholesterol can also induce macrophage apoptosis through the Fas pathway. Among all the pathways mentioned previously, ER-stress-mediated cell injury has been proposed to play a prominent role in macrophage death in atherosclerosis (82).
OxLDL cytotoxicity
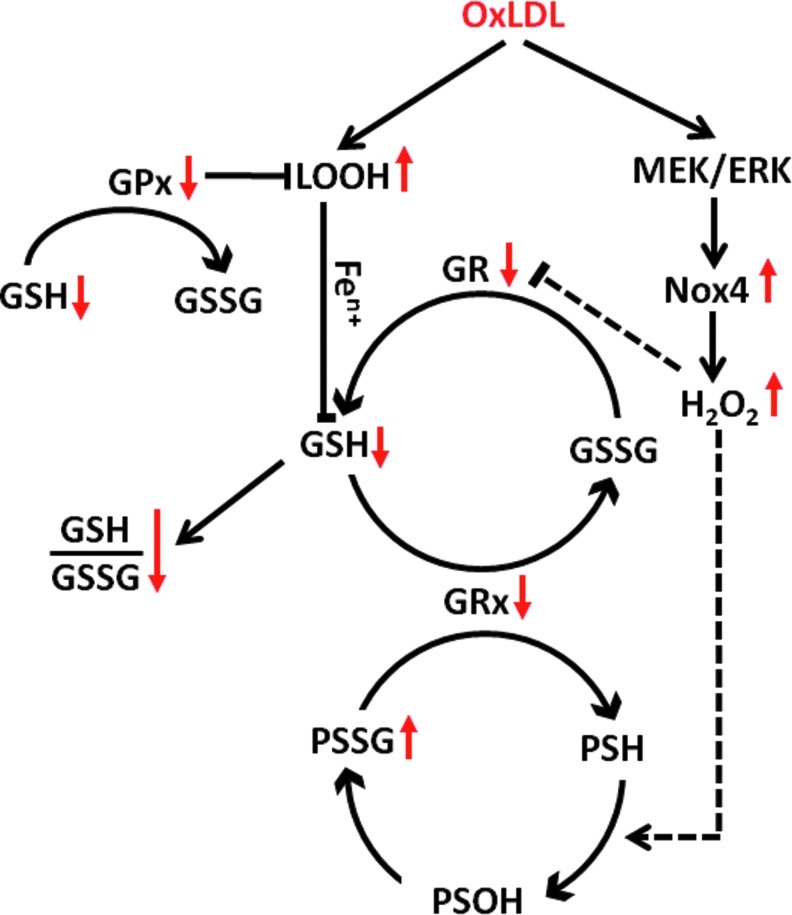
OxLDL is present in atherosclerotic plaques, and OxLDL isolated from these lesions is cytotoxic (83). Structural alterations of the LDL particles as a result of enzymatic and oxidative modifications destabilize the particles and promote (Ox)LDL aggregation and fusion (47). Interestingly, aggregation of cytotoxic OxLDL particles promotes their rapid phagocytic clearance by macrophages. Although inducing foam cell formation, this process does not provoke cell injury, allowing the macrophages to, at least temporarily, escape the immediate cytotoxicity of OxLDL (3). However, foam cell formation itself markedly enhances the sensitivity of human macrophages to OxLDL-induced cytotoxicity (4). A schematic model of OxLDL cytotoxicity in human-monocyte-derived macrophage is shown in Figure 3. We demonstrated that OxLDL triggers cell death in human-monocyte-derived macrophages through a caspase-independent pathway, which is mediated by increased peroxide formation, mitochondrial dysfunction, and the depletion of intracellular GSH and the collapse of GSH redox buffer (2, 79). The importance of the GSH redox buffer for macrophage function and survival is highlighted by the fact that overexpression of GSH reductase in macrophages of dyslipidemic LDL-R−/− mice protects against atherosclerosis (57). In cultured macrophages, overexpression of GSH reductase also prevents OxLDL-induced mitochondrial hyperpolarization, suggesting a role for mitochondrial dysfunction in macrophage death in atherosclerotic plaque (57). OxLDL cytotoxicity has also been shown in a variety of other cells, including endothelial cells (22), smooth muscle cells (22), lymphocytes (1), and fibroblasts.
FIG. 3.
OxLDL-induced cell death in human-monocyte-derived macrophages. Lipid hydroperoxides (LOOH) derived from OxLDL are transferred to macrophages, where they, presumably after metal ion catalyzed conversion into reactive aldehydes, promote the depletion of glutathione (GSH). Increased utilization of GSH as an electron donor for antioxidant enzymes, including glutathione peroxidases (GPx) and glutaredoxins (Grx), results in the accumulation of glutathione disulfide (GSSG). Increased GSSG formation combined with GSH depletion promote the collapse of the GSH/GSSG ratio, disrupting the cellular thiol redox system. In a second, mitogen activated kinase kinase of extracellular signal-regulated kinase (MEK)/extracellular signal-regulated kinase (ERK)-dependent pathway, OxLDL induces Nox4-derived H2O2 production. Both the depletion of GSH and Nox4-mediated ROS production are required for OxLDL-induced macrophage death, but by themselves are not sufficient to promote cell death. Peroxide-mediated oxidation of protein thiols (PSH) leads to the formation and accumulation of mixed disulfides between protein thiols and GSH (PSSG), presumably via protein sulfenates (PSOH) as intermediates. Sustained S-glutathionylation and subsequent inactivation of key macrophage proteins are likely to contribute to the toxic effect of OxLDL on macrophages.
Unlike native LDL, OxLDL strongly increases intracellular ROS formation that is critical in OxLDL-induced cytotoxicity (10, 59). Both enzymatic and nonenzymatic pathways appear to contribute to OxLDL-induced ROS formation (18). Lysophosphatidylcholine, a byproduct of LDL oxidation, is a potent Nox activator (21, 60) and promotes cell injury by increasing ROS formation in endothelial cells (31), vascular smooth muscle cells (46), and macrophages (52). We recently identified Nox4 as a novel Nox family member in monocytes and macrophages (32). The Nox4/p22phox system is induced by OxLDL in human monocytes and macrophages via the MEK/ERK pathway, and is the source of OxLDL-induced intracellular ROS generation and essential for OxLDL-induced macrophage death (32). Most members of Nox family, including Nox-2—which is the major source of ROS in macrophages—produce superoxide that is primarily directed toward extracellular space and phagosomes. Nox4, on the other hand, primarily generates H2O2, which is the major intracellular redox signaling messenger. The enzyme's intracellular localization, its inducibility, and its ability to generate H2O2 suggest that Nox4 may play a central role in macrophage redox signaling (32). The molecular targets of Nox4-derived H2O2 in OxLDL-stimulated macrophages have not been identified.
Autophagy
Autophagy is an evolutionary conserved catabolic process in eukaryotic cells that may occur constitutively at low level or be triggered by unfavorable environmental conditions, for example, hypoxia and starvation (61, 62). By allowing cells to degrade dysfunctional organelles and recycle the cytoplasmic macromolecules, autophagy may promote cell survival in a hostile environment (40, 61, 62). Autophagy has been implicated in both physiologic processes, for example, embryonic development, as well as pathologic conditions, including cancer, aging, myocardial ischemia/reperfusion injury, and atherosclerosis. Many known triggers of autophagy, including cytokines (e.g., TNF-α), ROS, and OxLDL, are present in atherosclerotic plaques and may induce autophagy in the inflammatory milieu of the plaques (62, 85). However, the role of autophagy in atherosclerosis, and macrophage biology in particular, is still not well characterized [recently reviewed in (62)]. It has been suggested that while baseline autophagy of vascular smooth muscle cells may be protective in atherosclerosis, excessive autophagy may promote plaque instability (62). Autophagy is also involved in cholesterol efflux from foam cells through an ABCA1-mediated process, suggesting a potential protective role of macrophage autophagy in atherosclerosis (48).
Conclusions
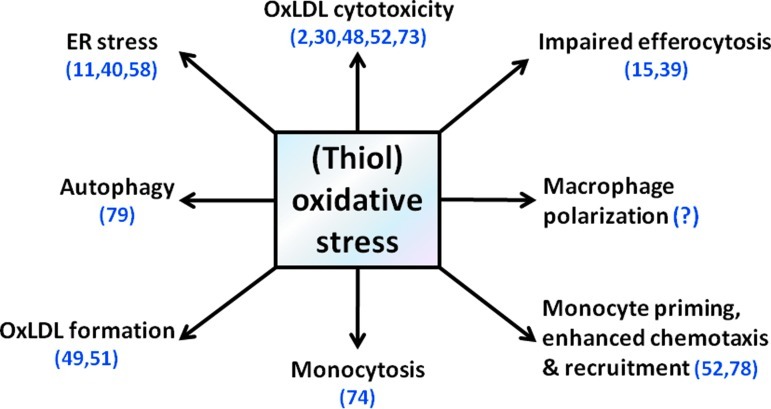
Macrophages are a prominent component of atherosclerotic plaques, from the earliest and clinically insignificant lesions, the fatty streaks, to complex, stenotic, or ruptured plaques. Recent data demonstrated that both circulating monocytes and macrophages accumulated in the plaques are heterogeneous groups of cells, which play diverse and at times opposing functions in different stages of atherosclerosis. ROS play a central role in most of these processes and contribute to the pathophysiology of atherosclerosis by regulating monocyte priming, adhesion, and recruitment, and macrophage differentiation, activation, and polarization and as well as macrophage death and autophagy (Fig. 4). There is growing evidence that oxidative-stress-induced thiol oxidation and protein S-glutathionylation are fundamental to the pathogenic roles monocyte and macrophage play in atherosclerosis (57).
FIG. 4.
(Thiol) oxidative stress and macrophage biology in atherosclerosis. Oxidative stress plays critical functions in the biology of monocytes and macrophages throughout different stages of atherogenesis. (To see this illustration in color the reader is referred to the web version of this article at www.liebertpub.com/ars).
Abbreviations Used
- ACAT
A:cholesterol acyltransferase
- CHOP
C/EBP homologous protein
- ER
endoplasmic reticulum
- GSH
glutathione
- GSSG
glutathione disulfide
- IFN-γ
interferon-γ
- IL
interleukin
- LDLs
low-density lipoproteins
- LDL-R
LDL-receptor
- LPS
lipopolysaccharide
- MCP-1
monocyte chemoattractant protein-1
- Nox
NADPH oxidase
- OxLDL
oxidatively modified LDL
- RANTES
Regulated upon Activation, Normal T-cell Expressed, and Secreted
- ROS
reactive oxygen species
- TNF-α
tumor necrosis factor α
- UPR
unfolded protein response
Acknowledgments
This work was supported by grants to R.A. from the NIH (HL-70963) and the AHA (0855011F). S.T. was supported by a research grant (RR11313) from the RSNA.
References
- 1.Alcouffe J. Caspar-Bauguil S. Garcia V. Salvayre R. Thomsen M. Benoist H. Oxidized low density lipoproteins induce apoptosis in PHA-activated peripheral blood mononuclear cells and in the Jurkat T-cell line. J Lipid Res. 1999;40:1200–1210. [PubMed] [Google Scholar]
- 2.Asmis R. Begley JG. Oxidized LDL promotes peroxide-mediated mitochondrial dysfunction and cell death in human macrophages: a caspase-3-independent pathway. Circ Res. 2003;92:e20–e29. doi: 10.1161/01.res.0000051886.43510.90. [DOI] [PubMed] [Google Scholar]
- 3.Asmis R. Begley JG. Jelk J. Everson WV. Lipoprotein aggregation protects human monocyte-derived macrophages from OxLDL-induced cytotoxicity. J Lipid Res. 2005;46:1124–1132. doi: 10.1194/jlr.M400485-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Asmis R. Jelk J. Vitamin E supplementation of human macrophages prevents neither foam cell formation nor increased susceptibility of foam cells to lysis by oxidized LDL. Arterioscler Thromb Vasc Biol. 2000;20:2078–2086. doi: 10.1161/01.atv.20.9.2078. [DOI] [PubMed] [Google Scholar]
- 5.Balce DR. Li B. Allan ER. Rybicka JM. Krohn RM. Yates RM. Alternative activation of macrophages by IL-4 enhances the proteolytic capacity of their phagosomes through synergistic mechanisms. Blood. 2011;118:4199–4208. doi: 10.1182/blood-2011-01-328906. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia VK. Yun S. Leung V. Grimsditch DC. Benson GM. Botto MB. Boyle JJ. Haskard DO. Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. Am J Pathol. 2007;170:416–426. doi: 10.2353/ajpath.2007.060406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boullier A. Li Y. Quehenberger O. Palinski W. Tabas I. Witztum JL. Miller YI. Minimally oxidized LDL offsets the apoptotic effects of extensively oxidized LDL and free cholesterol in macrophages. Arterioscler Thromb Vasc Biol. 2006;26:1169–1176. doi: 10.1161/01.ATV.0000210279.97308.9a. [DOI] [PubMed] [Google Scholar]
- 8.Brys L. Beschin A. Raes G. Ghassabeh GH. Noel W. Brandt J. Brombacher F. De Baetselier P. Reactive oxygen species and 12/15-lipoxygenase contribute to the antiproliferative capacity of alternatively activated myeloid cells elicited during helminth infection. J Immunol. 2005;174:6095–6104. doi: 10.4049/jimmunol.174.10.6095. [DOI] [PubMed] [Google Scholar]
- 9.Callegari A. Liu Y. White CC. Chait A. Gough P. Raines EW. Cox D. Kavanagh TJ. Rosenfeld ME. Gain and loss of function for glutathione synthesis: impact on advanced atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:2473–2482. doi: 10.1161/ATVBAHA.111.229765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clare K. Hardwick SJ. Carpenter KL. Weeratunge N. Mitchinson MJ. Toxicity of oxysterols to human monocyte-macrophages. Atherosclerosis. 1995;118:67–75. doi: 10.1016/0021-9150(95)05594-m. [DOI] [PubMed] [Google Scholar]
- 11.Combadiere C. Potteaux S. Rodero M. Simon T. Pezard A. Esposito B. Merval R. Proudfoot A. Tedgui A. Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 12.Devries-Seimon T. Li Y. Yao PM. Stone E. Wang Y. Davis RJ. Flavell R. Tabas I. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickhout JG. Hossain GS. Pozza LM. Zhou J. Lhotak S. Austin RC. Peroxynitrite causes endoplasmic reticulum stress and apoptosis in human vascular endothelium: implications in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25:2623–2629. doi: 10.1161/01.ATV.0000189159.96900.d9. [DOI] [PubMed] [Google Scholar]
- 14.Dickhout JG. Sood SK. Austin RC. Role of endoplasmic reticulum calcium disequilibria in the mechanism of homocysteine-induced ER stress. Antioxid Redox Signal. 2007;9:1863–1873. doi: 10.1089/ars.2007.1780. [DOI] [PubMed] [Google Scholar]
- 15.Feng B. Yao PM. Li Y. Devlin CM. Zhang D. Harding HP. Sweeney M. Rong JX. Kuriakose G. Fisher EA. Marks AR. Ron D. Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Boyanapalli RF. Frasch SC. McPhillips K. Vandivier RW. Harry BL. Riches DW. Henson PM. Bratton DL. Impaired apoptotic cell clearance in CGD due to altered macrophage programming is reversed by phosphatidylserine-dependent production of IL-4. Blood. 2009;113:2047–2055. doi: 10.1182/blood-2008-05-160564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu S. Yang L. Li P. Hofmann O. Dicker L. Hide W. Lin X. Watkins SM. Ivanov AR. Hotamisligil GS. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473:528–531. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galle J. Hansen-Hagge T. Wanner C. Seibold S. Impact of oxidized low density lipoprotein on vascular cells. Atherosclerosis. 2006;185:219–226. doi: 10.1016/j.atherosclerosis.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Gautier EL. Huby T. Witztum JL. Ouzilleau B. Miller ER. Saint-Charles F. Aucouturier P. Chapman MJ. Lesnik P. Macrophage apoptosis exerts divergent effects on atherogenesis as a function of lesion stage. Circulation. 2009;119:1795–1804. doi: 10.1161/CIRCULATIONAHA.108.806158. [DOI] [PubMed] [Google Scholar]
- 20.Gilmore WJ. Kirby GM. Endoplasmic reticulum stress due to altered cellular redox status positively regulates murine hepatic CYP2A5 expression. J Pharmacol Exp Ther. 2004;308:600–608. doi: 10.1124/jpet.103.060111. [DOI] [PubMed] [Google Scholar]
- 21.Heinloth A. Heermeier K. Raff U. Wanner C. Galle J. Stimulation of NADPH oxidase by oxidized low-density lipoprotein induces proliferation of human vascular endothelial cells. J Am Soc Nephrol. 2000;11:1819–1825. doi: 10.1681/ASN.V11101819. [DOI] [PubMed] [Google Scholar]
- 22.Hessler JR. Robertson AL., Jr. Chisolm GM., 3rd. LDL-induced cytotoxicity and its inhibition by HDL in human vascular smooth muscle and endothelial cells in culture. Atherosclerosis. 1979;32:213–229. doi: 10.1016/0021-9150(79)90166-7. [DOI] [PubMed] [Google Scholar]
- 23.Hiura TS. Li N. Kaplan R. Horwitz M. Seagrave JC. Nel AE. The role of a mitochondrial pathway in the induction of apoptosis by chemicals extracted from diesel exhaust particles. J Immunol. 2000;165:2703–2711. doi: 10.4049/jimmunol.165.5.2703. [DOI] [PubMed] [Google Scholar]
- 24.Imanishi T. Ikejima H. Tsujioka H. Kuroi A. Ishibashi K. Komukai K. Tanimoto T. Ino Y. Takeshita T. Akasaka T. Association of monocyte subset counts with coronary fibrous cap thickness in patients with unstable angina pectoris. Atherosclerosis. 2010;212:628–635. doi: 10.1016/j.atherosclerosis.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 25.Ingersoll MA. Spanbroek R. Lottaz C. Gautier EL. Frankenberger M. Hoffmann R. Lang R. Haniffa M. Collin M. Tacke F. Habenicht AJ. Ziegler-Heitbrock L. Randolph GJ. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:e10–e19. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnsen SH. Fosse E. Joakimsen O. Mathiesen EB. Stensland-Bugge E. Njolstad I. Arnesen E. Monocyte count is a predictor of novel plaque formation: a 7-year follow-up study of 2610 persons without carotid plaque at baseline the Tromso Study. Stroke. 2005;36:715–719. doi: 10.1161/01.STR.0000158909.07634.83. [DOI] [PubMed] [Google Scholar]
- 27.Kaneko H. Anzai T. Horiuchi K. Kohno T. Nagai T. Anzai A. Takahashi T. Sasaki A. Shimoda M. Maekawa Y. Shimizu H. Yoshikawa T. Okada Y. Yozu R. Fukuda K. Tumor necrosis factor-alpha converting enzyme is a key mediator of abdominal aortic aneurysm development. Atherosclerosis. 2011;218:470–478. doi: 10.1016/j.atherosclerosis.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Kaneko H. Anzai T. Morisawa M. Kohno T. Nagai T. Anzai A. Takahashi T. Shimoda M. Sasaki A. Maekawa Y. Yoshimura K. Aoki H. Tsubota K. Yoshikawa T. Okada Y. Ogawa S. Fukuda K. Resveratrol prevents the development of abdominal aortic aneurysm through attenuation of inflammation, oxidative stress, and neovascularization. Atherosclerosis. 2011;217:350–357. doi: 10.1016/j.atherosclerosis.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 29.Khallou-Laschet J. Varthaman A. Fornasa G. Compain C. Gaston AT. Clement M. Dussiot M. Levillain O. Graff-Dubois S. Nicoletti A. Caligiuri G. Macrophage plasticity in experimental atherosclerosis. PLoS One. 2010;5:e8852. doi: 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan MI. Pichna BA. Shi Y. Bowes AJ. Werstuck GH. Evidence supporting a role for endoplasmic reticulum stress in the development of atherosclerosis in a hyperglycaemic mouse model. Antioxid Redox Signal. 2009;11:2289–2298. doi: 10.1089/ars.2009.2569. [DOI] [PubMed] [Google Scholar]
- 31.Kugiyama K. Sugiyama S. Ogata N. Oka H. Doi H. Ota Y. Yasue H. Burst production of superoxide anion in human endothelial cells by lysophosphatidylcholine. Atherosclerosis. 1999;143:201–204. doi: 10.1016/s0021-9150(98)00288-3. [DOI] [PubMed] [Google Scholar]
- 32.Lee CF. Qiao M. Schroder K. Zhao Q. Asmis R. Nox4 is a novel inducible source of reactive oxygen species in monocytes and macrophages and mediates oxidized low density lipoprotein-induced macrophage death. Circ Res. 2010;106:1489–1497. doi: 10.1161/CIRCRESAHA.109.215392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li AC. Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nat Med. 2002;8:1235–1242. doi: 10.1038/nm1102-1235. [DOI] [PubMed] [Google Scholar]
- 34.Li G. Scull C. Ozcan L. Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J Cell Biol. 2010;191:1113–1125. doi: 10.1083/jcb.201006121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 36.Malhotra JD. Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 37.Malhotra JD. Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malhotra JD. Miao H. Zhang K. Wolfson A. Pennathur S. Pipe SW. Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mantovani A. Sica A. Locati M. New vistas on macrophage differentiation and activation. Eur J Immunol. 2007;37:14–16. doi: 10.1002/eji.200636910. [DOI] [PubMed] [Google Scholar]
- 40.Martinet W. De Meyer GR. Autophagy in atherosclerosis: a cell survival and death phenomenon with therapeutic potential. Circ Res. 2009;104:304–317. doi: 10.1161/CIRCRESAHA.108.188318. [DOI] [PubMed] [Google Scholar]
- 41.McMillen TS. Heinecke JW. LeBoeuf RC. Expression of human myeloperoxidase by macrophages promotes atherosclerosis in mice. Circulation. 2005;111:2798–2804. doi: 10.1161/CIRCULATIONAHA.104.516278. [DOI] [PubMed] [Google Scholar]
- 42.McPhillips K. Janssen WJ. Ghosh M. Byrne A. Gardai S. Remigio L. Bratton DL. Kang JL. Henson P. TNF-alpha inhibits macrophage clearance of apoptotic cells via cytosolic phospholipase A2 and oxidant-dependent mechanisms. J Immunol. 2007;178:8117–8126. doi: 10.4049/jimmunol.178.12.8117. [DOI] [PubMed] [Google Scholar]
- 43.Myoishi M. Hao H. Minamino T. Watanabe K. Nishihira K. Hatakeyama K. Asada Y. Okada K. Ishibashi-Ueda H. Gabbiani G. Bochaton-Piallat ML. Mochizuki N. Kitakaze M. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 44.Nahrendorf M. Jaffer FA. Kelly KA. Sosnovik DE. Aikawa E. Libby P. Weissleder R. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114:1504–1511. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 45.Nasir K. Guallar E. Navas-Acien A. Criqui MH. Lima JA. Relationship of monocyte count and peripheral arterial disease: results from the National Health and Nutrition Examination Survey 1999–2002. Arterioscler Thromb Vasc Biol. 2005;25:1966–1971. doi: 10.1161/01.ATV.0000175296.02550.e4. [DOI] [PubMed] [Google Scholar]
- 46.Ohara Y. Peterson TE. Zheng B. Kuo JF. Harrison DG. Lysophosphatidylcholine increases vascular superoxide anion production via protein kinase C activation. Arterioscler Thromb. 1994;14:1007–1013. doi: 10.1161/01.atv.14.6.1007. [DOI] [PubMed] [Google Scholar]
- 47.Oorni K. Pentikainen MO. Ala-Korpela M. Kovanen PT. Aggregation, fusion, and vesicle formation of modified low density lipoprotein particles: molecular mechanisms and effects on matrix interactions. J Lipid Res. 2000;41:1703–1714. [PubMed] [Google Scholar]
- 48.Ouimet M. Franklin V. Mak E. Liao X. Tabas I. Marcel YL. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2011;13:655–667. doi: 10.1016/j.cmet.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Outinen PA. Sood SK. Pfeifer SI. Pamidi S. Podor TJ. Li J. Weitz JI. Austin RC. Homocysteine-induced endoplasmic reticulum stress and growth arrest leads to specific changes in gene expression in human vascular endothelial cells. Blood. 1999;94:959–967. [PubMed] [Google Scholar]
- 50.Pacher P. Beckman JS. Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parathath S. Grauer L. Huang LS. Sanson M. Distel E. Goldberg IJ. Fisher EA. Diabetes adversely affects macrophages during atherosclerotic plaque regression in mice. Diabetes. 2011;60:1759–1769. doi: 10.2337/db10-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park CH. Kim MR. Han JM. Jeong TS. Sok DE. Lysophosphatidylcholine exhibits selective cytotoxicity, accompanied by ROS formation, in RAW 264.7 macrophages. Lipids. 2009;44:425–435. doi: 10.1007/s11745-009-3286-6. [DOI] [PubMed] [Google Scholar]
- 53.Parthasarathy S. Oxidation of low-density lipoprotein by thiol compounds leads to its recognition by the acetyl LDL receptor. Biochim Biophys Acta. 1987;917:337–340. doi: 10.1016/0005-2760(87)90139-1. [DOI] [PubMed] [Google Scholar]
- 54.Pelegrin P. Surprenant A. Dynamics of macrophage polarization reveal new mechanism to inhibit IL-1beta release through pyrophosphates. EMBO J. 2009;28:2114–2127. doi: 10.1038/emboj.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Podrez EA. Febbraio M. Sheibani N. Schmitt D. Silverstein RL. Hajjar DP. Cohen PA. Frazier WA. Hoff HF. Hazen SL. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J Clin Invest. 2000;105:1095–1108. doi: 10.1172/JCI8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiao M. Kisgati M. Cholewa JM. Zhu W. Smart EJ. Sulistio MS. Asmis R. Increased expression of glutathione reductase in macrophages decreases atherosclerotic lesion formation in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:1375–1382. doi: 10.1161/ATVBAHA.107.142109. [DOI] [PubMed] [Google Scholar]
- 57.Qiao M. Zhao Q. Lee CF. Tannock LR. Smart EJ. LeBaron RG. Phelix CF. Rangel Y. Asmis R. Thiol oxidative stress induced by metabolic disorders amplifies macrophage chemotactic responses and accelerates atherogenesis and kidney injury in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2009;29:1779–1786. doi: 10.1161/ATVBAHA.109.191759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rabbani R. Topol EJ. Strategies to achieve coronary arterial plaque stabilization. Cardiovasc Res. 1999;41:402–417. doi: 10.1016/s0008-6363(98)00279-x. [DOI] [PubMed] [Google Scholar]
- 59.Reid VC. Mitchinson MJ. Toxicity of oxidised low density lipoprotein towards mouse peritoneal macrophages in vitro. Atherosclerosis. 1993;98:17–24. doi: 10.1016/0021-9150(93)90219-k. [DOI] [PubMed] [Google Scholar]
- 60.Rueckschloss U. Galle J. Holtz J. Zerkowski HR. Morawietz H. Induction of NAD(P)H oxidase by oxidized low-density lipoprotein in human endothelial cells: antioxidative potential of hydroxymethylglutaryl coenzyme A reductase inhibitor therapy. Circulation. 2001;104:1767–1772. doi: 10.1161/hc4001.097056. [DOI] [PubMed] [Google Scholar]
- 61.Scherz-Shouval R. Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 62.Schrijvers DM. De Meyer GR. Martinet W. Autophagy in atherosclerosis: a potential drug target for plaque stabilization. Arterioscler Thromb Vasc Biol. 2011;31:2787–2791. doi: 10.1161/ATVBAHA.111.224899. [DOI] [PubMed] [Google Scholar]
- 63.Seimon TA. Nadolski MJ. Liao X. Magallon J. Nguyen M. Feric NT. Koschinsky ML. Harkewicz R. Witztum JL. Tsimikas S. Golenbock D. Moore KJ. Tabas I. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12:467–482. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith JD. Trogan E. Ginsberg M. Grigaux C. Tian J. Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci U S A. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stoneman V. Braganza D. Figg N. Mercer J. Lang R. Goddard M. Bennett M. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ Res. 2007;100:884–893. doi: 10.1161/01.RES.0000260802.75766.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sugamura K. Keaney JF., Jr Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011;51:978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swirski FK. Weissleder R. Pittet MJ. Heterogeneous in vivo behavior of monocyte subsets in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1424–1432. doi: 10.1161/ATVBAHA.108.180521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tabas I. Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. J Clin Invest. 2002;110:905–911. doi: 10.1172/JCI16452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tabas I. Macrophage apoptosis in atherosclerosis: consequences on plaque progression and the role of endoplasmic reticulum stress. Antioxid Redox Signal. 2009;11:2333–2339. doi: 10.1089/ars.2009.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takao S. Smith EH. Wang D. Chan CK. Bulkley GB. Klein AS. Role of reactive oxygen metabolites in murine peritoneal macrophage phagocytosis and phagocytic killing. Am J Physiol. 1996;271:C1278–C1284. doi: 10.1152/ajpcell.1996.271.4.C1278. [DOI] [PubMed] [Google Scholar]
- 72.Tavakoli S. Sadeghi MM. Imaging of vascular biology in the heart. Curr Cardiovasc Imaging Rep. 2009;2:40–49. [Google Scholar]
- 73.Thorp E. Li G. Seimon TA. Kuriakose G. Ron D. Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 2009;9:474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thorp E. Tabas I. Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J Leukoc Biol. 2009;86:1089–1095. doi: 10.1189/jlb.0209115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsukano H. Gotoh T. Endo M. Miyata K. Tazume H. Kadomatsu T. Yano M. Iwawaki T. Kohno K. Araki K. Mizuta H. Oike Y. The endoplasmic reticulum stress-C/EBP homologous protein pathway-mediated apoptosis in macrophages contributes to the instability of atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2010;30:1925–1932. doi: 10.1161/ATVBAHA.110.206094. [DOI] [PubMed] [Google Scholar]
- 76.Ullevig S. Zhao Q. Lee CF. Kim HS. Zamora D. Asmis R. NADPH oxidase 4 mediates monocyte priming and accelerated chemotaxis induced by metabolic stress. Arterioscler Thromb Vasc Biol. 2012;32:415–426. doi: 10.1161/ATVBAHA.111.238899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Violi F. Basili S. Nigro C. Pignatelli P. Role of NADPH oxidase in atherosclerosis. Future Cardiol. 2009;5:83–92. doi: 10.2217/14796678.5.1.83. [DOI] [PubMed] [Google Scholar]
- 78.Waldo SW. Li Y. Buono C. Zhao B. Billings EM. Chang J. Kruth HS. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am J Pathol. 2008;172:1112–1126. doi: 10.2353/ajpath.2008.070513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y. Qiao M. Mieyal JJ. Asmis LM. Asmis R. Molecular mechanism of glutathione-mediated protection from oxidized low-density lipoprotein-induced cell injury in human macrophages: role of glutathione reductase and glutaredoxin. Free Radic Biol Med. 2006;41:775–785. doi: 10.1016/j.freeradbiomed.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 80.Wildgruber M. Lee H. Chudnovskiy A. Yoon TJ. Etzrodt M. Pittet MJ. Nahrendorf M. Croce K. Libby P. Weissleder R. Swirski FK. Monocyte subset dynamics in human atherosclerosis can be profiled with magnetic nano-sensors. PLoS One. 2009;4:e5663. doi: 10.1371/journal.pone.0005663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yakubenko VP. Bhattacharjee A. Pluskota E. Cathcart MK. AlphaMbeta integrin activation prevents alternative activation of human and murine macrophages and impedes foam cell formation. Circ Res. 2011;108:544–554. doi: 10.1161/CIRCRESAHA.110.231803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yao PM. Tabas I. Free cholesterol loading of macrophages induces apoptosis involving the fas pathway. J Biol Chem. 2000;275:23807–23813. doi: 10.1074/jbc.M002087200. [DOI] [PubMed] [Google Scholar]
- 83.Yla-Herttuala S. Palinski W. Rosenfeld ME. Parthasarathy S. Carew TE. Butler S. Witztum JL. Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84:1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang D. Jiang X. Fang P. Yan Y. Song J. Gupta S. Schafer AI. Durante W. Kruger WD. Yang X. Wang H. Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine beta-synthase-deficient mice. Circulation. 2009;120:1893–1902. doi: 10.1161/CIRCULATIONAHA.109.866889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhaorigetu S. Yang Z. Toma I. McCaffrey TA. Hu CA. Apolipoprotein L6, induced in atherosclerotic lesions, promotes apoptosis and blocks Beclin 1-dependent autophagy in atherosclerotic cells. J Biol Chem. 2011;286:27389–27398. doi: 10.1074/jbc.M110.210245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou J. Lhotak S. Hilditch BA. Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2005;111:1814–1821. doi: 10.1161/01.CIR.0000160864.31351.C1. [DOI] [PubMed] [Google Scholar]