Abstract
Type I and type II focal cortical dysplasias (FCDs) exhibit distinct histopathological features that suggest different pathogenic mechanisms. Type I FCDs are characterized by mild laminar disorganization and hypertrophic neurons whereas type II FCDs exhibit dramatic laminar disorganization and cytomegalic cells (balloon cells). Both FCD types are associated with intractable epilepsy; therefore, identifying cellular or molecular differences between these lesion types that explains the histological differences could provide new diagnostic and therapeutic insights. Type II FCDs express nestin, a neuroglial progenitor protein that is modulated in vitro by the stem cell proteins c-Myc, SOX2, and Oct-4 following activation of mammalian target of rapamycin complex 1 (mTORC1). Since mTORC1 activation has been demonstrated in type II FCDs, we hypothesized that c-Myc, SOX2, and Oct-4 expression would distinguish type II from type I FCDs. In addition, we assayed the expression of progenitor cell proteins FOXG1, KLF4, Nanog, and SOX3. Differential expression of 7 stem cell proteins and aberrant phosphorylation of 2 mTORC1 substrates, S6 and S6 kinase 1 proteins, clearly distinguished type II from type I FCDs (n = 10 each). Our results demonstrate new potential pathogenic pathways in type II FCDs and suggest biomarkers for diagnostic pathology in resected epilepsy specimens.
Keywords: Cortical dysplasia, Epilepsy, mTOR, STRADa, Tuberous sclerosis
INTRODUCTION
Focal cortical dysplasias (FCDs) are sporadic developmental malformations of the cortex that are the most common cause of intractable epilepsy in children (1-3). FCDs have been classified histopathologically as either type I (subtype A or B), characterized by mild cortical disorganization and dyslamination and/or hypertrophic neurons, or type II, characterized by total loss of cortical lamination and the presence of cytomegalic-dysmorphic neurons (CDNs; type IIA) and/or balloon cells (BCs; type IIB) (2, 4). The histopathological differences between type I and type II FCD suggest that there are distinct mechanistic differences that lead to their formation, but the molecular pathogenesis of sporadic type I and type II FCDs has not been fully elucidated. Understanding the molecular mechanisms that underlie the histopathological differences between type I and type II FCDs would provide important insights into diagnostic biomarkers and potentially, new therapeutic strategies for patients with intractable epilepsy.
The identification of 3 autosomal FCD subtypes associated with single gene defects provides insights into sporadic FCDs. For example, tubers in tuberous sclerosis complex (TSC) represent an autosomal dominant form of type IIB dysplasia resulting from mutations in TSC1 or TSC2, and are characterized by extensive laminar disorganization, CDNs, and the presence of BCs, also known as giant cells (GCs) in TSC (5). TSC1 or TSC2 gene mutations lead to constitutive activation of the mammalian target of rapamycin complex 1 (mTORC1) as evidenced by aberrant phosphorylation of several downstream signaling proteins including S6 kinase 1 (S6K1), ribosomal protein S6 (S6) and 4-elongation factor binding protein-1 (4E-BP1) in tubers (6-8) (Fig.1). The polyhydramnios, megalencephaly, symptomatic epilepsy syndrome (PS) is an autosomal recessive type II FCD characterized by cytomegalic cells and heterotopic cells in the subcortical white matter that results from a deletion in STRADa, an upstream activator of the TSC1:TSC2 complex (9) (Fig.1). Like TSC1 and TSC2, mutations in STRADa also lead to hyperactivation of mTORC1 (9). Interestingly, enhanced phosphorylation of S6 protein is observed in sporadic type II FCDs and suggests that hyperactivated mTORC1 signaling may also be a pathogenic mechanism in this FCD subtype (10). In contrast, the cortical dysplasia focal epilepsy (CDFE) syndrome is a type I FCD that results from mutations in the contactin-associated protein-like 2 gene (CNTNAP2) encoding a scaffolding protein for the Kv1.1 channel, which has no known link to mTORC1 signaling (11). mTORC1 pathway activation has not been assessed in sporadic type I FCDs.
Figure 1.

Schematic of mTORC1 signaling and genetic mutations at specific sites in the pathway. Cytoplasmic mTOR signaling is activated in normal cells by growth factors and nutrients. Loss-of-function mutations in either TSC1 or TSC2 lead to constitutive mTORC1 signaling and cause tuberous sclerosis complex (TSC). Loss-of-function deletions in STRADa cause polyhydramnios, megalencephaly, symptomatic epilepsy syndrome (PS) and result in hypoactivation of the TSC1:TSC2 complex and enhanced mTORC1 signaling. Hyperactive mTORC1 signaling results in enhanced S6K1 and S6 phosphorylation, leading to increased cell size, such as balloon cells (BCs) and giant cells (GCs). mTORC1 mediated inhibition of 4E-BP1 results in enhanced translation of c-Myc, which in turn translocates into the nucleus to regulate expression of Octamer-4 (Oct-4), sex-determining region Y box 2 (SOX2), and nestin, perhaps conferring an immature cellular phenotype on many type II focal cortical dysplasia (FCD) cells. The molecular event causing sporadic FCD type II has yet to be identified but the phenotypic and histological similarities between sporadic FCD II, PS, and TSC suggest that sporadic FCD type II may be caused by dysfunction of an mTORC1 regulatory gene.
Proteins that are typically expressed in neural progenitor cells (e.g. nestin, vimentin, CD133, and Mcm2) are found in GCs in TSC and CDNs and BCs in type II FCDs (12-17) and may be linked to mTORC1 signaling (13, 18). Specifically, mTORC1 activation results in enhanced translation of the transcriptional activator c-Myc (19, 20). c-Myc is involved in transcriptional regulation of certain stem cell marker proteins, such as sex-determining region Y-box 2 (SOX2) and Octamer-4 (Oct-4) (21). SOX2 regulates nestin expression by binding to its enhancer domain (22) and sustained Oct-4 expression promotes differentiation of nestin-positive neural precursors (23). Moreover, exogenous expression of c-Myc promotes proliferation of nestin-positive neural progenitor cells in vitro (24) and in vivo (25). Thus, we hypothesized that differential phosphorylation of mTORC1 signaling proteins and expression of SOX2, Oct-4, c-Myc could provide a mechanistic and pathophysiological distinction between type II and type I FCDs. Furthermore, because expression of the stem cell proteins SOX2, Oct-4, and c-Myc could also suggest a phenotypic characteristic of GCs and BCs, we also examined 4 additional stem cell protein markers: the winged-helix transcription factor forkhead box G1 (FOXG1), Kruppel-like factor 4 (KLF4), Nanog, and sex-determining region Y-box 3 (SOX3). Our results demonstrate a clear distinction in stem cell protein expression and mTORC1 signaling between type I and type II FCDs that underscores the histological differences between these dysplasia subtypes.
MATERIALS AND METHODS
Human Tissue Specimens
Sporadic type I and type II FCD specimens were obtained from 20 patients (type I, mean age 7.8 years, 6 males, 4 females; type II, mean age 5.5 years, 6 males, 4 females; Table 1), following epilepsy surgery at the Academic Medical Center, University of Amsterdam, the Netherlands, Children’s Hospital of Philadelphia, Philadelphia, PA, or the National Hospital for Neurology and Neurosurgery, London, UK. Surgical localization of the resection site in all cases reflected the seizure focus as determined by standard pre-surgical evaluation including scalp or intracranial EEG monitoring. Tissue specimens were removed from frontal, temporal, or parietal lobes (Table 1). All patients exhibited Engel class I or II surgical outcomes (26).
Table 1.
Clinical Features of Focal Cortical Dysplasia Patients
| Age (y) at Surgery/sex | Age at seizure Onset | Epilepsy Duration (y) | FCD type | Location | Engel Class* |
|---|---|---|---|---|---|
| 2/M | birth | 2 | IA | Fr | I |
| 5/M | 3 mo | 5 | IA | Fr | II |
| 5/M | 2 mo | 5 | IA | T | II |
| 6/F | 3 y | 3 | IB | T | I |
| 7/M | birth | 7 | IB | T | II |
| 8/F | 1 y | 7 | IB | Fr | II |
| 9/M | 1 y | 8 | IB | Fr | I |
| 10/M | 3 y | 7 | IB | Fr | II |
| 11/F | 4 y | 7 | IB | T-P | II |
| 15/F | 2 y | 13 | IB | Fr | II |
| 1/F | birth | 1 | IIA | Fr-P | I |
| 1/F | birth | 1 | IIA | Fr | II |
| 2/M | birth | 2 | IIA | P | I |
| 3/M | birth | 3 | IIB | T-P | I |
| 4/M | 3 mo | 4 | IIB | T | I |
| 4/M | 3 mo | 4 | IIB | T | I |
| 5/F | 2 y | 3 | IIB | Fr | II |
| 6/F | birth | 6 | IIB | T | I |
| 14/M | birth | 14 | IIB | Fr | I |
| 15/M | 1 y | 14 | IIB | P | I |
(26)
FCD, focal cortical dysplasia; M, male; F, female; Fr, frontal lobe; T, temporal lobe; P, parietal lobe.
Type I FCD cases were defined histologically by cortical dyslamination and heterotopic neurons in layer I and the subcortical white matter. Type II FCD cases were defined by loss of cortical lamination, blurring of the gray-white junction and the presence of CDNs or BCs (1). CDFE specimens (n = 3; mean age 4 years; 2 males, 1 female) were obtained following surgical resection (all 3 with Engel class II outcomes) and exhibited altered cortical lamination and heterotopic neurons similar to sporadic type I FCD. A single PS specimen from a 7-month-old female was available for analysis. This case exhibited aberrant cortical lamination, heterotopic neurons in the subcortical white matter and oval cytomegalic cells that were similar to those found in type II FCD. Cortical tubers were obtained from TSC patients (n = 8, mean age 7.2 years; 4 males, 4 females; Table 2) with TSC defined by clinical criteria. Tubers exhibited loss of cortical lamination and contained CDNs and GCs consistent with type II FCD. In addition, 4 regions of non-tuber cortex exhibiting normal histopathologic features were obtained postmortem from 4 TSC patients.
Table 2.
Clinical Features of Tuberous Sclerosis Complex Patients
| Age (y) at Surgery/sex | Age at seizure Onset | Epilepsy Duration (y) | Location | Engel Class | Genotype |
|---|---|---|---|---|---|
| 2/M | birth | 2 | Fr | I | TSC1 |
| 4/M | 2 y | 2 | T | II | TSC2 |
| 8/M | 2 y | 6 | T | II | TSC2 |
| 9/M | 5 y | 4 | T | I | TSC2 |
| 4/F | 3 mo | 4 | Fr | II | TSC1 |
| 7/F | 1 y | 6 | Fr | II | TSC2 |
| 14/F | 4 y | 10 | Fr | II | TSC2 |
| 10/F | 2 y | 8 | Fr | II | TSC2 |
M, male, F, female, Fr, frontal lobe, T, temporal lobe; TSC1 or TSC2 refers to clinical genetic testing results in these patients.
There were 2 control patient tissue groups. First, specimens were obtained following focal cortical resection (n = 6; mean age 7.2 years) for intractable seizures (Children’s Hospital of Philadelphia; Academic Medical Center, University of Amsterdam) from patients with no radiographic evidence of FCD or altered brain structure. Histological analysis of these samples revealed intact cortical cytoarchitecture and no evidence of type I or II FCD. These samples did not differ in gender (n = 4 males, 2 females), age of seizure onset, duration of seizures, or numbers of antiepileptic drugs from the type I or type II FCD patients (Table 3), and were classified as “epilepsy controls.” Epilepsy control specimens were resected from frontal or temporal neocortices and all resulted in Engel class I or II outcomes. The second control group consisted of frontal and temporal neocortex specimens obtained from 6 patients at necropsy who died of non-neurologic causes (mean age 5.8 years; 3 males, 3 females; Brain and Tissue Bank for Developmental Disorders, University of Maryland; Children’s Hospital of Philadelphia; mean postmortem interval = 14 hours, range = 11-16 hours). Seizures were not terminal events in these patients and none had a personal or family history of epilepsy. The cortical cytoarchitecture of these specimens was intact; these cases were classified as “postmortem controls”.
Table 3.
Comparison of Focal Cortical Dysplasia, Epilepsy Control, and Postmortem Control Samples
| FCD | Epilepsy | Postmortem | Significance (t-test) | |
|---|---|---|---|---|
| Age (surgery) | 6.6 | 7.2 | 5.8 (at death) | NS |
| Seizure Duration | 5.3 | 4.6 | NA | NS |
| Age at Onset | 10.7 | 8.9 | NA | NS |
| AEDs Used | 4 | 4 | NA | NS |
FCD = focal cortical dysplasia; mean age at surgery or death, in y; mean seizure duration, y; mean age at seizure onset (months); mean number of antiepileptic drugs (AEDs) used; NS, non-significant (p > 0.05, Student t-test); NA, not applicable.
All human tissue was obtained in accordance with protocols approved by Academic Medical Center, University of Amsterdam, and the Joint Research Ethics Committee of the National Hospital for Neurology and Neurosurgery and Institute of Neurology, Children’s Hospital of Philadelphia, and the University of Pennsylvania Institutional Review Board and Committee on Human Research.
Human Cell Culture
In 1 FCDIIB specimen (14-year-old male, Table 1), a portion of the resected tissue was used to generate dissociated cultures immediately following surgical removal. Tissue was digested in 0.25% Trypsin-EDTA (Gibco, Invitrogen, Carlsbad, CA) at 37°C for 20 minutes, titrated with flame-constricted glass pipettes, and plated on poly-L-lysine coated coverslips. Cells were cultured in one of 3 types of media: DMEM/F12, 1%N2, 1% FBS, 1% P/S; DMEM, 10% FBS, 1% P/S; or Neurobasal, 5% FBS, 2% B-27 supplement, 2 mM GlutaMAX-I Supplement (Gibco). Cells were maintained at 37°C, pH 7.4 and analyzed after 24 and 72 hours in vitro.
Genotype Analysis
Genomic DNA was extracted (Qiagen, Valencia, CA) from all TSC and type I and type II FCD specimens. Exon specific flanking PCR primers (forward and reverse) were generated to TSC1 and TSC2 and amplicons from all exons were sequenced (ABI Prism, Invitrogen). All PCR and sequencing reactions were performed in duplicate. Sequence results were compared with existing database sequence for TSC1 and TSC2 (http://chromium.liacs.nl/lovd/index.php?select_db=TSC1 or db=TSC2). Genotype analysis revealed that 2 TSC cases were associated with TSC1 mutations; 6 cases were associated with TSC2 mutations. These findings corroborated the mutations identified by available clinical testing for 5 TSC patients. Genotype analysis in the 4 postmortem TSC specimens revealed 1 TSC1 and 3 TSC2 mutations. Previous studies identified a 5-exon terminal deletion in the LYK5 gene in all PS patients, including the patient in our sample (11). Published sequence analysis of CNTNAP2 in the 3 patients with CDFE revealed a single-base deletion at nucleotide 3709 (coding sequence 3709delG) in exon 22, resulting in a frame-shift mutation and a premature stop codon (10).
Tissue Processing, Immunohistochemistry, and Immunocytochemistry
All tissue specimens were placed in 4% paraformaldehyde fixative for a defined period (based on the size of the tissue block) and then embedded in paraffin. Portions of several specimens were rapidly frozen on dry ice for Western analysis. Paraffin-embedded blocks were sectioned at 7 mm; 5 representative sections per case were incubated overnight at 4°C with antibodies to c-Myc (Abcam, Cambridge, MA; mouse monoclonal, 1:250), FoxG1 (AbCam, rabbit polyclonal, 1:500), KLF4 (Abgent, San Diego, CA; rabbit polyclonal; 1:25), Nanog (Cosmo Bio Company, mouse polyclonal; 1:100), nestin (Chemicon, Temecula, CA; mouse monoclonal; 1:200), Oct-4 (Chemicon, rabbit polyclonal; 1:200), phospho-S6K1 (Thr389; Novus Biologicals, Littleton, CO; rabbit monoclonal, 1:50), phospho-S6 (Ser235/236; Bethyl Laboratories, Montgomery, TX; rabbit polyclonal; 1:50), SOX2 (Abcam, rabbit polyclonal; 1:100), and SOX3 (Chemicon, rabbit polyclonal, 1:100). Sections were then probed with biotinylated secondary antibodies for 1 hour at room temperature and visualized using avidin-biotin conjugation (Vectastain ABC Elite; Vector Labs, Burlingame, CA) with 3,3’-diaminobenzidine. Dehydrated sections were mounted with coverslips (Permount). Light-microscopy images were acquired using a Leica DM4000 B microscope. All immunohistochemistry (IHC) was performed using batch processing for each antibody.
Cells cultured from FCD IIB were fixed with 4% PFA for 15 minutes after 1 and 3 days of growth, permeabilized in 0.3% Triton X-100 and blocked in 2.5% normal goat serum and 2.5% normal horse serum for 4 hours at room temperature. Cells were probed with the following primary antibodies overnight: βIII-Tubulin (Abcam, mouse monoclonal, 1:500), MAP2 (Abcam, mouse monoclonal, 1:500), nestin (Abcam, mouse monoclonal, 1:200), phospho-S6 (Ser235/236; Cell Signaling, Danvers, MA; rabbit monoclonal, 1:100). Cells were then incubated in secondary antibodies, Texas Red goat anti-rabbit (Vector Labs, Burlingame, CA) or Fluorescein horse anti-mouse (Vector Labs) for 2 hours and co-stained with Hoechst 33342 (Invitrogen, 0.0001 μg/uL). Coverslips were mounted on microscope slides (Fluoromount-G) and images were acquired using a Leica DM4000 microscope with a Leica DFC340 FX camera.
Cell Counts
Quantitative cell counts of SOX2, Oct-4, and c-Myc immunolabeled cells in postmortem control, epilepsy control, sporadic type I FCD, and sporadic type II FCD tissue specimens were performed as previously described (27). A 1 cm2 region of interest (ROI) was identified in each tissue section (n = 3 sections per case) that extended from the pial surface to the edge of the resection margin and to the subcortical white matter and exhibited representative histological features of each control or FCD type. Cells were analyzed under light microscopy (20x magnification) from each section using image acquisition and analysis software (Spot RT CCD camera, Diagnostic Instruments, Inc. and Phase 3 Imaging System integrated with Image Pro Plus; Media Cybernetics, Silver Spring MD). All immunoreactive cells were counted in these specimens. In addition, the BCs in sporadic FCD IIB that expressed each of the progenitor cell protein markers were counted. BCs were identified by morphological parameters. For all counts, each ROI was visually inspected and any cellular elements (e.g. blood vessels) erroneously included in the computerized count analysis were deleted.
Western Analysis
Frozen control (n = 3 epilepsy and n = 3 postmortem) and FCD (n = 6) tissue samples were thawed on ice in radio-immunoprecipitation assay lysis buffer containing protease and phosphatase inhibitors. Whole tissue lysates were prepared by continual passage through an 18 g 1½” needle using a 1 ml syringe followed by multiple centrifugations at 12,000 rpm at 4°C and the supernatant fractions were collected. Total protein concentrations were determined using the DC Protein Assay Kit (Bio-Rad, Foster City, CA). Aliquots of total protein (40-50 mg) were combined with 4X reducing sample loading buffer and denatured at 95°C for 5 minutes. The samples were then subjected to electrophoresis on a 4%–15% SDS-PAGE and transferred onto polyvinylidene difluoride membranes at 4°C. Membranes were blocked in TBS, 0.1% Tween 20 (TBS-T), 5% nonfat dry milk (TBS-T, 5% NFDM) for 1 hour and washed in TBS-T. The membranes were then probed with antibodies against c-Myc (Abcam, mouse monoclonal, 1:750), Oct-4 (Abcam, rabbit polyclonal; 1μg/ml), phospho-S6 (Ser235/236; Cell Signaling, rabbit polyclonal, 1:2000), or SOX2 (Abcam, rabbit polyclonal; 1:2000) overnight at 4°C, washed 3 times in TBS-T, and incubated with an HRP-conjugated anti-rabbit or anti-mouse secondary antibody (GE Healthcare, Piscataway, NJ, 1:1500-1:3000) for 1 hour. The ECL detection system (GE Healthcare) was used for immunodetection by exposure of the membrane to X-ray film (Kodak, Rochester, NY). To ensure equal loading, membranes were subsequently stripped in Restore Western Blot Stripping Buffer (Thermo Scientific, Waltham, MA), blocked in PBS-T, 5% NFDM, subjected to Western blot analysis as described above, probed with an HRP-conjugated antibody against β-actin (Abcam, mouse monoclonal; 1:5000) or glyceraldehyde phosphate dehydrogenase (GAPDH) (Cell Signaling, rabbit polyclonal; 1:5000), and visualized using the ECL detection system (GE Healthcare).
Single Cell Microdissection, mRNA Amplification, and RT-PCR
Following SOX2 immunolabeling, sporadic FCD type II tissue sections were treated with Proteinase K (50 mg/ml) at 37°C for 30 minutes and then washed in diethylpyrocarbonate-treated water. Sections were processed for mRNA amplification, beginning with in situ reverse transcription of cellular poly (A) mRNA into cDNA directly on the tissue section, as previously described (28). To initiate reverse transcription in situ, an oligo-dT primer coupled to a T7 RNA polymerase promoter (Epicentre Technologies, Madison, WI; 2500 U/ml, 50000 U) was annealed to cellular poly (A) mRNA overnight at room temperature. cDNA synthesis was then performed with avian myeloblastosis virus reverse transcriptase (Seikagaku America; 0.5U/ml). Thereafter, sections were washed in 0.5X SSC buffer and placed in RNAse-free water.
Single SOX2 labeled neurons were microdissected from 2 representative FCD (n = 10 cells) or from postmortem control cortex sections (n = 10 cells) under light microscopy using a stainless steel microscalpel and a joystick micromanipulator. Single cells were aspirated into a pipette tip, transferred to a microfuge tube containing in situ reverse transcription buffer and avian myeloblastosis virus reverse transcriptase and incubated at 4°C for 90 minutes to generate single stranded cDNA. Double stranded template cDNA was generated with T4 DNA polymerase I (Amersham, Piscataway, NJ) from cDNA. Specific primers recognizing SOX2 and Oct-4 transcripts were generated for RT-PCR. Following PCR amplification, amplicons were resolved on a 1% agarose gel and products were sequenced (ABIPrism) to confirm their identity.
RESULTS
Aberrant phosphorylation of S6K1 and S6 proteins and robust expression of all 7 stem cell marker proteins was observed in the type II but not type I FCD specimens. The distribution of cells immunoreactive for each of the proteins assayed in type II FCDs was not uniform in each case and reflected the distribution of CDNs, BCs or GCs. For example, in some specimens CDNs, BCs or GCs were observed throughout the thickness of the dysplasia (i.e. from white matter to pial surface), whereas in other cases these cells were seen in deeper portions of the lesion. In general, there were many more BCs or GCs, (and hence immunoreactive cells) in the deeper portions and white matter within the dysplasia than closer to the pial surface. Morphologically normal cortex at the edge of FCD type II or tuber resections that did not contain CDNs, BCs or GCs did not exhibit immunoreactivity for any of the assayed proteins.
Type II But Not Type I FCD Express Progenitor Cell Proteins
We first analyzed mTORC1 signaling and progenitor cell proteins in an FCD IIB specimen rapidly processed from the operating room. Aberrantly enhanced phosphorylation of S6K1 and S6 proteins and robust c-Myc, SOX2, and nestin expression was detected by IHC and was corroborated by Western assay (Fig. 2). Several very large cultured neurons with a laterally displaced nucleus that were morphologically similar to CDNs in fixed FCD specimens co-expressed both phosphorylated S6 and nestin or phosphorylated S6 and β-III tubulin or MAP2 (14) (Fig. 2). We submit that the ultra-rapid processing of the tissue for Western assay and IHC, as well as the detection of enhanced phospho-S6 in vitro, provides a view of phospho-protein and progenitor cell protein expression that approximates levels of these proteins in vivo.
Figure 2.
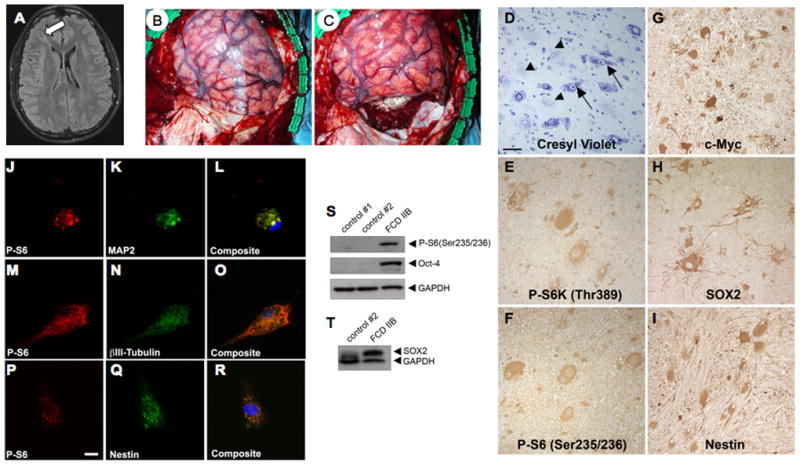
Hyperactive mTORC1 signaling and progenitor cell marker protein expression in rapidly processed, surgically resected type IIB focal cortical dysplasia (FCD). (A) Axial FLAIR MRI of a 14-year-old male patient with intractable epilepsy demonstrating a cortical dysplasia extending from the right frontal horn of the lateral ventricle to the right frontal cortex (arrow). (B, C) Intra-operative photographs showing pre-operative (B) and post-operative (C) images of the focal cortical dysplasia depicted radiographically in (A). (D-I) Histology and immunohistochemistry of the resected FCD IIB demonstrating numerous balloon cells (BCs) (arrowheads) and dysmorphic neurons (arrows) in a cresyl violet-stained section (D), enhanced immunoreactivity for mTORC1 signaling proteins phospho-p70S6Kinase (P-S6K1) (E), phospho-ribosomal S6 (P-S6) (F), and c-Myc (G), and expression of sex-determining region Y-box 2 (SOX2) (H) and nestin (I) by BCs and dysmorphic neurons (20x objective, scale bar = 50 mm). (J-R) Cells cultured from the resected FCD IIB specimen express microtubule associated protein-2 (MAP2) and β-III tubulin (K, N) and nestin (Q) and are highly immunoreactive for P-S6 (J, M, P). Cells were co-stained with Hoechst (L, O, R) to visualize the nuclei. Note the abnormal morphology of the cells and laterally displaced nuclei (L, O). Scale bar = 10 mm. (S, T) Representative Western blot analysis of whole tissue lysates from epilepsy control cortex (control #1), postmortem control cortex (control #2), and the FCD IIB specimen. FCD IIB demonstrates enhanced P-S6, Octamer-4 (Oct-4) (S), and SOX2 (T) expression vs. the control samples. Glyceraldehyde phosphate dehydrogenase (GAPDH) served as an internal loading control.
Because prior studies have identified robust nestin expression in BCs and GCs in FCD type II and TSC, respectively (13, 14, 30), we next investigated and compared the expression profile of SOX2 and Oct-4 (2 proteins modulate nestin expression) in FCD type I and FCD type II specimens (n = 10 of each type) by IHC and Western assay. SOX2 (Figs. 2, 3) and Oct-4 expression (Figs. 2, 4) was detected in all sporadic and syndromic type II FCDs and was largely confined to CDNs and BCs in FCD II or GCs in tubers. The subcellular distribution of SOX2 and Oct-4 immunoreactivity was both nuclear and cytoplasmic in sporadic FCD II or TSC, whereas it was largely nuclear in PS. In contrast, there was minimal expression of SOX2 or Oct-4 in sporadic type I FCD, CDFE specimens, non-tuber cortex, or postmortem and epilepsy controls (Figs. 3, 4). Western analysis demonstrated an increase in SOX2 and Oct-4 expression in tuber and FCD IIB specimens vs. control samples. The 43 and 70 kDa isoforms of Oct-4 were detected in tuber and FCD IIB but not FCD type I or control cortex.
Figure 3.

Sex-determining region Y-box 2 (SOX2) is expressed in polyhydramnios, megalencephaly, symptomatic epilepsy syndrome (PS), type II focal cortical dysplasia (FCD), and tuberous sclerosis complex (TSC) but not in control, type I FCD, or cortical dysplasia focal epilepsy (CDFE) cortex. (A-F) SOX2 expression detected by immunohistochemistry in neocortical brain tissue samples (large panel photos, 5x objective; bar = 200 mm; inset photos, 40x objective; bar = 50 mm). SOX2 expression is virtually absent in control (A), type I FCD (B), and CDFE2 (C) cases and abundant in PS (D), type II FCD (E), and TSC (F). Bottom left, Western blot analysis of SOX2 expression in tissue lysates from postmortem control cortex, FCD IIB, and 2 tuber specimens. Glyceraldehyde phosphate dehydrogenase (GAPDH) served as an internal loading control. Bottom center, RT-PCR amplification of SOX2 transcripts (225 bp amplicon, arrow) from single microdissected SOX2+ balloon cells (BCs) in 2 FCD IIB specimens but not a pyramidal neuron from postmortem control cortex. First lane is size marker. Bottom right, cell counts of SOX2+ cells in postmortem (PM) control, epilepsy (Epi) control, type I FCD, and type II FCD specimens. Counts reflect mean cell number across 3 regions of interest per specimen; mean ± SEM, *p < 0.05).
Figure 4.

Octamer-4 (Oct-4) is expressed in polyhydramnios, megalencephaly, symptomatic epilepsy syndrome (PS), type II focal cortical dysplasia (FCD), and tuberous sclerosis complex (TSC) but not in control, type I FCD, or cortical dysplasia focal epilepsy (CDFE) cortex. Immunohistochemistry for Oct-4 expression in neocortical brain tissue samples (large panel photos, 5x objective; bar = 200 mm; inset photos, 40x objective; bar = 50 mm). Oct-4 expression is virtually absent in postmortem control (A), type I FCD (B), and CDFE (C) cases and abundant in PS (D), type II FCD (E), and TSC (F). Bottom left, Western blot analysis of Oct-4 depicting enhanced expression in tissue lysates from FCD IIB and 2 tuber specimens compared with epilepsy control cortex (control #1) and postmortem control (control #2) cortex and FCD I. Glyceraldehyde phosphate dehydrogenase (GAPDH) served as an internal loading control. Bottom center, RT-PCR amplification of Oct-4 transcript (290 bp amplicon) from single microdissected SOX2-immunoreactive balloon cell (BC) in an FCD IIB specimen but not a pyramidal neuron from postmortem control cortex. Bottom right, cell counts of Oct-4+ cells in postmortem (PM) control, epilepsy (Epi) control, type I FCD, and type II FCD specimens. Counts reflect mean cell number across 3 regions of interest per specimen; mean ± SEM, *p < 0.05).
To corroborate the IHC, single SOX2 labeled cells were microdissected from sporadic FCD IIB samples and RT-PCR amplification of SOX2 and Oct-4 transcripts was performed. SOX2 and Oct-4 amplicons of appropriate size were detected in all dissected SOX2 labeled BCs, but not from control pyramidal neurons (Figs. 3, 4) and were by confirmed by cDNA sequencing.
c-Myc was robustly expressed in all type II FCD specimens and the IHC pattern closely paralleled that of SOX2 and Oct-4 expression (Figs. 2, 5). Low to nearly absent levels of c-Myc expression were observed in sporadic type I FCD and CDFE specimens that did not differ from postmortem and epilepsy control specimens or non-tuber TSC cortex. Enhanced c-Myc protein expression detected by IHC was confirmed by Western analysis (Fig. 5).
Figure 5.

c-Myc expression is prominent in polyhydramnios, megalencephaly, symptomatic epilepsy syndrome (PS), type II focal cortical dysplasia (FCD), and tuberous sclerosis complex (TSC) but not control, type I FCD, or cortical dysplasia focal epilepsy (CDFE) cortex. c-Myc expression in neocortical brain tissue samples (large panel photos, 5x objective; bar = 200 mm; inset photos, 40x objective; bar = 50 mm). c-Myc immunoreactivity is virtually absent in control (A), type I FCD (B), and CDFE (C) cases and abundant in PS (D), type II FCD (E), and TSC (F). Bottom left, Western blot analysis of c-Myc showing enhanced expression in FCD IIB and tuber specimen compared with FCD I or postmortem control cortex. Bottom right, cell counts of c-Myc+ cells in postmortem (PM) control, epilepsy (Epi) control, type I FCD, and type II FCD specimens. Counts reflect mean cell number across 3 regions of interest per specimen; mean ± SEM, *p < 0.05).
IHC revealed that there was minimal expression of KLF4, FOXG1, SOX3, or Nanog in control specimens or in the sporadic or autosomal recessive type I FCD specimens. In contrast, expression of KLF4 (Fig. 6), SOX3 (Fig. 6), FOXG1 (Fig. 7), or Nanog (Fig. 8) proteins was detected in BCs in sporadic FCD type II and in GCs in all TSC tuber specimens.
Figure 6.

Sex-determining region Y-box 3 (SOX3) (A-C) and Kruppel-like factor 4 (KLF4) (D-F) immunolabeling in control (A, D), type II focal cortical dysplasia (FCD) (B, E), and tuberous sclerosis complex (TSC) (C, F). Inset in (B) shows higher magnification image of SOX3 labeling. In (C), there are both SOX3 labeled (arrow) and unlabeled (arrowhead) cells. There is strong KLF4 labeling in type II FCD and tubers. SOX3 and KLF4 expression is absent in control cortex. Bar: A, B, D, F = 300 mm; C = 100 mm.
Figure 7.

Forkhead box G1 (FOXG1) expression in control cortex, type I, and type II focal cortical dysplasia (FCD). Expression is absent in control and type I FCD specimens vs. strong protein immunoreactivity in type II FCD (large panel photos, 5x objective; bar = 200 mm; inset photos, 40x objective; bar = 50 mm).
Figure 8.

Expression of Nanog in type II dysplasias. Nanog immunoreactivity is virtually absent in control (A), type I focal cortical dysplasia (FCD) (B), and cortical dysplasia focal epilepsy (CDFE) (C) cases and abundant in polyhydramnios, megalencephaly, symptomatic epilepsy syndrome (PS) (D), type II FCD (E), and tuberous sclerosis complex (TSC) (F) (large panel photos, 5x objective; bar = 200 mm; inset photos, 40x objective; bar = 50 mm).
We were particularly interested in the numbers of BCs that expressed each progenitor cell protein marker and thus focused cell quantification on BCs within defined 1-cm2 ROIs for each FCD IIB case that extended from the pial surface to the edge of the resection margin and to the subcortical white matter (n = 3 sections per case). The mean maximal cell soma diameter of the BCs was 117.6 mm (range 104-125 mm) and this parameter was used to generate cell counts for each individual protein marker. Across the 10 sporadic FCD II specimens, 93% of cells meeting morphological criteria for BCs expressed SOX2, 89% expressed c-Myc, 86% expressed FOXG 1, 82% expressed SOX3, 77% expressed KLF4, 61% expressed Oct-4, and 37% expressed Nanog (Fig. 9).
Figure 9.
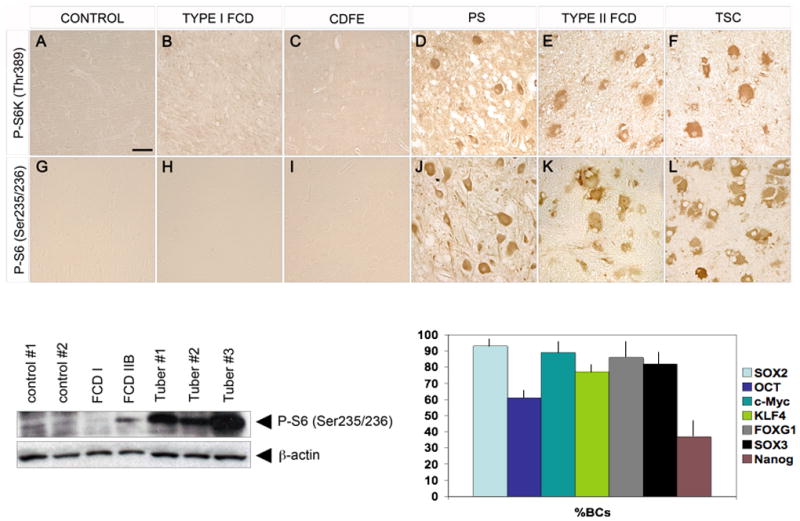
mTRC1 activation distinguishes type I from type II focal cortical dysplasia (FCD) as evidenced by enhanced immunoreactivity for phospho-p70S6Kinase (P-S6K1; Thr389) and phospho-ribosomal S6 (P-S6; Ser235/236) in the latter. (A-H) Aberrant phosphorylation of S6K1 is prominent in polyhydramnios, megalencephaly, symptomatic epilepsy syndrome (PS) (D), type II FCD (E), and tuberous sclerosis complex (TSC) (F) but is virtually absent in postmortem control (A), type I FCD (B), and cortical dysplasia focal epilepsy (CDFE) (C) samples. Hyperphosphorylation of the S6K1 target S6 is evident in PS (J), type II FCD (K), and TSC (L) but is virtually absent in control (G), type I FCD (H), and CDFE (I) samples (20x objective, bar = 50 mm). Bottom left, Western blot analysis shows enhanced S6 protein phosphorylation in FCD IIB and 3 tuber specimens vs. FCD I, epilepsy control cortex (control #1) and postmortem control cortex (control #2). Bottom right, graph depicting percent of morphologically defined balloon cells (BCs) in sporadic type IIB FCD expressing each stem cell marker protein (± SEM).
Type II But Not Type I FCD Exhibits mTORC1 Activation
The expression of progenitor cell protein markers distinguished type I and type II FCDs. Since expression of c-Myc, SOX2, and Oct4 have been linked to mTORC1 signaling, we investigated whether aberrant phosphorylation of downstream proteins such as S6K1 and S6 could also delineate these subtypes. There was a clear difference in phosphorylation of S6K1 and S6 between type I and type II FCDs (Fig. 9). More than 90% of BCs in sporadic type II FCDs or GCs in tubers exhibited enhanced S6 and S6K1 phosphorylation, in accordance with previous studies (9, 30, 31). In addition, phospho-S6 and phospho-S6K1 immunoreactivity was detected in PS brain tissue (9). Aberrant S6K1 and S6 protein phosphorylation was not detected in sporadic or autosomal recessive (CDFE) type I FCD, or in control specimens (Fig. 9). Greater S6 phosphorylation was identified by Western assay in FCD type IIB and 3 tuber specimens than in controls and sporadic type I FCD (Fig. 9). Another activated kinase, phosphorylated p90RSK (Ser380), is known to phosphorylate S6 in an mTORC1-independent fashion. There was no difference in phospho-p90RSK (Ser380) immunoreactivity in type I FCD, type II FCD, or control specimens (data not shown).
Genotype Analysis of Type I and Type II FCDs
Genotype analysis of TSC1 and TSC2 in the 20 sporadic FCD type I and type II cases did not reveal mutations in either gene, thereby confirming that these dysplasias did not represent somatic mosaic forms of TSC (28). However, sequencing was confined to exons only; splice site mutations were not identified. Several single nucleotide polymorphisms previously identified in the TSC sequence database that are known to be present in normal individuals were found in type I FCD (Table 4).
Table 4.
Representative TSC1 and TSC2 Polymorphisms in Sporadic Type I Focal Cortical Dysplasia
| Exon | Base Change | Amino Acid Change | SNP Type |
|---|---|---|---|
| TSC1 14 | C>T | H>Y | NS |
| TSC1 14 | A>G | G>G | S |
| TSC1 17 | C>T | L>L | S |
| TSC2 12 | C>T | F>F | S |
| TSC2 17 | G>A | V>V | S |
NS, non-synonymous; S, synonymous; SNP, Single nucleotide polymorphism.
DISCUSSION
We demonstrate that the expression of SOX2, Oct-4, c-Myc, FOXG1, KLF4, Nanog, and SOX3, as well as of hyperphosphorylated S6K1 and S6 proteins distinguishes sporadic, autosomal dominant (TSC) and autosomal recessive (PS) forms of type II FCD from sporadic or autosomal recessive (CDFE) forms of type I FCD or control cortex. SOX2 and SOX3 are markers for multipotent neural stem cells, and FOXG1 is a known repressor of differentiation in neural progenitor cells, whereas c-Myc, KLF4, Oct-4, and Nanog are typically identified in pluripotent stem cells (29). Thus, these results suggest that BCs and GCs exhibit a protein expression phenotype similar to multipotent or pluripotent stem cells. The concomitant aberrant phosphorylation of S6K1 and S6 proteins (known downstream effectors of mTORC1 signaling in type II but not type I dysplasias) mirrors the expression profiles of the 7 stem cell proteins. This suggests that hyperactive mTORC1 signaling may be linked to a progenitor cell phenotype in type II FCD and highlights a mechanistic distinction between type I and type II FCDs.
There are several caveats to the interpretation of our results. First, while the generation of dissociated cultures from 1 case of type II FCD provides a compelling novel strategy to study FCD in vitro, further work is necessary to define the phenotype of these cells. The experiments in the dissociated cultures were designed to demonstrate colocalization of nestin and phospho-S6 in live cells and to show the feasibility of cell culture from resected FCD specimens. Nestin, c-Myc, SOX2, and Oct-4 expression as well as phosphorylation of S6K1 and S6 were corroborated in this same sample by IHC and Western assay. Second, in view of the rarity of PS, only a single case specimen was available for analysis. However, due to the direct link between STRADa and mTORC1 signaling via the TSC1:TSC2 complex (11), we suggest that the findings in the PS case support our hypothesis that hyperactive mTORC1 signaling is a central feature of type II FCD. Third, it is unlikely that seizures or anti-epileptic drug exposure alone accounted for altered protein expression; for example, c-Myc, SOX2, and Oct-4 were not detected in our epilepsy control group or in non-tuber cortex from TSC patients. We previously reported minimal S6K1 phosphorylation in type II FCD (30), but now revise these observations using a different antibody to phospho-S6K1 that has given us more reproducible results (P. Crino, unpublished observations). Indeed, other investigators have demonstrated aberrant phosphorylation of S6K1 as well as of eIFG4 in FCD IIB (10, 31, 32). The absence of immunoreactivity of phospho-p90RSK (Ser308), another kinase that phosphorylates S6 on Ser235/236 in an mTORC1-independent fashion, in any of the dysplasia specimens lends support to the notion that enhanced S6 phosphorylation specifically reflects mTORC1 activation. Finally, the expression of progenitor cell marker proteins in FCD type II may not demarcate a specific cell differentiation state but instead may reflect aberrant gene transcription or protein translation as a consequence of the profound abnormality of cortical lamination in type II FCD.
Our findings have implications for diagnostic pathology and clinical outcome following surgical resection of FCDs. A clear designation as type I or type II FCD may pose a challenge, especially in the absence of BCs. The distinction between type I and type II FCD based on both histology and signal cascade activation may help to stratify patients and to identify FCD subtypes with greater accuracy. Although larger cohorts will be needed to determine the functional diagnostic sensitivity and specificity of these marker proteins, our findings coupled with those of other investigators (10, 12-16, 32. 33) suggest that probing resected tissue with a panel of antibodies (e.g. phospho-S6K1, phospho-S6, nestin, c-Myc, FOXG1, KLF4, Oct-4, SOX2, and SOX3) might enhance detection and diagnostic accuracy of FCDs. Furthermore, in some recent studies, tissue resected from patients with normal pre-operative MRI scans (so-called non-lesional cases) was found to contain type I or type II FCD (34). Thus, expression of mTORC1 signaling or progenitor cell proteins may enhance detection of small or radiographically occult sporadic type II FCDs. Finally, in a recent comprehensive review of surgical treatment of FCDs (35) it was observed that complete resection is the most consistent predictor of seizure freedom following epilepsy surgery for type I and type II FCD. Since the edge of the surgical margin is currently defined on morphological grounds alone, clear definition of the resection edge using mTORC1 signaling proteins as biomarkers may aid in defining the extent of FCD type II.
Further experiments will be needed to determine whether BCs and GCs are stem cells; the ability to maintain FCD IIB cells in culture supports the feasibility of this approach. Our findings are bolstered by literature that shows expression of other progenitor cell markers such as vimentin, CD133, and Mcm2 in type II FCD (12-17). Although nestin, Vimentin, Mcm2, CD133, c-Myc, FOXG1, KLF4, Nanog, Oct-4, SOX2, and SOX3 expression may reflect a stem cell phenotype, true definition of stem cells will require further in vitro studies that demonstrate the capacity of these cells for self-renewal as well as the ability to differentiate into neuroglial (i.e. neurons, astrocytes, and oligodendrocytes) or other cell lineages. One compelling observation was that many more BCs or GCs in the subcortical white matter expressed the 7 progenitor cell proteins and that there appeared to be a labeling gradient between deep and more superficial portions. Conceivably, it may indicate that there are different pools of cells within the lesion or that some BCs or GCs undergo further differentiation and cease to express these protein markers.
The detection of SOX2 and Oct-4 in BCs and GCs provides clues to expression of other progenitor cell markers (e.g. nestin and vimentin) that are also found in BCs and GCs (13, 14, 17, 33). SOX2 regulates nestin expression by binding to the neural enhancer of nestin during different stages of differentiation (23, 36) and occupies the vimentin promoter and enhances vimentin expression (36). Sustained Oct-4 expression promotes differentiation and proliferation of nestin+ progenitor cells (36). Thus, the strong expression of SOX2 and Oct-4 in type II FCDs suggests 2 potential transcription factors that may account for nestin expression in this dysplasia subtype. SOX2 and Oct-4 expression is directly modulated by mTORC1 activation via c-Myc, the translation of which is controlled by 4E-BP1/eIF-4E in an mTORC1-dependent manner (Fig. 1; 21, 22). Exogenous expression of c-Myc in GFAP+ astrocytes has been shown to promote an undifferentiated phenotype, characterized by nestin and vimentin expression (22). Interestingly, KLF4 has recently been shown to bind with SOX2 and Oct-4 to regulate Nanog expression in embryonic stem cells (37).
Previous studies have demonstrated upstream activation of the mTORC1 pathway in type II FCD (10, 30-32). Our present data demonstrate that pathway activation is extended downstream to both transcriptional, as evidenced by SOX2 and Oct-4 expression, and translational, as evidenced by c-Myc expression, activation. Knockout of Tsc1 or Pten in the mouse leads to mTOR activation and models some of the features of type II FCD such as cytomegaly, laminar disorganization, and clinically, spontaneous seizures (38, 39). In contrast, we find no evidence of mTORC1 cascade activation, either transcriptional or translational, in type I FCD. Indeed, we show for the first time that somatic mutations in TSC1 and TSC2 do not occur in FCD type I. These findings suggest that the the molecular mechanisms leading to type I FCD formation during brain development are likely to be distinct from those in type II FCD and unrelated to mTORC1.
Acknowledgments
We thank Ralph J. Monfort, Jia Yu MD, and Leah Marcotte for technical assistance.
This work was supported by NS045877, NS045022, and Department of Defense CDMRP TSC Initiative (P.B.C.) and by the National Epilepsy Fund (NEF 05-11) and Stichting Michelle (M06.011and M07 016; E.A.).
References
- 1.Palmini A, Najm I, Avanzini G, et al. Terminology and classification of the cortical dysplasias. Neurology. 2004;62:S2–8. doi: 10.1212/01.wnl.0000114507.30388.7e. [DOI] [PubMed] [Google Scholar]
- 2.Krsek P, Maton B, Korman B, et al. Different features of histopathological subtypes of pediatric focal cortical dysplasia. Ann Neurol. 2008;63:758–69. doi: 10.1002/ana.21398. [DOI] [PubMed] [Google Scholar]
- 3.Fauser S, Huppertz HJ, Bast T, et al. Clinical characteristics in focal cortical dysplasia: A retrospective evaluation in a series of 120 patients. Brain. 2006;129:1907–16. doi: 10.1093/brain/awl133. [DOI] [PubMed] [Google Scholar]
- 4.Andre VM, Flores-Hernandez J, Cepeda C, et al. NMDA receptor alterations in neurons from pediatric cortical dysplasia tissue. Cereb Cortex. 2004;14:634–46. doi: 10.1093/cercor/bhh024. [DOI] [PubMed] [Google Scholar]
- 5.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–56. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 6.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 7.Goncharova EA, Goncharov DA, Eszterhas A, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM) J Biol Chem. 2002;277:30958–67. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- 8.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Puffenberger EG, Strauss KA, Ramsey KE, et al. Polyhydramnios, megalencephaly and symptomatic epilepsy caused by a homozygous 7-kilobase deletion in LYK5. Brain. 2007;130:1929–41. doi: 10.1093/brain/awm100. [DOI] [PubMed] [Google Scholar]
- 10.Schick V, Majores M, Koch A, et al. Alterations of phosphatidylinositol 3-kinase pathway components in epilepsy-associated glioneuronal lesions. Epilepsia. 2007;48(Suppl 5):65–73. doi: 10.1111/j.1528-1167.2007.01291.x. [DOI] [PubMed] [Google Scholar]
- 11.Strauss KA, Puffenberger EG, Huentelman MJ, et al. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N Engl J Med. 2006;354:1370–7. doi: 10.1056/NEJMoa052773. [DOI] [PubMed] [Google Scholar]
- 12.Taylor JP, Sater R, French J, et al. Transcription of intermediate filament genes is enhanced in focal cortical dysplasia. Acta Neuropathol. 2001;102:141–8. doi: 10.1007/s004010000348. [DOI] [PubMed] [Google Scholar]
- 13.Ying Z, Gonzalez-Martinez J, Tilelli C, et al. Expression of neural stem cell surface marker CD133 in balloon cells of human focal cortical dysplasia. Epilepsia. 2005;46:1716–23. doi: 10.1111/j.1528-1167.2005.00276.x. [DOI] [PubMed] [Google Scholar]
- 14.Crino PB, Trojanowski JQ, Eberwine J. Internexin, MAP1B, and nestin in cortical dysplasia as markers of developmental maturity. Acta Neuropathol. 1997;93:619–27. doi: 10.1007/s004010050660. [DOI] [PubMed] [Google Scholar]
- 15.Thom M, Martinian L, Sisodiya SM, et al. Mcm2 labelling of balloon cells in focal cortical dysplasia. Neuropathol Appl Neurobiol. 2005;31:580–8. doi: 10.1111/j.1365-2990.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 16.Mizuguchi M, Yamanouchi H, Becker LE, et al. Doublecortin immunoreactivity in giant cells of tuberous sclerosis and focal cortical dysplasia. Acta Neuropathol. 2002;104:418–24. doi: 10.1007/s00401-002-0575-z. [DOI] [PubMed] [Google Scholar]
- 17.Lamparello P, Baybis M, Pollard J, et al. Developmental lineage of cell types in cortical dysplasia with balloon cells. Brain. 2007;130:2267–76. doi: 10.1093/brain/awm175. [DOI] [PubMed] [Google Scholar]
- 18.Hambardzumyan D, Becher OJ, Rosenblum MK, et al. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22:436–48. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Benedetti A, Graff JR. eIF-4E expression and its role in malignancies and metastases. Oncogene. 2004;23:3189–99. doi: 10.1038/sj.onc.1207545. [DOI] [PubMed] [Google Scholar]
- 20.Kuang X, Shen J, Wong PK, Yan M. Deregulation of mTOR signaling is involved in thymic lymphoma development in Atm-/- mice. Biochem Biophys Res Commun. 2009;383:368–72. doi: 10.1016/j.bbrc.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Kidder BL, Yang J, Palmer S. Stat3 and c-Myc genome-wide promoter occupancy in embryonic stem cells. PLoS One. 2008;3:e3932. doi: 10.1371/journal.pone.0003932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin Z, Liu L, Bian W, et al. Different transcription factors regulate nestin gene expression during P19 cell neural differentiation and central nervous system development. J Biol Chem. 2009;284:8160–73. doi: 10.1074/jbc.M805632200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimozaki K, Nakashima K, Niwa H, Taga T. Involvement of Oct3/4 in the enhancement of neuronal differentiation of ES cells in neurogenesis-inducing cultures. Development. 2003;130:2505–12. doi: 10.1242/dev.00476. [DOI] [PubMed] [Google Scholar]
- 24.Lassman AB, Dai C, Fuller GN, et al. Overexpression of c-MYC promotes an undifferentiated phenotype in cultured astrocytes and allows elevated Ras and Akt signaling to induce gliomas from GFAP-expressing cells in mice. Neuron Glia Biol. 2004;1:157–63. doi: 10.1017/s1740925x04000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fults D, Pedone C, Dai C, Holland EC. MYC expression promotes the proliferation of neural progenitor cells in culture and in vivo. Neoplasia. 2002;4:32–39. doi: 10.1038/sj.neo.7900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engel J, Van Ness PC, Rasmussen TB, et al. Outcome with respect to epileptic seizures. In: Engel J, editor. Surgical Treatment of the Epilepsies. New York: Raven Press; 1993. pp. 609–21. [Google Scholar]
- 27.Kyin R, Hua Y, Baybis M, et al. Differential cellular expression of neurotrophins in cortical tubers of the tuberous sclerosis complex. Am J Pathol. 2001;159:1541–54. doi: 10.1016/S0002-9440(10)62539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becker AJ, Urbach H, Scheffler B, et al. Focal cortical dysplasia of Taylor’s balloon cell type: mutational analysis of the TSC1 gene indicates a pathogenic relationship to tuberous sclerosis. Ann Neurol. 2002;52:29–37. doi: 10.1002/ana.10251. [DOI] [PubMed] [Google Scholar]
- 29.Boyer LA, Lee TI, Cole MF, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–56. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baybis M, Yu J, Lee A, et al. mTOR cascade activation distinguishes tubers from focal cortical dysplasia. Ann Neurol. 2004;56:478–87. doi: 10.1002/ana.20211. [DOI] [PubMed] [Google Scholar]
- 31.Miyata H, Chiang AC, Vinters HV. Insulin signaling pathways in cortical dysplasia and TSC-tubers: tissue microarray analysis. Ann Neurol. 2004;56:510–19. doi: 10.1002/ana.20234. [DOI] [PubMed] [Google Scholar]
- 32.Ljungberg MC, Bhattacharjee MB, Lu Y, et al. Activation of mammalian target of rapamycin in cytomegalic neurons of human cortical dysplasia. Ann Neurol. 2006;60:420–9. doi: 10.1002/ana.20949. [DOI] [PubMed] [Google Scholar]
- 33.Crino PB, Trojanowski JQ, Dichter MA, Eberwine J. Embryonic neuronal markers in tuberous sclerosis: single-cell molecular pathology. Proc Natl Acad Sci U S A. 1996;93:14152–7. doi: 10.1073/pnas.93.24.14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SK, Lee SY, Kim KK, et al. Surgical outcome and prognostic factors of cryptogenic neocortical epilepsy. Ann Neurol. 2005;58:525–32. doi: 10.1002/ana.20569. [DOI] [PubMed] [Google Scholar]
- 35.Lerner JT, Salamon N, Hauptman JS, et al. Assessment and surgical outcomes for mild type I and severe type II cortical dysplasia: A critical review and the UCLA experience. Epilepsia. 2009;50:1310–35. doi: 10.1111/j.1528-1167.2008.01998.x. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka S, Kamachi Y, Tanouchi A, et al. Interplay of SOX and POU factors in regulation of the nestin gene in neural primordial cells. Mol Cell Biol. 2004;24:8834–8846. doi: 10.1128/MCB.24.20.8834-8846.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei Z, Yang Y, Zhang P, et al. Klf4 Interacts directly with Oct4 and Sox2 to promote reprogramming stem cells. 2009;27:2969–78. doi: 10.1002/stem.231. [DOI] [PubMed] [Google Scholar]
- 38.Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–53. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwon CH, Zhu X, Zhang J, Baker SJ. mTor is required for hypertrophy of Pten-deficient neuronal soma in vivo. Proc Natl Acad Sci U S A. 2003;100:12923–8. doi: 10.1073/pnas.2132711100. [DOI] [PMC free article] [PubMed] [Google Scholar]


