Abstract
The combination of radiation therapy and immunotherapy holds enticing promise as a strategy for cancer treatment. Preclinical studies have shown that radiation may act synergistically with immunotherapy to enhance or broaden antitumor immune responses, in part due to radiation-induced phenotypic alterations of tumor cells that render them more susceptible to immune-mediated killing. Clinical trials employing the combination of therapeutic vaccines with radiation have confirmed many of these findings, and clinical endpoint human studies are both ongoing and planned. This review examines a) the evidence that radiation induces immunologic death, b) the mechanisms by which radiation therapy can induce or augment antitumor immune responses, and c) translational studies demonstrating that immunotherapy can be effectively combined with radiation therapy. Finally, we review recent and current clinical trials combining radiation therapy with immunotherapy.
Keywords: therapeutic vaccine, radiotherapy, cancer, immunopotentiation
Introduction
Conventional cancer therapies can have numerous toxicities. While they kill cancer cells, they also destroy normal hematopoietic, endothelial, and stromal cells. Immunotherapy offers a unique approach in cancer therapeutics because it utilizes the immune system to specifically identify tumor cells via expression of tumor antigen, and is therefore less toxic. In spite of this, immunotherapies as single agents, when given in the setting of high tumor burden, have proved less than satisfactory in providing clinical benefit. This is likely a result of multiple factors. Prior chemotherapy can compromise the immune system’s ability to generate a robust immune response. In addition, tumor cells can negatively affect T cells by rendering them inactive and limiting cell division, cell differentiation, and cytokine production, leading to T-cell anergy [1]. T-cell function and penetration into the tumor are further affected by the tumor vasculature and interstitial tumor pressure. Moreover, the tumor microenvironment can downregulate the expression of major histocompatibility complex (MHC) class I molecules and tumor-associated antigens (TAAs) and deplete suppressive cells and immunosuppressive cytokines, thus greatly decreasing T cell-mediated immune recognition and tumor attack.
Radiation therapy may be able to overcome avenues of escape from immune recognition. Local tumor radiation is standard care for many cancers because of its direct cytotoxic effect. Due to toxicity restraints, some individual tumor cells within a tumor mass may receive a sublethal dose of radiation. This dose, however, may be capable of inducing multiple changes within the cell, facilitating recognition of the cell by the immune system and immune-mediated killing.
Radiation therapy induces immunologic cell death
Radiation treatment that is lethal to tumor cells can modulate the immune system’s ability to activate effective antigen presentation by inducing recognition and phagocytosis signals for dendritic cells (DCs) [2]. First, antigens released by dying irradiated tumor cells can be taken up, processed, and presented by antigen-presenting cells (APCs) in the microenvironment and draining lymph nodes. Second, dying tumor cells release a specific “danger” signal that acts upon DCs to stimulate antigen processing and presentation to T cells [3, 4].
A study by Nesslinger et al. showed that standard cancer treatments can induce antigen-specific immune responses. They examined serum samples from men with prostate cancer who had been treated with definitive radiation therapy or surgery. The men who received brachytherapy or external beam radiation therapy developed immune serological changes with new antibody responses after treatment, as measured by Western blot analysis (4 of 29 and 5 of 20 respectively), whereas none of those treated with radical prostatectomy (n = 14) had such responses. Equally important, responses were seen within 4 to 9 months of treatment and observed across the range of disease groups [5].
Another clinical study recently demonstrated that radiation increases tumor-specific T-cell reactivity in patients with both colorectal cancer (CRC) and prostate cancer [6]. Schaue et al. [6] evaluated the level of CD8+ T cells specific for the TAA survivin, which is overexpressed in both cancer types. Lymphocytes were collected before, during, and after radiotherapy. In the CRC group, 9 of 13 patients showed an increase in the percent of survivin-specific CD8+ T cells, while 7 of 11 prostate cancer patients had an increase in survivin-specific CD8+ T cells. Biopsy samples from CRC patients whose tumors were downstaged following radiation expressed more survivin and had more promising increases in survivin-specific CD8+ T cells than patients whose tumors were not downstaged. Thus, this study demonstrated that radiation does not induce tolerance to survivin, but rather is associated with induction or augmentation of TAA immune responses.
Local radiation has also been reported to affect metastatic sites distant from the site of radiation in a phenomenon called the “abscopal effect” [3]. Among a cohort of 28 patients with metastatic renal cell carcinoma in which some were treated with stereotactic radiotherapy, the abscopal effect was seen in 4 patients who displayed regression of nonradiated lesions [7]. It should be noted that no patient was being treated concomitantly with systemic therapy.
While the exact mechanism of this phenomenon remains unclear, it is thought to be immunologically mediated. Taken together, these data provide evidence that cellular and humoral immune responses can be seen following radiation alone. However, these relatively low-level immune responses are suboptimal for causing clinically significant antitumor activity in the majority of cases.
Radiation therapy induces immunotherapy-potentiating phenotypic changes
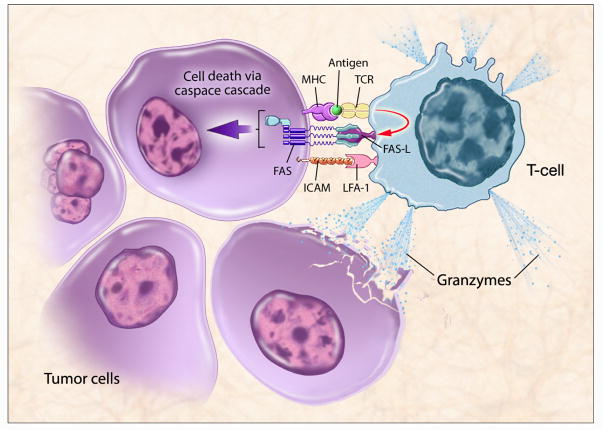
Immune-mediated tumor killing requires functional tumor-specific T cells to travel to the tumor, recognize the tumor, and kill the tumor. Recognition of tumor by CD8+ cytotoxic T lymphocytes (CTLs) occurs when the T-cell receptor binds to an MHC molecule with a tumor-specific peptide in its cleft. Killing is generally done via the Fas pathway or through the local release of granzymes (Figure 1). Tumor cells often evade the host immune system because they do not adequately express the MHC molecules necessary for antigen processing and presentation. MHC class I provides the basis for tumor-antigen recognition by CTLs. Therefore, upregulation of MHC molecules or TAAs makes it easier for T cells to recognize tumor. The Fas receptor is a mediator of apoptosis and is involved in CTL killing [8]. When Fas ligand on CTLs binds to the Fas on tumor cells, it causes trimerization of the Fas membrane protein, triggering an apoptotic cascade that leads to tumor-cell death. Thus upregulation of Fas on the tumor facilitates immune-mediated tumor lysis. Additionally, intercellular adhesion molecules such as ICAM-1 increase immune recognition by providing signals for T-cell trafficking to the tumor site. Sabzevari et al. also showed in vitro that upregulating ICAM-1 on tumor cells resulted in improved ability of antitumor CTLs to lyse tumor cells [9].
Figure 1. T cell-mediated killing of tumor cells.
T-cell recognition requires an MHC molecule with a T cell-specific tumor-associated antigen (TAA) peptide in its cleft. This triggers immune-mediated killing either through Fas or the release of granzymes. Radiation-induced upregulation of MHC, TAA, ICAM and Fas on the tumor cell can facilitate immune-mediated killing. (Figure courtesy of NIH Medical Arts.)
Radiation has the ability to alter nonlethally radiated tumor cells and tissues in a way that stimulates immune-cell recognition and killing of the tumor and may also facilitate immune-cell trafficking to the tumor. Indeed, sublethal doses of ionizing radiation have the ability to alter the phenotype of target tissue by upregulating immune modulators and rendering tumor cells more susceptible to immune T-cell attack (Table 1). In a study by Garnett et al., 23 human carcinoma cell lines (12 colon, 7 lung, and 4 prostate) were analyzed for their response to low-dose, nonlytic radiation (either 10 or 20 Gy). It was shown that radiation changes the expression of Fas (CD95), MHC class I, and other cell surface molecules, specifically ICAM-1 and TAAs such as carcinoembryonic antigen (CEA) and mucin-1 (MUC-1) [10]. Radiation affected 21 of 23 cell lines by upregulating one or more surface molecules that facilitate T cell-mediated recognition or killing of tumor. It has also been shown that radiation can improve T-cell trafficking to tumor by upregulating chemokines [11].
Table 1.
Radiation augments immune-mediated killing.
| Effects of radiation | T-cell response |
|---|---|
| ↑ chemokines, adhesion molecules | ↑ Migration |
| ↑ MHC, peptides/TAAs | ↑ Recognition |
| ↑ Fas, adhesion molecules | ↑ Killing |
A study by Reits et al. [12] showed that expression of MHC class I molecules was upregulated in a radiation dose-dependent manner, and that the intracellular peptide repertoire increased following radiation. Initially, a pool of intracellular peptides was created as a result of increased degradation of proteins. Then, activation of the mTOR pathway led to increased protein translation, subsequent peptide production, and creation of a new peptide repertoire. The results of this study provide evidence that targeted radiotherapy can improve the overall efficacy of immunotherapy and augment the immunological response.
Strontium-89 and samarium-153 (153Sm) are FDA-approved bone-seeking radionuclides frequently used as palliative treatment of pain caused by metastatic lesions to bone. This form of radiotherapy has also been shown to cause phenotypic modulation of tumor cells [13]. In a recently reported study, 10 human tumor-cell lines representing tumors that metastasize to bone (4 prostate, 2 breast, and 4 lung) were exposed to clinically equivalent palliative doses of 153Sm-EDTMP for 4 days. Expression of several cell surface molecules and TAAs was examined by flow cytometry and PCR. Of the 10 cell lines, 100% upregulated Fas, 90% upregulated CEA, 60% upregulated MUC-1, 50% upregulated MHC class I, and 40% upregulated ICAM-1. These results show that exposure to 153Sm-EDTMP induces phenotypic changes in tumor cells, rendering them more susceptible to T-cell immune-mediated killing [13].
The studies described above demonstrate radiation’s ability to upregulate MHC molecules, TAAs, adhesion molecules, and Fas expression on tumor cells, which can make the immune system more proficient at recognizing and destroying cancer cells. These findings have led to in vivo studies combining vaccine and radiation.
In vivo studies of vaccine plus radiation
In vivo preclinical studies have shown that the antitumor effects of the combination of external beam radiation and vaccine are greater than those of single-agent therapies. Chakraborty et al. studied CEA transgenic mice and a murine carcinoma cell line transfected with CEA. Mice were given a vaccine expressing CEA and a TRIad of COstimulatory Molecules (B7-1, ICAM-1, and LFA-3; TRICOM) along with low-dose radiation (8 Gy). Mice receiving the combination treatment had a 50% reduction in tumor mass and massive infiltration of T cells, while vaccine or radiation given as a single modality was ineffective [14]. The regimen used in this study upregulated Fas on tumor cells, leading to improved vaccine-mediated killing of tumor. Additionally, Chakraborty et al. demonstrated a subsequent broadening of the immune response, referred to as antigen cascade, whereby mice cured of tumors developed CD4+ and CD8+ T-cell responses not only to CEA, but also to p53 and gp70, antigens that are also overexpressed by tumor cells. Interestingly, the vaccine did not contain p53 or gp70, and the immune response to gp70 was greater than the response to CEA peptide, indicating that antigen cascade serves as a critical step in the immune response.
Chakraborty et al. also examined the effect of a radiolabeled monoclonal antibody (mAb) on tumor-cell phenotype [15]. CEA transgenic mice were injected with MC38-CEA+ tumor cells, then treated with an anti-CEA mAb labeled with yttrium-90, either alone or in combination with the above-described CEA/TRICOM vaccine. In mice that received no treatment, tumors grew progressively (average tumor volume on day 28 was 3311 mm3). Moreover, 100% of the animals died by day 30. In mice treated with vaccine alone, the vaccination regimen did not significantly inhibit tumor growth. Radiolabeled mAb given as a monotherapy significantly inhibited tumor growth compared to no treatment. However, treating tumors with a combination of vaccine and radiolabeled mAb resulted in a marked decrease in tumor growth rate and tumor volume. Moreover, mice treated with the combination therapy not only showed delayed tumor progression, but 20% experienced a resolution of tumor mass by endogenous host response and remained tumor-free for the duration of the experiment (77 days). In mice transplanted with MC38-CEA+ tumor cells that were defective in Fas signaling, treatment with vaccine and radiolabeled mAb failed to mediate tumor regression, defining the crucial role of the Fas signaling pathway in the murine model. As reported in other studies [14, 16], T-cell responses were detected not only to the CEA encoded in the vaccine vector, but also to other tumor antigens, including p53 and gp70. These and other peripheral tumor antigens can enter the MHC class I pathway via antigen presentation and cross priming to affect CD8+ T cells [17]. This study also showed that there was no increase in apoptosis of antigen-specific tumor-infiltrating lymphocytes in mice treated with vaccine and radiolabeled mAb compared with vaccine alone. This study is consistent with another study which showed that memory T cells are more resistant to radiation than naïve T cells [18].
In a study by Sega et al., mice with subcutaneous 300 mm3 tumors were treated with a folate-targeted hapten immunotherapy (FTHI) concurrent with interleukin (IL)-2, interferon-α, and low-dose radiotherapy (3 Gy). An analysis of the tumor microenvironment revealed that mice treated with the combination therapy had large increases in immune cell infiltration—specifically CD4+ T cells, CD8+ T cells, and macrophages—compared with either modality alone. A significant decrease in tumor volume was observed with FTHI, cytokines, and low-dose radiation therapy (P = 0.004 compared to either modality alone) [19]. Furthermore, the growth rate of nonradiated tumor was significantly less in mice treated with the combination therapy than in mice treated with radiation alone. Finally, inhibition of primary tumor growth caused by the combination therapy was significantly greater than the sum of the 2 individual therapies (FTHI alone or radiation alone), indicating that the 2 treatment modalities may work synergistically.
Clinical trials
The combination of radiation and vaccine has been explored in several clinical trials (Table 2). One recent study in men with localized prostate cancer used a recombinant cancer vaccine combined with standard definitive radiation therapy to determine if vaccine could induce an immune response in the presence of tumor irradiation [20]. The trial was designed as a randomized phase II study, with the primary endpoint of immunologic response and secondary endpoints of safety and clinical response. Nineteen patients received the combination of vaccine plus radiation and 11 patients received radiation alone. Patients in the combination arm received a priming vaccine of recombinant vaccinia (rV) expressing prostate-specific antigen (rV-PSA) admixed with rV expressing the costimulatory molecule B7-1. This was followed by monthly boosts with recombinant fowlpox (rF)-PSA. The vaccines were administered with local granulocyte-macrophage colony-stimulating factor and low-dose systemic IL-2. Standard external beam radiation was given between the fourth and sixth vaccinations. The 13 of 17 patients in the combination arm who received the full 8 vaccinations showed a 3-fold increase in circulating PSA-specific T cells. In the radiation-alone arm, no detectable increases in PSA-specific cells were observed (P < 0.0005). This study also demonstrated that patients receiving both the vaccine and radiation had evidence of de novo generation of T cells to prostate-associated antigens not present in the vaccine, providing further evidence of immune-mediated tumor killing via antigen cascade. Immunological responses (including antigen cascade) were seen almost entirely in the combination treatment arm, indicating that vaccine and radiation together are much more efficient at generating immunity than radiation alone. Lechleider et al. conducted a follow-up study using the same vaccine regimen and radiation schedule described above, with a metronomic dose of IL-2 (0.6 MIU/M2), and reported similar immune-mediated activity with less toxicity than the higher dose of IL-2 used previously [21].
Table 2.
Clinical trials showing that radiation enhances immune response.
| Study | Cancer type | Treatment | Results |
|---|---|---|---|
| Nesslinger [5] | Prostate | Radiation or hormonal | Development of new antibodies. |
| Schaue [6] | Colorectal and prostate | Radiation alone | Increase in TAA (survivin)-specific CD8+ cells. |
| Wersall [7] | Renal | Radiation alone | Evidence of absopal effect; regression of nonradiated tumor lesions |
| Gulley [20] | Prostate | Combination therapy: radiation + vaccine | Increase in PSA-specific T cells. Evidence of antigen cascade (de novo generation of antigens not found in vaccine). |
| Okawa [22] | Cervical | Combination therapy: radiation + vaccine | Tumor reduction and histological changes. |
| Chi [23] | Hepatoma | Combination therapy: radiation + vaccine | Increase in natural killer cell activity. Increase in TAA (alpha-fetoprotein)-specific immune response. |
| Gulley [20] | Prostate | Combination therapy: radionuclide + vaccine | Preliminary data: phenotypic changes in tumor cells. |
Okawa et al. conducted a phase II randomized study of LC9018 (a biologic response modifier prepared from heat-killed Lactobacillus casei YTTT018) and radiation in patients with carcinoma of the uterine cervix. Patients were randomized to receive radiation alone or radiation plus vaccine. The combination therapy demonstrated significant tumor reduction and histological changes compared with radiation alone at cumulative doses of 15 and 30 Gy [22]. This study suggests that these treatment modalities, when used together, produce an enhanced immunotherapeutic response.
In a phase I study, patients with advanced hepatoma were given 8 Gy of radiation, followed 2 days later by an intratumoral injection of autologous immature DCs. Of 10 patients evaluated for immune response, 6 showed increased natural killer cell activity, 8 had increases in alpha-fetoprotein (AFP)-specific immune responses by cytokine-release assay, and 7 showed increased AFP-specific immune responses by ELISPOT. Of 14 patients enrolled, 4 had minor responses and 2 additional patients had partial responses, including a patient who had a decrease in AFP from 128 ng/mL to 1.6 ng/mL. These results demonstrate that the treatment was safe and could induce tumor-specific T-cell immunity [23]. While all patients in this study had the combination of intratumoral DCs and radiation, recent preclinical data by the same group have demonstrated that while intratumoral DCs inhibited tumor growth, the combination of intratumoral DCs plus radiation significantly inhibited tumor growth compared with intratumoral DCs without radiation [24]. There was no decrease in average tumor size with radiation alone, compared with control. Furthermore, in a rechallenge experiment, only the combination of intratumoral DCs and radiation led to long-term tumor-free mice compared with radiation alone, indicating induction of a memory response.
An ongoing trial at the National Cancer Institute is evaluating the combination of radionuclide and vaccine in patients with castrate-resistant prostate cancer metastatic to bone. Patients are given 153Sm lexidronam (Quadramet®), a bone-seeking radionuclide, alone or in combination with vaccine containing PSA plus the 3 costimulatory molecules B7-1, ICAM-1, and LFA-3 (PSA-TRICOM) [25]. As mentioned earlier, preclinical studies have demonstrated that the FDA-approved dose of Quadramet® delivers a sufficient amount of radiation to induce immunomodulating phenotypic changes in tumor cells [13]. This study is currently enrolling patients.
Conclusions
Radiation from a variety of different sources can not only induce tumor-cell death in a manner consistent with antitumor immune activation, but can also phenotypically modify the cells not killed in a way that facilitates both immune recognition and immune-mediated killing. This ability of radiation to lower the threshold for immune-mediated killing lies at the heart of the synergy seen with combination therapies in preclinical models. Immune-mediated killing of tumor cells can lead to an antigen cascade (also known as epitope spreading) in which the immune system becomes activated to multiple tumor antigens that may be more relevant and more immunologically potent than those found in the vaccine. This phenomenon has been identified both in murine models and in clinical trials.
Capitalizing on the immunological effects induced by radiation treatment by adding potent antitumor vaccines may lead to synergistic approaches to cancer management that offer feasible, well-tolerated therapeutic options for cancer patients. Randomized clinical endpoint studies comparing the combination of radiation plus immunotherapy with radiation alone are ongoing [25] or planned.
Acknowledgments
The authors acknowledge the expert editorial assistance of Bonnie L. Casey in the preparation of this manuscript.
References
- 1.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 2.Hodge JW, Guha C, Neefjes J, Gulley JL. Synergizing radiation therapy and immunotherapy for curing incurable cancers. Opportunities and challenges. Oncology (Williston Park) 2008;22(9):1064–1070. discussion 1075, 1080–1061, 1084. [PMC free article] [PubMed] [Google Scholar]
- 3.Demaria S, Bhardwaj N, McBride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys. 2005;63(3):655–666. doi: 10.1016/j.ijrobp.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tesniere A, Apetoh L, Ghiringhelli F, Joza N, Panaretakis T, Kepp O, Schlemmer F, Zitvogel L, Kroemer G. Immunogenic cancer cell death: a key-lock paradigm. Curr Opin Immunol. 2008;20(5):504–511. doi: 10.1016/j.coi.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Nesslinger NJ, Sahota RA, Stone B, Johnson K, Chima N, King C, Rasmussen D, Bishop D, Rennie PS, Gleave M, Blood P, et al. Standard treatments induce antigen-specific immune responses in prostate cancer. Clin Cancer Res. 2007;13(5):1493–1502. doi: 10.1158/1078-0432.CCR-06-1772. [DOI] [PubMed] [Google Scholar]
- 6.Schaue D, Comin-Anduix B, Ribas A, Zhang L, Goodglick L, Sayre JW, Debucquoy A, Haustermans K, McBride WH. T-cell responses to survivin in cancer patients undergoing radiation therapy. Clin Cancer Res. 2008;14(15):4883–4890. doi: 10.1158/1078-0432.CCR-07-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wersall PJ, Blomgren H, Pisa P, Lax I, Kalkner KM, Svedman C. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol. 2006;45(4):493–497. doi: 10.1080/02841860600604611. [DOI] [PubMed] [Google Scholar]
- 8.Kojima H, Shinohara N, Hanaoka S, Someya-Shirota Y, Takagaki Y, Ohno H, Saito T, Katayama T, Yagita H, Okumura K, et al. Two distinct pathways of specific killing revealed by perforin mutant cytotoxic T lymphocytes. Immunity. 1994;1(5):357–364. doi: 10.1016/1074-7613(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 9.Slavin-Chiorini DC, Catalfamo M, Kudo-Saito C, Hodge JW, Schlom J, Sabzevari H. Amplification of the lytic potential of effector/memory CD8+ cells by vector-based enhancement of ICAM-1 (CD54) in target cells: implications for intratumoral vaccine therapy. Cancer Gene Ther. 2004;11(10):665–680. doi: 10.1038/sj.cgt.7700741. [DOI] [PubMed] [Google Scholar]
- 10.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64(21):7985–7994. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 11.Zong ZW, Cheng TM, Su YP, Ran XZ, Li N, Ai GP, Xu H. Crucial role of SDF-1/CXCR4 interaction in the recruitment of transplanted dermal multipotent cells to sublethally irradiated bone marrow. J Radiat Res (Tokyo) 2006;47(3–4):287–293. doi: 10.1269/jrr.0531. [DOI] [PubMed] [Google Scholar]
- 12.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, Griekspoor A, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborty M, Wansley EK, Carrasquillo JA, Yu S, Paik CH, Camphausen K, Becker MD, Goeckeler WF, Schlom J, Hodge JW. The use of chelated radionuclide (samarium-153-ethylenediaminetetramethylenephosphonate) to modulate phenotype of tumor cells and enhance T cell-mediated killing. Clin Cancer Res. 2008;14(13):4241–4249. doi: 10.1158/1078-0432.CCR-08-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64(12):4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 15.Chakraborty M, Gelbard A, Carrasquillo JA, Yu S, Mamede M, Paik CH, Camphausen K, Schlom J, Hodge JW. Use of radiolabeled monoclonal antibody to enhance vaccine-mediated antitumor effects. Cancer Immunol Immunother. 2008;57(8):1173–1183. doi: 10.1007/s00262-008-0449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudo-Saito C, Schlom J, Hodge JW. Induction of an antigen cascade by diversified subcutaneous/intratumoral vaccination is associated with antitumor responses. Clin Cancer Res. 2005;11(6):2416–2426. doi: 10.1158/1078-0432.CCR-04-1380. [DOI] [PubMed] [Google Scholar]
- 17.Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, Frelinger JA, Robinson BW. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170(10):4905–4913. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 18.Grayson JM, Harrington LE, Lanier JG, Wherry EJ, Ahmed R. Differential sensitivity of naive and memory CD8+ T cells to apoptosis in vivo. J Immunol. 2002;169(7):3760–3770. doi: 10.4049/jimmunol.169.7.3760. [DOI] [PubMed] [Google Scholar]
- 19.Sega EI, Lu Y, Ringor M, Leamon CP, Low PS. Low-dose radiation potentiates the therapeutic efficacy of folate receptor-targeted hapten therapy. Int J Radiat Oncol Biol Phys. 2008;71(2):559–566. doi: 10.1016/j.ijrobp.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, Tsang KY, Yokokawa J, Hodge JW, Menard C, Camphausen K, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11(9):3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 21.Lechleider RJ, Arlen PM, Tsang KY, Steinberg SM, Yokokawa J, Cereda V, Camphausen K, Schlom J, Dahut WL, Gulley JL. Safety and immunologic response of a viral vaccine to prostate-specific antigen in combination with radiation therapy when metronomic-dose interleukin 2 is used as an adjuvant. Clin Cancer Res. 2008;14(16):5284–5291. doi: 10.1158/1078-0432.CCR-07-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okawa T, Kita M, Arai T, Iida K, Dokiya T, Takegawa Y, Hirokawa Y, Yamazaki K, Hashimoto S. Phase II randomized clinical trial of LC9018 concurrently used with radiation in the treatment of carcinoma of the uterine cervix. Its effect on tumor reduction and histology. Cancer. 1989;64(9):1769–1776. doi: 10.1002/1097-0142(19891101)64:9<1769::aid-cncr2820640902>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 23.Chi KH, Liu SJ, Li CP, Kuo HP, Wang YS, Chao Y, Hsieh SL. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J Immunother. 2005;28(2):129–135. doi: 10.1097/01.cji.0000154248.74383.5e. [DOI] [PubMed] [Google Scholar]
- 24.Wang YS, Tsang YW, Chi CH, Chang CC, Chu RM, Chi KH. Synergistic anti-tumor effect of combination radio- and immunotherapy by electro-gene therapy plus intra-tumor injection of dendritic cells. Cancer Lett. 2008;266(2):275–285. doi: 10.1016/j.canlet.2008.02.063. [DOI] [PubMed] [Google Scholar]
- 25.National Cancer Institute. Clinical Trials at NIH. A Randomized Phase 2.5 Study of [153]Sm-EDTMP (Quadramet) With or Without a PSA TRICOM Vaccine in Men With Androgen-Insensitive Metastatic Prostate Cancer. http://bethesdatrials.cancer.gov/clinical-research/search_detail.aspx?ProtocolID=NCI-07-C-0106.