Abstract
Although sessile in nature, plants are able to use a number of mechanisms to modify their morphology in response to changing environmental conditions. Differential growth is one such mechanism. Despite its importance in plant development, little is known about the molecular events regulating the establishment of differential growth. Here we report analyses of the nph4 (nonphototropic hypocotyl) mutants of Arabidopsis that suggest that the NPH4 protein plays a central role in the modulation of auxin-dependent differential growth. Results from physiological studies demonstrate that NPH4 activity is conditionally required for a number of differential growth responses, including phototropism, gravitropism, phytochrome-dependent hypocotyl curvature, apical hook maintenance, and abaxial/adaxial leaf-blade expansion. The nph4 mutants exhibited auxin resistance and severely impaired auxin-dependent gene expression, indicating that the defects associated with differential growth likely arise because of altered auxin responsiveness. Moreover, the auxin signaling events mediating phototropism are genetically correlated with the abundance of the NPH4 protein.
Most, if not all, plant growth can be considered differential in the sense that not all cells within a given organ are elongating equally at any given time. However, “differential growth responses” have been classically defined by the bending or movement of an organ resulting from unequal cellular growth in one position of the organ relative to an opposing position. As such, the generation of differential growth represents one adaptive mechanism by which plants are able to modify their morphology rapidly in response to changing environmental conditions. Examples of such responses include tropisms, modification of apical hook structures, and nastic movements of leaves (for reviews, see Darwin and Darwin, 1896; Firn and Digby, 1980; Palmer, 1985). Results from physiological studies conducted during the past 60 years have shown that auxins likely play an important role(s) in the establishment of differential growth (for reviews, see Trewavas, 1992; Hobbie and Estelle, 1994; Kaufman et al., 1995). Perhaps the most well-known interpretation of such data is found in the Cholodny-Went theory (Went and Thimann, 1937). This theory holds that tropic curvatures develop in response to an unequal distribution of auxin in the two sides of a curving organ, which arises as a result of lateral auxin transport. Despite considerable effort aimed at testing this theory (see Trewavas, 1992), very little is known about the coordinated regulation of differential growth at the molecular level by auxin or any other growth-promoting/ -inhibiting substances.
In recent years the study of mutants has played an increasingly important role in the analysis of differential growth (for reviews, see Hobbie and Estelle, 1994; Estelle, 1996; Leyser, 1998). The aux1, axr3, and hls1 mutants of Arabidopsis are especially notable. These mutants exhibit altered root gravitropic and thigmotropic responses (Maher and Martindale, 1980; Okada and Shimura, 1990; Pickett et al., 1990; Timpte et al., 1995), altered root and hypocotyl gravitropic responses (Leyser et al., 1996), and altered apical hook formation/maintenance (Guzman and Ecker, 1990; Hou et al., 1993; Lehman et al., 1996), respectively. The corresponding wild-type genes have been cloned for these mutants, and each of the encoded proteins has been hypothesized to regulate auxin-dependent processes. Specifically, the AUX1/amino acid permease-like protein may function in the basipetal transport of IAA (Bennett et al., 1996; Yamamoto and Yamamoto, 1998), the AXR3/IAA17 protein may act as an auxin-responsive transcriptional regulator (Rouse et al., 1998), and the putative HLS1/N-acetyltransferase may modify the transport or chemical structure of IAA in planta (Lehman et al., 1996).
Despite the intriguing nature of these gene products and their possible roles, phenotypic analyses of mutants indicate that the proteins encoded by these loci function in only one or a limited number of differential growth responses. Furthermore, the hls1 and axr3 mutants exhibit several additional defects not directly related to differential growth, including decreased hypocotyl and primary inflorescence length and changes in apical dominance (Lehman et al., 1996; Leyser et al., 1996). Hence, none of these proteins appears to have functions that are common to the suite of differential growth responses a plant possesses.
Another class of Arabidopsis mutants in which differential growth can be studied are the nph (nonphototropic hypocotyl) mutants (Liscum and Briggs, 1995). Of particular interest are the nph4 mutants, which have been shown to exhibit not only disrupted hypocotyl and root phototropism, but also impaired hypocotyl gravitropism (Liscum and Briggs, 1996). It has been hypothesized that the NPH4 protein might act close to, or directly on, the differential growth response, giving rise to tropic curvatures (Liscum and Briggs, 1996). In this paper we present results from a number of physiological analyses of the nph4 mutants that implicate NPH4 as a specific regulator of multiple auxin-dependent differential growth responses. Genetic and molecular studies further indicate that NPH4 represents a temporally early-acting, concentration-dependent modulator of an auxin-response pathway(s) leading to differential growth.
MATERIALS AND METHODS
All mutants used in these studies were of the Columbia ecotype of Arabidopsis and have been described elsewhere: nph1-4 (Liscum and Briggs, 1995); nph1-5 (Huala et al., 1997); nph4-1, nph4-2, and nph4-3 (Liscum and Briggs, 1996); tir5-1 (nph4-4) (Ruegger et al., 1997); msg1-2 (nph4-102) (Watahiki and Yamamoto, 1997); etr1-1 (Bleecker et al., 1988); hy4-101 (Liscum and Hangarter, 1991); and phyB-9 (Reed et al., 1993).
Growth Conditions
For seedling experiments, seeds were surface-sterilized and plated on nutrient medium solidified with 1.0% (w/v) agar, as described previously (Liscum and Briggs, 1995). One-half-strength Murashige and Skoog nutrient medium (Murashige and Skoog, 1962) without Suc was used for all but the auxin-sensitivity experiments. In these latter experiments, full-strength Murashige and Skoog medium supplemented with 2.0% (w/v) Suc was used. Cold treatment and RL exposure to induce uniform germination were as described previously by Liscum and Briggs (1995).
After induction of germination plates were handled in several different ways, depending on the experiment. For phototropic assays, plates were placed in darkness for 71.5 h and then transferred to unilateral BL (0.1 μmol m−2 s−1) for 8 h. For assays of dark growth and apical hook structure, plates were placed in a vertical position in darkness for the indicated times. Vertical plate orientation caused seedlings to grow along the surface of the agar medium, thus allowing seedling images to be traced on the back side of the plates. When seedlings were to be exposed to ethylene, plates were placed in a desiccator to which 1 mL of pure ethylene was added each day after purging with ambient air (daily ethylene exposure was approximately 50 μL/L). For de-etiolation experiments, plates were placed in darkness for 23.5 h and then transferred to BL or RL for 96 h at the indicated fluence rates. For assays of RL-induced hypocotyl curvature, plates were placed in darkness for 60 h and then transferred to RL for 20 h at the indicated fluence rates. For auxin-sensitivity experiments, plates lacking auxin were incubated in darkness for 23.5 h and then transferred to YL (30 ± 5 μmol m−2 s−1). After 48 h seedlings were transferred to vertically oriented plates containing auxin (IAA, 2,4-D, or NAA) at the indicated concentrations. After marking the positions of hypocotyl and root termini, plates were returned to YL for 72 h. For assays of gene expression, plates were placed in darkness for 23.5 h and then transferred to WL (45 ± 4 μmol m−2 s−1) for 7 d before exposure to auxin.
For mature plant experiments, seeds were sown directly on Pro-Mix (Premier Horticulture, Red Hill, PA) saturated with 0.3% (w/v) Peter's nutrient solution (Scotts-Sierra Horticultural Products, Marysville, OH) and grown under constant WL (100–150 μmol m−2 s−1). Plants were watered twice weekly with distilled water and once every other week with nutrient solution.
Light Sources
For the induction of germination and phytochrome-dependent hypocotyl growth inhibition experiments, RL was obtained by filtering light from gold fluorescent bulbs (F40/GO, Sylvania) through one layer of red acrylic (Rohm and Haas no. 2423, 3.18 mm thick; Cope Plastics, St. Louis, MO). For phototropism experiments, BL was obtained as described previously (Liscum and Briggs, 1995), and for cryptochrome-dependent hypocotyl-growth-inhibition experiments, BL was obtained by filtering light from blue fluorescent bulbs (F40B, Sylvania) through one layer of blue acrylic (Rohm and Haas no. 2424, 3.18 mm thick; Cope Plastics). For auxin-sensitivity experiments, YL was obtained by filtering light from cool-white fluorescent bulbs (F40CW.RS.WM, Sylvania) through one layer of yellow acrylic (Rohm and Haas no. 2208, 3.18 mm thick; Cope Plastics). For the growth of seedlings for RNA experiments, WL was obtained from unfiltered, cool-white fluorescent bulbs, and for the growth of adult plants, WL was obtained from Trimline T8 fluorescent bulbs (F32T8SP41, General Electric).
Genetic Analysis
Heterozygous (nph4-1/NPH4-1) F1 plants were generated by pollinating wild-type plants with pollen from homozygous nph4-1 plants. Complementation tests were performed using F1 seedlings from crosses between homozygous mutants. The genetic mapping stock consisted of nph4-1 individuals (aphototropic/agravitropic seedlings) selected from an F2 population arising from self-pollination of F1 plants derived from a cross of a nph4-1/nph4-1 plant of the Columbia ecotype to a wild-type Landsberg erecta plant. The nph4 genotype of F2 mapping individuals was verified in the F3 generation. PCR primers for simple-sequence-length polymorphism marker-based mapping (Bell and Ecker, 1994) were obtained from Research Genetics (Huntsville, AL). Linkage to the flanking markers nga106 and nga139 was determined by examination of 250 and 290 chromosomes, respectively. Map positions are relative to the latest recombinant-inbred map (http://genome-www.stanford.edu/Arabidopsis/ww/Feb98Rimaps/html/chrom5.html).
Growth Measurements
Hypocotyl curvature responses (phototropism and RL-induced curvatures) were determined as described previously for phototropic and gravitropic responses of etiolated Arabidopsis seedlings (Liscum and Briggs, 1995). For the analysis of etiolated hypocotyl growth, seedling images traced on the back of growth plates (see above) were measured with a ruler to the nearest millimeter. Apical hook angles were measured as described by Liscum and Hangarter (1993a). Light-dependent hypocotyl growth inhibition was determined as described by Young et al. (1992). Growth of hypocotyls and roots after exposure to exogenous hormones was determined essentially as described by Lincoln et al. (1990).
Microscopy
Three-day-old etiolated seedlings were fixed and embedded in butyl-methyl-methacrylate, and ultramicrotome sections were made as described by Baskin and Wilson (1997). Sections were then stained using a modified periodic acid-Schiff's reagent method. Sections were first placed in acetone for 10 min to extract the embedded material, and then transferred to 1.0% (v/v) periodic acid for 15 min. After a 9-min wash in tap water, sections were placed in Schiff's reagent (Sigma) for 30 min, followed by a 9-min wash in tap water. Sections were finally washed for 1 min in distilled water.
Northern-Blot Analysis
Plates containing 7-d-old WL-grown seedlings were flooded with 10 mL of 100 μm IAA or solvent (0.04% [v/v] ethanol) and returned to WL for an additional 1 h. Seedlings were then immediately frozen in liquid nitrogen, and total RNA was extracted as described by Ausubel et al. (1995). RNA samples (20 μg) were separated on a 1.0% (w/v) agarose formaldehyde/Mops gel and transferred to a nylon membrane (Nytran, Schleicher & Schuell), according to the method of Ausubel et al. (1995). Prehybridization, hybridization, and washing of blots were performed as described by Ausubel et al. (1995). Probes were labeled with 32P by random priming (Prime-a-Gene Labeling System, Promega) and then purified from unincorporated label by chromatography (NucTrap columns, Stratagene). Hybridized membranes were exposed to Kodak X-Omat x-ray film.
RESULTS
Genetic Analysis of the nph4 Locus
It was shown previously that nph4 alleles do not segregate as simple recessive Mendelian traits in F2 populations (Liscum and Briggs, 1995). Here we demonstrate that etiolated NPH4/nph4 heterozygotes (F1 plants) exhibit phototropic curvatures that are intermediate between, and significantly different from, those of either parental homozygote (Table I). These results indicate that nph4 alleles are semidominant with respect to phototropism.
Table I.
Phototropic curvature in wild-type, heterozygous, and homozygous nph4 mutants
| Genotype | Curvature | t | Pa |
|---|---|---|---|
| degrees | |||
| NPH4/NPH4 | 52.3 ± 12.9 (65) | 12.64b | <0.001 |
| NPH4/nph4-1 | 32.2 ± 8.9 (124) | – | – |
| nph4-1/nph4-1 | 2.2 ± 5.3 (60) | 24.19c | <0.001 |
Three-day-old seedlings were exposed to 7 h of continuous, unilateral BL at a fluence rate of 0.1 μmol m−2 s−1, and then phototropic curvatures were determined (see Methods). Data represent the mean response ± sd. Numbers of seedlings are given in parentheses.
Genotypes being compared were considered significantly different if P ≤ 0.05.
Student's t test comparison of mean responses between wild-type and heterozygous seedlings.
Student's t test comparison of mean responses between homozygous and heterozygous mutant seedlings.
The nph4 locus was mapped to the proximal arm of chromosome 5 between simple-sequence-length polymorphism markers (Bell and Ecker, 1994) nga106 and nga139 at approximately position 44 centimorgans (data not shown). A similar map position has been reported for two recently identified Arabidopsis mutants, msg1 (Watahiki and Yamamoto, 1997) and tir5 (Ruegger et al., 1997). The msg1 mutants were identified in a screen for mutants that failed to exhibit hypocotyl curvature in response to unilaterally applied auxin, whereas the tir5 mutants were identified by their resistance to auxin-transport inhibitors. Neither the msg1-2 nor the tir5-1 mutant was capable of complementing the nph4 mutants in the F1 generation, and no wild-type seedlings have segregated in the F2 progeny from such F1 plants (data not shown). Thus, the msg1 and tir5 mutants represent independently identified alleles of the nph4 locus. The two tir5 alleles (Ruegger et al., 1997) have been renamed nph4-4 and nph4-5 (M. Estelle, unpublished data), and the msg1 mutants (Watahiki and Yamamoto, 1997) have been given nph4 allele designations beginning with allele number 101 (K. Yamamoto, unpublished data).
Overall Morphogenesis of Dark- and Light-Grown nph4 Plants Is Normal
It was proposed previously that the NPH4 gene product might play an essential (Watahiki and Yamamoto, 1997), if not direct (Liscum and Briggs, 1996), role in the establishment of differential growth in Arabidopsis. This hypothesis was based mainly on data related to hypocotyl tropisms. Alternatively, it could be argued that the observed mutant tropic phenotypes arose because of changes in overall growth properties or cellular/tissue organization, rather than from specific defects related directly to differential growth. In an attempt to reconcile these opposing hypotheses, we examined a number of morphogenic properties in both dark- and light-grown nph4 plants.
Etiolated Growth
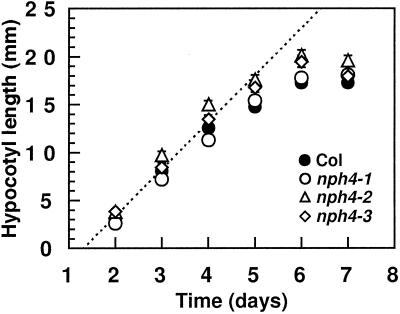
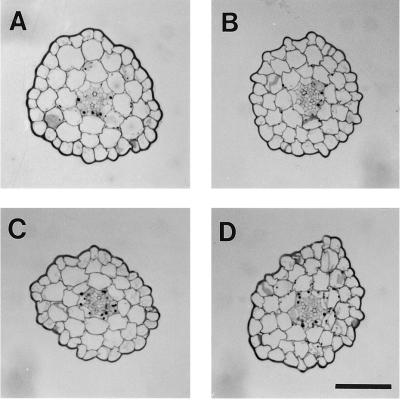
As shown in Figure 1, hypocotyls of etiolated wild-type and nph4 seedlings exhibit similar straight-growth kinetics. Moreover, the overall cellular morphology and anatomical structure are similar between hypocotyls of wild-type and nph4 seedlings (Fig. 2). Thus, it appears unlikely that disrupted tropic responses of etiolated nph4 seedlings result from gross changes in hypocotyl morphogenesis.
Figure 1.
Time course of hypocotyl growth of wild-type and nph4 seedlings in darkness. At the indicated times after induction of germination, seedlings were removed and hypocotyl lengths were measured to the nearest millimeter. Data represent the mean response of a minimum of 60 seedlings from two replicate experiments. The vertical error bars represent the se values. Because the symbols often overlap, some individual symbols and error bars are not visible. The dotted line represents a regression (r2 = 0.942) calculated for combined wild-type and nph4 data during the period of linear growth (d 2, 3, and 4). Col, Wild-type Columbia ecotype.
Figure 2.
Cellular morphology of wild-type (A), nph4-1 (B), nph4-2 (C), and nph4-3 (D) hypocotyls. All sections were taken from a region just below the apical hook of 3-d-old dark-grown seedlings, where phototropism is initiated (Orbovic and Poff, 1991), stained with periodic acid-Schiff's reagent, and viewed with bright-field optics. Morphologies are similar to those reported previously for etiolated Arabidopsis seedlings (Gendreau et al., 1997). Bar = 100 μm.
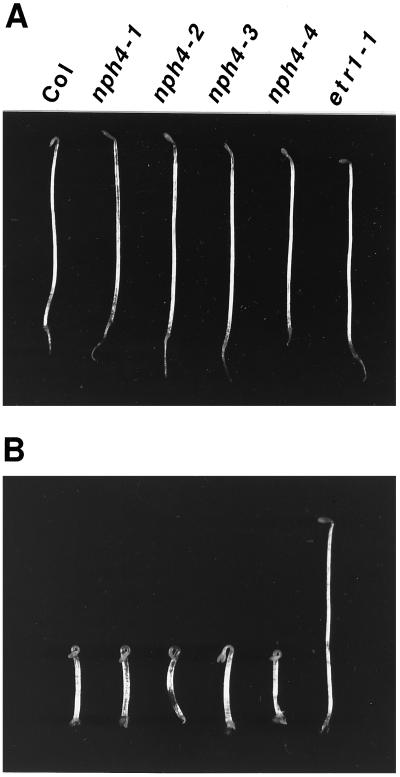
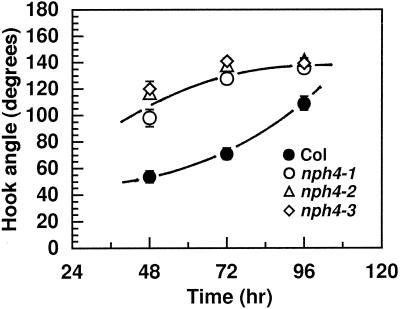
Because apical hooks of etiolated seedlings are formed by the continual differential growth of cells on the inner and outer edges of the hook as they flow through the apical region (Silk and Erickson, 1978), we examined the apical hooks of nph4 mutants. As illustrated in Figure 3A, nph4 seedlings were partially hookless, indicating that this response is disrupted by nph4 mutations. The dark-dependent hook-opening response of nph4 seedlings was saturated after about 2.5 to 3 d, rather than 4 to 5 d as in the wild-type (Fig. 4; see also Liscum and Hangarter, 1993a). Apical hooks of nph4 seedlings, however, were similar in appearance to the wild type upon germination (data not shown). Thus, it appears that the hookless phenotype of nph4 seedlings resulted from an accelerated phase of opening. It is interesting that both wild-type and nph4 seedlings exhibited an exaggerated apical hook when grown in the presence of ethylene (Fig. 3B). This result demonstrates that the cells within the apical hook of nph4 seedlings are capable of exhibiting differential growth, and indicates that the hookless phenotype of air-grown nph4 seedlings is not a result of a general defect in apical hook structure/maintenance. Taken together, the apical hook phenotypes of nph4 seedlings suggest that NPH4 acts as a conditional modulator of differential growth.
Figure 3.
Morphogenesis of etiolated wild-type and nph4 seedlings grown in air (A) and 50 μL/L ethylene (B). Photographs were taken after 4 d of growth in darkness. The ethylene receptor mutant etr1-1 is shown as a negative control for ethylene responsiveness. Col, Wild-type Columbia ecotype.
Figure 4.
Time course of apical hook opening in dark-grown wild-type and nph4 seedlings. Seedlings were grown in darkness on vertical plates. At the indicated times (after induction of germination), apical hook angles were determined (see Methods). Data represent the mean response of a minimum of 21 seedlings from two replicate experiments. The vertical error bars represent the se values. Because the symbols often overlap, some individual symbols and error bars are not visible. Col, Wild-type Columbia ecotype.
De-Etiolated Growth
As was observed with etiolated seedlings (Fig. 1), hypocotyl growth of deetiolated nph4 seedlings was indistinguishable from that of the wild type (Fig. 5). In general, the growth and development of adult light-grown nph4 plants also appeared normal. As shown in Table II, rosettes of mature nph4 plants were similar to those of the wild type with respect to size and number of leaves. The growth and development of reproductive structures were also unaffected by nph4 mutations, such that wild-type and nph4 plants flowered with a similar timing (data not shown) and the resultant inflorescences were similar in size and number (Table II).
Figure 5.
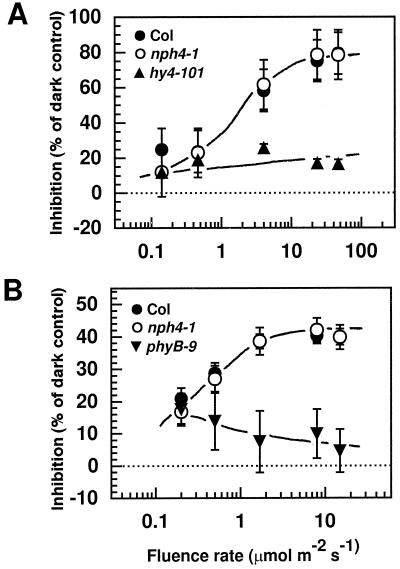
BL-dependent (A) and RL-dependent (B) hypocotyl growth inhibition in wild-type and nph4 seedlings. After 23 h of growth in darkness, seedlings were transferred to continuous light at the indicated fluence rates shown for an additional 96 h. Control seedlings were kept in darkness for the entire growth period. Hypocotyl lengths were measured from digitized images of seedlings (see Methods). Data represent the mean response of at least 33 seedlings from two replicate experiments. Vertical error bars represent the combined se values for dark- and light-grown seedlings. Because the symbols often overlap, some individual symbols and error bars are not visible. The response of cry1-deficient hy4-101 seedlings is presented as a negative control. The response of phyB-deficient phyB-9 seedlings is presented as a negative control. Col, Wild-type Columbia ecotype.
Table II.
Morphological features of adult wild-type and nph4 plants
| Feature | Col | nph4-2a |
|---|---|---|
| No. of rosette leaves | 11.8 ± 1.4 | 12.6 ± 1.6 |
| Rosette diameter (mm)b | 89.6 ± 13.6 | 80.4 ± 14.4 |
| No. of inflorescencesc | 4.5 ± 0.8 | 4.9 ± 0.5 |
| Length of inflorescence (cm)d | 36.6 ± 5.6 | 40.3 ± 6.7 |
| No. of lateral branchese | 17.0 ± 7.2 | 20.7 ± 4.4 |
Seeds were sown directly on soil and allowed to germinate under constant WL (120 μmol m−2 s−1) at 25°C. Measurements were made at 3 weeks (number of leaves) and 6 weeks (all other characteristics) after sowing. Data represent the mean response ± sd of a minimum of 16 plants. Col, Wild-type Columbia ecotype (genetic background of nph4-2).
Similar results were obtained with other nph4 alleles.
Measured across the two largest opposing leaves.
Measured as the number of bolts emerging from the rosette.
Measured on the longest bolt.
Measured as secondary inflorescences arising from axils of primary inflorescences.
The only abnormal morphological feature invariably associated with light-grown nph4 plants was the presence of epinastic or hyponastic rosette leaves (data not shown). The extent of leaf epinasty observed in this study was similar to that observed previously for the nph4-102 allele (see Watahiki and Yamamoto, 1997). Although hyponasty has been reported for the nph4-103 allele (Watahiki and Yamamoto, 1997), only epinasty was observed in other nph4 alleles (data not shown; Watahiki and Yamamoto, 1997). The morphology of mature nph4 plants, like that of seedlings, indicated that NPH4 is dispensable with respect to the overall morphological and developmental program of the plant. However, the epinastic/hyponastic character of nph4 rosette leaves, which likely occurred as a result of abnormal differential growth of adaxial and abaxial leaf surfaces (Palmer, 1985; Klee et al., 1987), provides additional evidence that NPH4 acts as a modulator of differential growth.
Phototropic Impairment Is a Common Feature of nph4 Mutants
Watahiki and Yamamoto (1997) reported that nph4-101, nph4-102, and nph4-103 seedlings exhibited normal phototropic responses in unilateral WL. However, it has been shown previously that under conditions in which significant phytochrome photoactivation occurs in addition to phototropic photoreceptor activation (i.e. unilateral WL), considerable phototropic response is observed in nph4 seedlings (Liscum and Briggs, 1996). Therefore, we examined the phototropic response of various nph4 mutants in unilateral BL. As shown in Table III, nph4-102 was only about 28% as responsive as the wild type after exposure to 8 h of unilateral BL, demonstrating that this allele is in fact phototropically impaired. The response of nph4-102, however, was more than twice as great as that observed in nph4-1 (Table III). nph4-4 seedlings exhibited a phototropic response that was intermediate between that of nph4-1 and nph4-102 (Table III). Thus, it appears that NPH4 is necessary for the generation of phototropic curvatures in unilateral BL, and that considerable quantitative variation occurs between the various nph4 alleles.
Table III.
BL-dependent hypocotyl phototropism in dark-grown wild-type and various nph4 seedlings
| Genotype | Curvature |
|---|---|
| degrees | |
| Col | 49.0 ± 1.4 (75) |
| nph4-1 | 4.4 ± 0.9 (59) |
| nph4-4 | 8.0 ± 1.1 (49) |
| nph4-102 | 13.5 ± 0.9 (75) |
| nph1-5a | 0.8 ± 0.9 (51) |
Three-day-old etiolated seedlings were exposed to 8 h of unilateral BL (0.1 μmol m−2 s−1), and then phototropic curvatures were determined (see Methods). Data represent the mean response ± se from a minimum of two replicate experiments. Numbers of seedlings are given in parentheses. Col, Wild-type Columbia ecotype.
The response of this genotype is given as a negative control.
Etiolated nph4 Seedlings Lack RL-Induced Hypocotyl Curvature
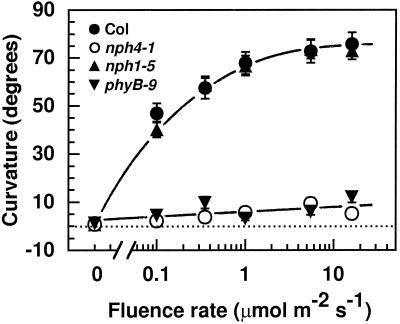
Although the hypocotyls of etiolated Arabidopsis seedlings normally grow vertically upward (Mizra et al., 1984; Liscum and Hangarter, 1993b), they bend away from this orientation when exposed to RL (Fig. 6; also see Hangarter, 1997). This differential growth response apparently requires phytochrome B photoconversion, since the response was virtually eliminated in a phyB null mutant (Fig. 6). However, it does not require functional NPH1, because a nph1 null mutant exhibited a wild-type response (Fig. 6). This latter result, together with the findings that the direction of RL-induced curvature was random (E. Liscum, unpublished data) and that phototropic curvatures in Arabidopsis were not induced by exposure to unilateral RL (Steinitz et al., 1985; Liscum and Briggs, 1996), indicates that this phytochrome-B-dependent curvature response is not a phototropic response. However, the nph4 mutants lack this RL-induced curvature (Fig. 6), indicating that at least one component is shared between this response and phototropism. This result also illustrates an additional condition in which NPH4 function is required for the generation of differential growth.
Figure 6.
RL-dependent hypocotyl curvature in dark-grown wild-type and mutant seedlings. Sixty-hour-old seedlings were exposed to 20 h of continuous RL at the indicated fluence rates, and then curvatures were determined (see Methods). Data represent the mean response of a minimum of 33 seedlings from two replicate experiments. Vertical error bars represent the se values. Because the symbols often overlap, some individual symbols and error bars are not visible. Col, Wild-type Columbia ecotype.
Auxin Responsiveness Is Disrupted in nph4 Seedlings
Auxin-Dependent Growth
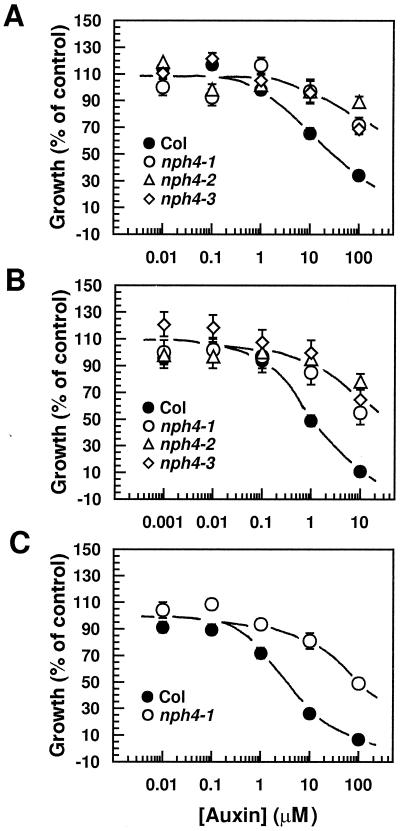
The nph4 mutants have been shown to represent a unique class of auxin-resistant mutants that are not crossresistant to other growth regulators, and exhibit auxin resistance only in aerial organs (Watahiki and Yamamoto, 1997). Figure 7 shows the auxin resistance exhibited by hypocotyls of the nph4-1, nph4-2, and nph4-3 mutants. Using a 50% inhibitory concentration as a measure of resistance we found that the nph4-1, nph4-2, and nph4-3 mutants were 15- to 20-fold more resistant to IAA, 2,4-D, and 1-NAA than the wild type. In comparison, the nph4-101, nph4-102, and nph4-103 mutants were only about 5-fold more resistant to 2,4-D than the wild type (Watahiki and Yamamoto, 1997). Together, these findings, along with those from analyses of apical hook structure and BL-dependent phototropism in etiolated seedlings (Figs. 3–5; Watahiki and Yamamoto, 1997), indicate that the nph4-101, nph4-102, and nph4-103 mutants represent weak alleles, whereas the nph4-1, nph4-2, and nph4-3 mutants represent strong alleles.
Figure 7.
Dose responses of wild-type and nph4 hypocotyls to exogenous IAA (A), 2,4-D (B), and 1-NAA (C). Three-day-old YL-grown seedlings were transferred to medium containing various concentrations of auxins (see Methods). Hypocotyl growth was measured 3 d later. Data represent the mean response (as a percent of controls) of a minimum of 90 seedlings from at least three replicate experiments. Vertical error bars represent the se values. Controls were seedlings transferred to plates containing only solvent (0.04% ethanol). Because the symbols often overlap, some individual symbols and error bars are not visible. Col, Wild-type Columbia ecotype.
Auxin-Dependent Gene Expression
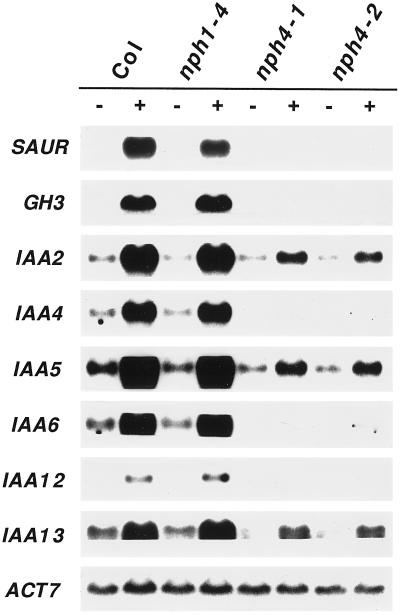
A number of genes have been identified in higher plants that are transcriptionally activated within 5 to 60 min of exposure to auxin (for review, see Abel and Theologis, 1996). In an attempt to determine how early in the auxin-response pathway(s) NPH4 functions, the steady-state mRNA levels of a number of these rapid primary-response genes were examined. As shown in Figure 8, expression of such genes was severely impaired in the nph4 mutant background. In particular, mRNAs of SAUR-AC1 and IAA12 were undetectable, and GH3, IAA4, and IAA6 mRNAs were detectable only after extended autoradiographic exposures (data not shown). IAA2, IAA5, and IAA13 mRNAs, however, were clearly detectable in total RNAs from nph4-1 and nph4-2, but were reduced in level relative to the wild type. The basal levels of expression (i.e. expression that was dependent on endogenous auxin) of all of the primary-response genes examined were also reduced. However, in some cases the effects were much smaller than those observed with respect to induction by exogenous auxin. For example, although a dramatic reduction in the abundance of IAA2 and IAA5 mRNAs was observed in auxin-treated nph4 seedlings, the basal level of expression of these genes was only slightly reduced relative to the wild type (Fig. 8).
Figure 8.
Expression of auxin-induced genes in wild-type and nph4 seedlings. Total RNA was isolated from 7-d-old WL-grown seedlings that had been exposed to 100 μm IAA (+) or solvent (0.04% ethanol; −) for 1 h. Samples (20 μg each) were separated on a 1.0% agarose formaldehyde/Mops gel and then blotted to nylon. The blot was then hybridized with 32P-labeled gene-specific probes against various auxin-induced genes: SAUR-AC1 (Gil et al., 1994); GH3 (Hagen et al., 1984); and IAA2, IAA4, IAA5, IAA6, IAA12, and IAA13 (Abel et al., 1995). The blot was also hybridized with a labeled actin probe (ACT7; McDowell et al., 1996) as a loading control. RNA from nph1-4 seedlings was used as an additional positive control. Similar overall results were observed in replicate experiments with both WL- and dark-grown seedlings (data not shown). The blot was rehybridized with multiple probes between strippings; thus, artificially flat upper and/or lower edges were generated on the IAA5, IAA6, and IAA13 transcripts when individual panels for these genes were cropped from the entire blot for photographs. Col, Wild-type Columbia ecotype; SAUR, SAUR-AC1.
In contrast to the nph4 mutants, no differences in mRNA abundances of the auxin primary-response genes were observed in the nph1-4 mutant (Fig. 8). Although this finding was not unexpected, given the proposed role of NPH1 as an early step in the signaling pathway controlling phototropism (Liscum and Briggs, 1995; Huala et al., 1997), it does indicate that the molecular phenotypes of the nph4 mutants were not simply a consequence of their aphototropic physiology (Liscum and Briggs, 1996). It remains to be determined if any of the early-response genes examined are actually necessary for the establishment of differential growth.
DISCUSSION
NPH4 Acts as a Conditional Modulator of Multiple Differential Growth Responses
Previous studies have shown that in addition to altered hypocotyl and root phototropism (Liscum and Briggs, 1996), nph4 mutant seedlings exhibit impaired hypocotyl gravitropism (Liscum and Briggs, 1996; Watahiki and Yamamoto, 1997). In this study we demonstrate that apical hook and phytochrome-dependent hypocotyl curvatures of etiolated seedlings are also disrupted by nph4 mutations. Furthermore, adaxial/abaxial leaf-surface expansion is altered in adult nph4 plants, such that rosette leaves are either epinastic or hyponastic in appearance (Watahiki and Yamamoto, 1997; this paper). Under laboratory conditions all of these nph4-dependent alterations occur in the absence of any obvious changes in general growth or development. One interpretation of these results might be that differential growth responses are dispensable with respect to the overall morphological and developmental program of Arabidopsis. However, the conditional nature of the nph4 phenotypes (i.e. aphototropism in BL versus considerable phototropism in WL, and a hookless phenotype in air versus a normal exaggerated hook in ethylene) indicate that redundant mechanisms exist to regulate differential growth. Hence, progression of a normal developmental program in the nph4 background probably reflects the function of these redundant differential growth pathways, rather than a “noneffect” of the nph4 mutations and dispensability of differential growth. Because NPH4 acts as a conditional modulator of differential growth, it represents an attractive molecule for future studies of differential growth regulation in the absence of potentially confounding pleiotropic effects, as occurs with many of the other apparent regulators of differential growth. Furthermore, the conditional nature of NPH4 action should allow us to genetically identify redundant modulators that are functioning under other conditions.
The nph4 Mutants Comprise a Phenotypically Variable Allele Series
Although the nph4 mutants were first identified by their ability to disrupt hypocotyl phototropism in etiolated seedlings (Liscum and Briggs, 1995, 1996), complementation studies presented here demonstrate that several additional nph4 alleles have recently been identified in screens for seedlings exhibiting reduced auxin-induced hypocotyl curvature (Watahiki and Yamamoto, 1997) or sensitivity to auxin-transport inhibitors (Ruegger et al., 1997). Analyses of the different nph4 mutants indicate that considerable phenotypic variation exists within this allele series. For instance, seedlings homozygous for the nph4-102 allele (previously designated msg1-2) exhibit hypocotyl phototropism and apical hook closure that is considerably more like the wild type (Watahiki and Yamamoto, 1997) than the mutants homozygous for any of the originally identified nph4 alleles, such as nph4-1. Furthermore, whereas “weak” nph4 alleles (i.e. nph4-102) are more resistant to exogenously applied auxin than the wild type, they retain auxin sensitivity that is three to four times greater than that observed with “strong” nph4 alleles (i.e. nph4-1). Such differences in auxin sensitivity are probably causal determinants of the aforementioned phenotypic differences between these alleles.
Allelic variation within the nph4 allele series should not be surprising considering how the different mutant alleles were generated. The nph4-1, nph4-2, and nph4-3 mutants were generated by fast-neutron bombardment (Liscum and Briggs, 1995, 1996), whereas the nph4-4, nph4-5, nph4-101, nph4-102, and nph4-103 mutants were generated by EMS mutagenesis (Ruegger et al., 1997; Watahiki and Yamamoto, 1997). Fast neutrons usually induce deletions and/or large chromosomal rearrangements (Rédei and Koncz, 1992; Bruggemann et al., 1996), which result in the severe dysfunction or lack of the protein encoded by the mutated gene. As expected, all of the fast-neutron-generated nph4 mutants are phenotypically strong mutants. In contrast, EMS usually causes G:C to A:T base substitutions that result in either missense or nonsense mutations (DuBridge and Calos, 1987). As would be predicted, both weak (i.e. nph4-102) and strong (i.e. nph4-4) alleles have been identified within the collection of EMS-generated nph4 mutants.
Strong nph4 Alleles Define a Threshold Step in the Phototropic Signal-Response Pathway
It was concluded from earlier studies that NPH4 likely functions as a signal transduction/response element acting downstream of the photoperception event(s) mediating phototropism (Liscum and Briggs, 1995, 1996). Because nph4 mutants exhibit alterations in multiple differential growth responses, it is probable that NPH4 acts late in the phototropic signal-response pathway. The semidominant inheritance exhibited by the fast-neutron-generated nph4 alleles indicates that the phototropic response is sensitive to the gene dosage of NPH4, and further implies that the magnitude of phototropic curvature is directly related to the abundance of the NPH4 protein. Therefore, we hypothesize that NPH4 is a concentration-dependent modulator of differential growth that functions late in the signal-response process(es) leading to phototropic curvatures. Previous photophysiological studies of phototropism in Arabidopsis (Steinitz and Poff, 1986; Janoudi and Poff, 1991; Janoudi et al., 1992) and other species such as maize (Iino, 1987, 1990) have shown that the magnitude of phototropic curvature is kinetically limited by a postphotoperception component in the signal transduction chain. It is possible that NPH4 represents, or regulates the activity of, this previously predicted but unidentified gene product.
It is interesting to note that although the fast-neutron-generated nph4 alleles exhibited semidominant inheritance, the EMS-generated alleles have been reported to segregate as simple recessive loci (Ruegger et al., 1997; Watahiki and Yamamoto, 1997). Of several plausible explanations for these apparently contradictory data, one in which allele strength determines the pattern of inheritance seems most likely. For example, heterozygotes carrying a weak nph4 allele (i.e. nph4-102) could make enough active NPH4 protein to exceed a threshold level required for the establishment of a wild-type phototropic response, whereas heterozygotes having a strong nph4 allele (i.e. nph4-1) would not and thus would appear partially mutant. Alternatively, the segregation of nph4 alleles could be dependent on the physiological response being examined. As an example, all alleles might segregate as semidominant loci with respect to phototropism. A second alternative is that all nph4 alleles are semidominant, independent of response, but that the “mutant” and “wild-type” classifications used in the initial genetic characterizations of the EMS-generated nph4 mutations (Ruegger et al., 1997; Watahiki and Yamamoto, 1997) were too broad to clearly distinguish between recessive and semidominant inheritance. To test these latter possibilities we are currently generating a population that is heterozygous for the nph4-102 allele (an apparent recessive allele), and will examine the dominance of this weak allele relative to phototropic response, for which semidominance has been observed.
Changes in Auxin Sensitivity of the nph4 Mutants Likely Account for the Alterations in Differential Growth
Although most of the previously identified auxin-response mutants are disrupted with respect to at least one differential growth response (for reviews, see Hobbie and Estelle, 1994; Estelle, 1996; Leyser, 1998), nearly all are highly pleiotropic and exhibit multiple defects in addition to altered differential growth. Moreover, many apparently secondary effects of nonauxin growth regulators are observed in these mutants. In contrast, the nph4 mutants represent a class of auxin-response mutants that can be used to assess the potential role(s) of auxin in the generation of differential growth in the absence of confounding phenotypic effects.
In addition to being resistant to exogenously applied auxin, the nph4 mutants exhibit alterations in multiple differential growth responses. Moreover, changes in hormone responsiveness of the nph4 mutants are limited to auxins, and among the phenotypes examined to date, most morphological defects appear to be confined to differential growth responses. The finding that auxin primary-response genes are expressed at dramatically reduced (or undetectable) steady-state levels in the nph4 background indicates that NPH4 functions temporally early in an auxin-response pathway(s). Because many of the genes that were examined are normally transcriptionally activated within 5 min of exposure to auxin (for review, see Abel and Theologis, 1996), and the lag period between tropic stimulation and measurable curvature of hypocotyls and roots in Arabidopsis is approximately 5 to 20 min (Kiss et al., 1989; Orbovic and Poff, 1991), NPH4 activity is apparently required before, or concomitant with, cellular changes that actually drive differential growth. These observations, together with those gathered from analyses of other auxin-response mutants (for reviews, see Hobbie and Estelle, 1994; Estelle, 1996; Leyser, 1998), provide clear genetic evidence that auxin plays a critical role in the generation of differential growth patterns. Our results suggest further that the magnitude of differential growth is related directly to the auxin responsiveness of the plant, which can be modulated by NPH4. These conclusions are consistent with a broad interpretation of the Cholodny-Went theory (see Trewavas, 1992).
Whereas a definitive biochemical function for NPH4 awaits cloning of the NPH4 locus, two obvious possible functions can be proposed based on the data accumulated to date: (a) NPH4 regulates lateral auxin transport (influx and/or efflux) or (b) NPH4 modulates auxin sensitivity at some step after the establishment of an auxin gradient. In terms of auxin influx, previous studies have shown that IAA and 2,4-D enter cells through an active influx carrier, whereas NAA enters via passive diffusion (Delbarre et al., 1996). Thus, if NPH4 regulates auxin influx, the nph4 mutants would be expected to exhibit greater sensitivity to NAA than either IAA or 2,4-D. However, all of the nph4 mutants examined were found to exhibit equivalent levels of resistance to IAA, 2,4-D, and NAA. With respect to auxin efflux, loss-of-function mutations affecting either the efflux carrier itself or some positively acting regulatory protein would be expected to cause increases in intracellular auxin concentration (see Lomax et al., 1995), thereby promoting dramatic changes in morphology. Increases in both hypocotyl elongation and apical dominance have been observed in light-grown Arabidopsis plants that overproduce IAA (Romano et al., 1995). However, no such morphological changes were observed in the strong nph4 mutants. Moreover, to achieve the reduced basal levels of expression of the IAA4 and IAA6 genes observed in the nph4 mutants, intracellular auxin concentrations might need to increase as much as 4.5 orders of magnitude (see Abel et al., 1995), which seems improbable. Therefore, it is unlikely that either auxin influx or efflux is dependent on NPH4 activity; therefore, we hypothesize that NPH4 plays a role in the modulation of auxin sensitivity. Such an activity could arise through direct binding of auxin or at some step removed from auxin binding (i.e. regulation of intracellular auxin signaling or auxin-dependent gene expression). These types of activities are consistent with the observed genetic and physiological phenotypes of the nph4 mutants. We are currently in the midst of a chromosome walk to clone the NPH4 locus, and in the near future would like to address these possibilities at the molecular level.
In conclusion, the results presented here demonstrate that the NPH4 locus encodes an important conditional modulator of auxin-dependent differential growth. Further analysis of this locus and the encoded protein will certainly provide insight into the basic regulation of these adaptive growth responses, and lead to a more comprehensive understanding of the coordinated regulation of cellular growth by auxins.
ACKNOWLEDGMENTS
We are grateful to Ms. Jan Wilson and Dr. Tobias Baskin for tissue embedding/sectioning/staining and for help with microscopy. We are also grateful to those who contributed materials for these studies: Dr. Mark Estelle for seed of tir5-1 (nph4-4); Dr. Kotaro Yamamoto for seed of msg1-2 (nph4-102); Dr. Pam Green for the SAUR-AC1 clone; Dr. Gretchen Hagen for the GH3 clone; Dr. Sakis Theologis for the various IAA clones; and Dr. Julie Stone for the ACT7 clone. We thank Ms. Lelia Flagg, Ms. Jamie Sommer, and Ms. Carrie Vaughn for plant care and other technical assistance. We also thank Drs. Tobias I. Baskin, Winslow R. Briggs, James A. Birchler, Tom J. Guilfoyle, Gretchen Hagen, Jane Murfett, John C. Walker, and Mr. Kevin Lease for critical reading of the manuscript.
Abbreviations:
- BL
blue light
- EMS
ethyl methanesulfonate
- RL
red light
- WL
white light
- YL
yellow light
Footnotes
This work was supported by grants from the National Science Foundation (NSF) (no. MCB-9723124), the U.S. Department of Agriculture (USDA)-National Research Initiative Competitive Grants Program (no. 9602628), and the University of Missouri Research Board (no. RB96-055) to E.L. E.L.S.-E. was supported by a predoctoral fellowship from the University of Missouri Maize Biology Training Program, a unit of the Department of Energy/NSF/USDA Collaborative Research in Plant Biology Program. R.M.H. was partially supported by the University of Missouri Food for the 21st Century Program.
LITERATURE CITED
- Abel S, Nguyen MD, Theologis A. The PS-IAA4/5-like family of early auxin inducible messenger RNAs in Arabidopsis thaliana. J Mol Biol. 1995;251:533–549. doi: 10.1006/jmbi.1995.0454. [DOI] [PubMed] [Google Scholar]
- Abel S, Theologis A. Early genes and auxin action. Plant Physiol. 1996;111:9–17. doi: 10.1104/pp.111.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel F, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Short Protocols in Molecular Biology. New York: John Wiley & Sons; 1995. [Google Scholar]
- Baskin TI, Wilson JE. Inhibitors of protein kinases and phosphatases alter root morphology and disorganize cortical microtubules. Plant Physiol. 1997;113:493–502. doi: 10.1104/pp.113.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C, Ecker JR. Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics. 1994;19:137–144. doi: 10.1006/geno.1994.1023. [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Marchant A, Green HJ, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA. Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science. 1996;273:948–950. doi: 10.1126/science.273.5277.948. [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Estelle MA, Somerville CR, Kende H. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science. 1988;241:1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- Bruggemann E, Handwerger K, Essex C, Storz G. Analysis of fast neutron-generated mutants at the Arabidopsis thaliana HY4 locus. Plant J. 1996;10:755–760. doi: 10.1046/j.1365-313x.1996.10040755.x. [DOI] [PubMed] [Google Scholar]
- Darwin C, Darwin F (1896) The Power of Movements in Plants. D Appleton and Co., New York
- Delbarre A, Muller P, Imhoff V, Guern J. Comparison of mechanisms controlling uptake and accumulation of 2,4-dichlorophenoxy acetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta. 1996;198:532–541. doi: 10.1007/BF00262639. [DOI] [PubMed] [Google Scholar]
- DuBridge RB, Calos MP. Molecular approaches to the study of gene mutation in human cells. Trends Genet. 1987;3:293–297. [Google Scholar]
- Estelle M. Plant tropisms: the ins and outs of auxin. Curr Biol. 1996;6:1589–1591. doi: 10.1016/s0960-9822(02)70780-x. [DOI] [PubMed] [Google Scholar]
- Firn RD, Digby J. The establishment of tropic curvatures in plants. Annu Rev Plant Physiol. 1980;31:131–148. [Google Scholar]
- Gendreau E, Traas J, Desnos T, Grandjean O, Caboche M, Hàfte H. Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol. 1997;114:295–305. doi: 10.1104/pp.114.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil P, Yang L, Orbovic V, Verkamp E, Poff KL, Green PJ. Characterization of the auxin inducible SAUR-AC1 gene for use as a molecular genetic tool in Arabidopsis. Plant Physiol. 1994;104:777–784. doi: 10.1104/pp.104.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman P, Ecker JR. Exploiting the triple response to identify ethylene-related mutants. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G, Kleinschmidt A, Guilfoyle T. Auxin-regulated gene expression in intact soybean hypocotyl and excised hypocotyl sections. Planta. 1984;162:147–153. doi: 10.1007/BF00410211. [DOI] [PubMed] [Google Scholar]
- Hangarter RP. Gravity, light and plant form. Plant Cell Environ. 1997;20:796–800. doi: 10.1046/j.1365-3040.1997.d01-124.x. [DOI] [PubMed] [Google Scholar]
- Hobbie L, Estelle M. Genetic approaches to auxin action. Plant Cell Environ. 1994;17:525–540. doi: 10.1111/j.1365-3040.1994.tb00147.x. [DOI] [PubMed] [Google Scholar]
- Hou Y, von Arnim AG, Deng X-W. A new class of Arabidopsis constitutive photomorphogenic genes involved in regulating cotyledon development. Plant Cell. 1993;5:329–339. doi: 10.1105/tpc.5.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huala E, Oeller PW, Liscum E, Han I-S, Larsen E, Briggs WR. Arabidopsis NPH1: a protein kinase with a putative redox-sensing domain. Science. 1997;278:2120–2123. doi: 10.1126/science.278.5346.2120. [DOI] [PubMed] [Google Scholar]
- Iino M. Kinetic modeling of phototropism in maize coleoptiles. Planta. 1987;171:110–126. doi: 10.1007/BF00395074. [DOI] [PubMed] [Google Scholar]
- Iino M. Phototropism: mechanisms and ecological implications. Plant Cell Environ. 1990;13:633–650. [Google Scholar]
- Janoudi A-K, Konjevic R, Poff KL. Time threshold for second positive phototropism is decreased by a pre-irradiation with red light. Plant Physiol. 1992;99:1422–1425. doi: 10.1104/pp.99.4.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoudi A-K, Poff KL. Characterization of adaptation in phototropism of Arabidopsis thaliana. Plant Physiol. 1991;95:517–521. doi: 10.1104/pp.95.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman PB, Wu L-L, Brock TG, Kim D. Hormones and orientation of growth. In: Davies PJ, editor. Plant Hormones. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 547–571. [Google Scholar]
- Kiss JZ, Hertel R, Sack FD. Amyloplasts are necessary for full gravitropic sensitivity in roots of Arabidopsis thaliana. Planta. 1989;177:198–206. [PubMed] [Google Scholar]
- Klee HJ, Horsch RB, Hinchee MA, Hein MB, Hoffmann NL. The effects of overproduction of two Agrobacterium tumefaciens T-DNA auxin biosynthetic gene products in transgenic petunia plants. Genes Dev. 1987;1:86–96. [Google Scholar]
- Lehman A, Black R, Ecker JR. HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell. 1996;85:183–194. doi: 10.1016/s0092-8674(00)81095-8. [DOI] [PubMed] [Google Scholar]
- Leyser HMO, Pickett FB, Dharmasiri S, Estelle M. Mutations in the AXR3 gene of Arabidopsis result in altered auxin response including ectopic expression from the SAUR-AC1 promoter. Plant J. 1996;10:403–413. doi: 10.1046/j.1365-313x.1996.10030403.x. [DOI] [PubMed] [Google Scholar]
- Leyser O. Auxin: lessons from a mutant weed. Physiol Plant. 1998;100:407–414. [Google Scholar]
- Lincoln C, Britton JH, Estelle M. Growth and development of the axr1 mutants of Arabidopsis. Plant Cell. 1990;2:1071–1080. doi: 10.1105/tpc.2.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Briggs WR. Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic stimuli. Plant Cell. 1995;7:473–485. doi: 10.1105/tpc.7.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Briggs WR. Mutations of Arabidopsis in potential transduction and response components of the phototropic signaling pathway. Plant Physiol. 1996;112:291–296. doi: 10.1104/pp.112.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Hangarter RP. Arabidopsis mutants lacking blue light-dependent inhibition of hypocotyl elongation. Plant Cell. 1991;3:685–694. doi: 10.1105/tpc.3.7.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Hangarter RP. Light-stimulated apical hook opening in wild-type Arabidopsis thaliana seedlings. Plant Physiol. 1993a;101:567–572. doi: 10.1104/pp.101.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Hangarter RP. Genetic evidence that the Pr form of phytochrome B modulates gravitropism in Arabidopsis thaliana. Plant Physiol. 1993b;103:15–19. doi: 10.1104/pp.103.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax TL, Muday GK, Rubery PH. Auxin transport. In: Davies PJ, editor. Plant Hormones. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 509–530. [Google Scholar]
- Maher EP, Martindale SJB. Mutants of Arabidopsis thaliana with altered responses to auxin and gravity. Biochem Genet. 1980;18:1041–1053. doi: 10.1007/BF00484337. [DOI] [PubMed] [Google Scholar]
- McDowell JM, Huang S, McKinney EC, An Y-Q, Meagher RB. Arabidopsis thaliana contains ten actin genes encoding six ancient protein subclasses. Genetics. 1996;142:587–602. doi: 10.1093/genetics/142.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza JI, Olsen GM, Iversen T-H, Maher EP. The growth and gravitropic responses of wild-type and auxin-resistant mutants of Arabidopsis thaliana. Physiol Plant. 1984;60:516–522. doi: 10.1111/j.1399-3054.1984.tb04921.x. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Okada K, Shimura Y. Reversible root tip rotation in Arabidopsis seedlings induced by obstacle-touching stimulus. Science. 1990;250:274–276. doi: 10.1126/science.250.4978.274. [DOI] [PubMed] [Google Scholar]
- Orbovic V, Poff KL. Kinetics for phototropic curvature by etiolated seedlings of Arabidopsis thaliana. Plant Physiol. 1991;97:1470–1475. doi: 10.1104/pp.97.4.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JH. Epinasty, hyponasty, and related topics. In: Pharis RP, Reid DM, editors. Encyclopedia of Plant Physiology. Berlin: Springer-Verlag; 1985. pp. 139–168. [Google Scholar]
- Pickett FB, Wilson AK, Estelle M. The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol. 1990;94:1462–1466. doi: 10.1104/pp.94.3.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rédei G, Koncz C. Classical mutagenesis. In: Koncz C, Chua N-H, Schell J, editors. Methods in Arabidopsis Research. Singapore: World Scientific; 1992. pp. 16–82. [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano CP, Robson PRH, Smith H, Estelle M, Klee H. Transgene-mediated auxin overproduction in Arabidopsis: hypocotyl elongation phenotype and interactions with the hy6-1 hypocotyl elongation and axr1 auxin-resistant mutants. Plant Mol Biol. 1995;27:1071–1083. doi: 10.1007/BF00020881. [DOI] [PubMed] [Google Scholar]
- Rouse D, Mackay P, Strinberg P, Estelle M, Leyser O. Changes in auxin response from mutations in an AUX/IAA gene. Science. 1998;279:1371–1373. doi: 10.1126/science.279.5355.1371. [DOI] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M. Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell. 1997;9:745–757. doi: 10.1105/tpc.9.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk WH, Erickson RO. Kinematics of hypocotyl curvature. Am J Bot. 1978;65:310–319. [Google Scholar]
- Steinitz B, Poff KL. A single positive phototropic response induced with pulsed light in hypocotyls of Arabidopsis thaliana seedlings. Planta. 1986;168:305–315. doi: 10.1007/BF00392354. [DOI] [PubMed] [Google Scholar]
- Steinitz B, Ren Z, Poff KL. Blue and green light-induced phototropism in Arabidopsis thaliana and Lactuca sativa L. seedlings. Plant Physiol. 1985;77:248–251. doi: 10.1104/pp.77.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpte C, Lincoln C, Pickett FB, Turner J, Estelle M. The AXR1 and AUX1 genes of Arabidopsis function in separate auxin-response pathways. Plant J. 1995;8:561–569. doi: 10.1046/j.1365-313x.1995.8040561.x. [DOI] [PubMed] [Google Scholar]
- Trewavas AJ. What remains of the Cholodny-Went theory? Plant Cell Environ. 1992;15:761–794. [PubMed] [Google Scholar]
- Watahiki MK, Yamamoto KT. The massugu1 mutation of Arabidopsis identified with failure of auxin-induced growth curvature of hypocotyl confers auxin insensitivity to hypocotyl and leaf. Plant Physiol. 1997;115:419–426. doi: 10.1104/pp.115.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Went FW, Thimann KV (1937) Phytohormones. Macmillan, New York
- Yamamoto M, Yamamoto KT. Differential effects of 1-naphthaleneacetic acid, indole-3-acetic acid and 2,4-dichlorophenoxyacetic acid on the gravitropic response of roots in an auxin-resistant mutant of Arabidopsis, aux1. Plant Cell Physiol. 1998;39:660–664. doi: 10.1093/oxfordjournals.pcp.a029419. [DOI] [PubMed] [Google Scholar]
- Young JC, Liscum E, Hangarter RP. Spectral-dependence of light-inhibited hypocotyl elongation in photomorphogenic mutants of Arabidopsis: evidence for a UV-A photosensor. Planta. 1992;188:106–114. doi: 10.1007/BF00198946. [DOI] [PubMed] [Google Scholar]