Abstract
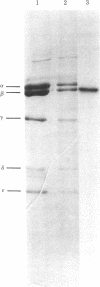
An analog of ADP containing an azido group at the C-2 position of the purine ring has been synthesized and used as an affinity probe of the membrane-bound coupling factor 1 of spinach chloroplast thylakoid membranes. The 2-azido-ADP inhibited light-induced dark binding of ADP at the tight nucleotide binding site on the thylakoid membranes. The 2-azido-ADP itself bound tightly to the thylakoid membranes, with 1 μM as the concentration giving 50% maximum binding. Tight binding of the analog required the thylakoid membranes to be energized, and the nucleotide remained bound after repeated washings of the membranes. The maximum extent of tight binding of the analog (1,2-1.3 nmol/mg of chlorophyll) was stoichiometric with the known coupling factor 1 content of thylakoid membranes but somewhat higher than that observed for ADP (0.5-0.9 nmol per mg of chlorophyll). Tight binding of 2-azido-ADP was decreased by the simultaneous addition of ADP. UV photolysis of washed thylakoid membranes containing tightly-bound 2-azido-[β-32P]ADP resulted in the covalent incorporation of label into the membranes. Isolation of the chloroplast coupling factor 1 from these membranes followed by NaDodSO4 gel electrophoresis demonstrated that the analog was covalently bound to the β subunit of the coupling factor complex.
Keywords: ATPase, adenine nucleotide analog
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird B. A., Hammes G. G. Structure of oxidative- and photo-phosphorylation coupling factor complexes. Biochim Biophys Acta. 1979 Jul 3;549(1):31–53. doi: 10.1016/0304-4173(79)90017-x. [DOI] [PubMed] [Google Scholar]
- Bruist M. F., Hammes G. G. Further characterization of nucleotide binding sites on chloroplast coupling factor one. Biochemistry. 1981 Oct 27;20(22):6298–6305. doi: 10.1021/bi00525a003. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Holowka D. A., Hammes G. G. Interaction of photoreactive and fluorescent nucleotides with chloroplast coupling factor 1. Biochemistry. 1979 Aug 7;18(16):3452–3457. doi: 10.1021/bi00583a003. [DOI] [PubMed] [Google Scholar]
- Cosson J. J., Guillory R. J. The use of arylazido-beta-alanyl-ATP as a photoaffinity label for the isolated and membrane-bound mitochondrial ATPase complex. J Biol Chem. 1979 Apr 25;254(8):2946–2955. [PubMed] [Google Scholar]
- Cusack N. J., Born G. V. Inhibition of adenosine deaminase and of platelet aggregation by 2-azidoadenosine, a photolysable analogue of adenosine. Proc R Soc Lond B Biol Sci. 1976 May 18;193(1112):307–311. doi: 10.1098/rspb.1976.0048. [DOI] [PubMed] [Google Scholar]
- Dunham K. R., Selman B. R. Regulation of spinach chloroplast coupling factor 1 ATPase activity. J Biol Chem. 1981 Jan 10;256(1):212–218. [PubMed] [Google Scholar]
- Dunn S. D., Futai M. Reconstitution of a functional coupling factor from the isolated subunits of Escherichia coli F1 ATPase. J Biol Chem. 1980 Jan 10;255(1):113–118. [PubMed] [Google Scholar]
- Dunn S. D., Heppel L. A. Properties and functions of the subunits of the Escherichia coli coupling factor ATPase. Arch Biochem Biophys. 1981 Sep;210(2):421–436. doi: 10.1016/0003-9861(81)90206-x. [DOI] [PubMed] [Google Scholar]
- Esch F. S., Allison W. S. Identification of a tyrosine residue at a nucleotide binding site in the beta subunit of the mitochondrial ATPase with p-fluorosulfonyl[14C]-benzoyl-5'-adenosine. J Biol Chem. 1978 Sep 10;253(17):6100–6106. [PubMed] [Google Scholar]
- Harris D. A., Gomez-Fernandez J. C., Klungsøyr L., Radda G. K. Specificity of nucleotide binding and coupled reactions utilising the mitochondrial ATPase. Biochim Biophys Acta. 1978 Dec 7;504(3):364–383. doi: 10.1016/0005-2728(78)90060-9. [DOI] [PubMed] [Google Scholar]
- Harris D. A., Slater E. D. Tightly bound nucleotides of the energy-transducing ATPase of chloroplasts and their role in photophosphorylation. Biochim Biophys Acta. 1975 May 15;387(2):335–348. doi: 10.1016/0005-2728(75)90114-0. [DOI] [PubMed] [Google Scholar]
- Harris D. A. The interactions of coupling ATPases with nucleotides. Biochim Biophys Acta. 1978 Mar 10;463(3-4):245–273. doi: 10.1016/0304-4173(78)90002-2. [DOI] [PubMed] [Google Scholar]
- Khym J. X. An analytical system for rapid separation of tissue nucleotides at low pressures on conventional anion exchangers. Clin Chem. 1975 Aug;21(9):1245–1252. [PubMed] [Google Scholar]
- Kozlov I. A., Milgrom Y. M. The non-catalytic nucleotide-binding site of mitochondrial ATPase is localised on the alpha-subunit(s) of factor F1. Eur J Biochem. 1980 May;106(2):457–462. doi: 10.1111/j.1432-1033.1980.tb04592.x. [DOI] [PubMed] [Google Scholar]
- Kumar G., Kalra V. K., Brodie A. F. Affinity labeling of coupling factor-latent ATPase from Mycobacterium phlei with 2',3'-dialdehyde derivatives of adenosine 5'-triphosphate and adenosine 5'-diphosphate. J Biol Chem. 1979 Mar 25;254(6):1964–1971. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lunardi J., Lauquin G. J., Vignais P. V. Interaction of azidonitrophenylaminobutyryl--ADP, a photoaffinity ADP analog, with mitochondrial adenosine triphosphatase. Identification of the labeled subunits. FEBS Lett. 1977 Aug 15;80(2):317–323. doi: 10.1016/0014-5793(77)80466-3. [DOI] [PubMed] [Google Scholar]
- MICHELSON A. M. SYNTHESIS OF NUCLEOTIDE ANHYDRIDES BY ANION EXCHANGE. Biochim Biophys Acta. 1964 Sep 11;91:1–13. doi: 10.1016/0926-6550(64)90164-1. [DOI] [PubMed] [Google Scholar]
- Macfarlane D. E., Mills D. C., Srivastava P. C. Binding of 2-azidoadenosine [beta-32P]diphosphate to the receptor on intact human blood platelets which inhibits adenylate cyclase. Biochemistry. 1982 Feb 2;21(3):544–549. doi: 10.1021/bi00532a020. [DOI] [PubMed] [Google Scholar]
- Magnusson R. P., McCarty R. E. Illumination of chloroplast thylakoids in the presence of ATP causes the binding of ADP to one of the large subunits of coupling factor 1. Biochem Biophys Res Commun. 1976 Jun 21;70(4):1283–1289. doi: 10.1016/0006-291x(76)91041-x. [DOI] [PubMed] [Google Scholar]
- Nelson N. Structure and function of chloroplast ATPase. Biochim Biophys Acta. 1976 Nov 30;456(3-4):314–338. doi: 10.1016/0304-4173(76)90003-3. [DOI] [PubMed] [Google Scholar]
- Ohta S., Tsubo M., Oshima T., Yoshida M., Kagawa Y. Nucleotide binding to isolated alpha and beta subunits of proton translocating adenosine triphosphatase studied with circular dichroism. J Biochem. 1980 Jun;87(6):1609–1617. doi: 10.1093/oxfordjournals.jbchem.a132904. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Mitochondrial ATPase. Adv Enzymol Relat Areas Mol Biol. 1979;49:223–280. doi: 10.1002/9780470122945.ch6. [DOI] [PubMed] [Google Scholar]
- Rosen G., Gresser M., Vinkler C., Boyer P. D. Assessment of total catalytic sites and the nature of bound nucleotide participation in photophosphorylation. J Biol Chem. 1979 Nov 10;254(21):10654–10661. [PubMed] [Google Scholar]
- Roy H., Moudrianakis E. N. Synthesis and discharge of the coupling factor.adenosine diphosphate complex in spinach chloroplast lamellae. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2720–2724. doi: 10.1073/pnas.68.11.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGER M. F., GUSS J. K. The dependence of reactions catalyzed by polynucleotide phosphorylase on oligonucleotides. J Biol Chem. 1962 Jan;237:182–189. [PubMed] [Google Scholar]
- Sarma R. H., Lee C. H., Evans F. E., Yathindra N., Sundaralingam M. Probing the interrelation between the glycosyl torsion, sugar pucker, and the backbone conformation in C(8) substituted adenine nucleotides by 1H and 1H-(31P) fast Fourier transform nuclear magnetic resonance methods and conformational energy calculations. J Am Chem Soc. 1974 Nov 13;96(23):7337–7348. doi: 10.1021/ja00830a028. [DOI] [PubMed] [Google Scholar]
- Schuster S. M., Ebel R. E., Lardy H. A. Kinetic studies on rat liver and beef heart mitochondrial ATPase. Evidence for nucleotide binding at separate regulatory and catalytic sites. J Biol Chem. 1975 Oct 10;250(19):7848–7853. [PubMed] [Google Scholar]
- Slater E. C., Kemp A., van der Kraan I., Muller J. L., Roveri O. A., Verschoor G. J., Wagenvoord R. J., Wielders J. P. The ATP-and ADP-binding sites in mitochondrial coupling factor F1 and their possible role in oxidative phosphorylation. FEBS Lett. 1979 Jul 1;103(1):7–11. doi: 10.1016/0014-5793(79)81239-9. [DOI] [PubMed] [Google Scholar]
- Strotmann H., Bickel-Sandkötter S. Energy-dependent exchange of adenine nucleotides on chloroplast coupling factor (CF1). Biochim Biophys Acta. 1977 Apr 11;460(1):126–135. doi: 10.1016/0005-2728(77)90158-x. [DOI] [PubMed] [Google Scholar]
- Strotmann H., Bickel S., Huchzermeyer B. Energy-dependent release of adenine nucleotides tightly bound to chloroplast coupling factor CF1. FEBS Lett. 1976 Jan 15;61(2):194–198. doi: 10.1016/0014-5793(76)81036-8. [DOI] [PubMed] [Google Scholar]
- Strotmann H., Hesse H., Edelmann K. Quantitative determination of coupling factor CF1 of chloroplasts. Biochim Biophys Acta. 1973 Aug 31;314(2):202–210. doi: 10.1016/0005-2728(73)90135-7. [DOI] [PubMed] [Google Scholar]
- Wagenvoord R. J., Kemp A., Slater E. C. The number and localisation of adenine nucleotide-binding sites in beef-heart mitochondrial ATPase (F1) determined by photolabelling with 8-azido-ATP and 8-azido-ADP. Biochim Biophys Acta. 1980 Dec 3;593(2):204–211. doi: 10.1016/0005-2728(80)90058-4. [DOI] [PubMed] [Google Scholar]
- Wagenvoord R. J., Verschoor G. J., Kemp A. Photolabelling with 8-azido-adenine nucleotides of adenine nucleotide-binding sites in isolated spinach chloroplast ATPase (CF1). Biochim Biophys Acta. 1981 Feb 12;634(2):229–236. doi: 10.1016/0005-2728(81)90141-9. [DOI] [PubMed] [Google Scholar]
- Williams N., Coleman P. S. Exploring the adenine nucleotide binding sites on mitochondrial F1-ATPase with a new photoaffinity probe, 3'-O-(4-benzoyl)benzoyl adenosine 5'-triphosphate. J Biol Chem. 1982 Mar 25;257(6):2834–2841. [PubMed] [Google Scholar]
- Yoshikawa M., Kato T., Takenishi T. A novel method for phosphorylation of nucleosides to 5'-nucleotides. Tetrahedron Lett. 1967 Dec;50:5065–5068. doi: 10.1016/s0040-4039(01)89915-9. [DOI] [PubMed] [Google Scholar]