Abstract
Purpose
A growing number of diabetic patients request laser in situ keratomileusis (LASIK) for elective vision correction each year. While the United States Food and Drug Administration considers diabetes a relative contraindication to LASIK surgery, there are several reports in the literature of LASIK being performed safely in this patient population. The purpose of this review was to examine whether diabetes should still be considered a contraindication to LASIK surgery by reviewing the ocular and systemic complications of diabetes, and examining the existing data on the outcomes of LASIK in diabetic patients.
Methods
A literature review was conducted through PubMed, Medline, and Ovid to identify any study on LASIK surgery in patients with diabetes mellitus. This search was conducted without date restrictions. The search used the Medical Subject Headings (MeSH®) term LASIK linked by the word “and” to the following MeSH and natural language terms: diabetes, diabetes mellitus, systemic disease, and contraindications. Abstracts for all studies meeting initial search criteria were reviewed for relevance. There were no prospective clinical studies identified. Three retrospective studies were identified. Key sources from these papers were identified, reviewed, and included as appropriate. An additional literature search was conducted to identify any study of ocular surgery on patients with diabetes using the MeSH terms refractive surgery, photorefractive keratectomy, radial keratotomy, cataract surgery, vitrectomy, and iridectomy linked by the word “and” to the following MeSH terms: diabetes, diabetes mellitus, and systemic disease. This search was conducted without date restrictions. Abstracts of studies meeting the initial search criteria were reviewed and articles deemed relevant to the subject were included in this review.
Conclusion
LASIK may be safe in diabetic patients with tight glycemic control and no ocular or systemic complications.
Keywords: diabetes mellitus, diabetic keratopathy, diabetic corneal neuropathy, refractive surgery, LASIK surgery
Introduction
Currently, an estimated 346 million people have diabetes, making it one of the most common medical conditions worldwide.1 Diabetic patients experience refractive visual complaints no less frequently than the general population, and with the popularity of laser vision correction procedures such as laser in situ keratomileusis (LASIK), a growing number of diabetic patients are requesting elective refractive surgery each year.
Currently the United States Food and Drug Administration (FDA) considers diabetes a relative contraindication to LASIK surgery.2 When this recommendation was issued in 2000, LASIK was a new procedure and limited data was available on its safety and efficacy in diabetic patients. As such, the recommendation was primarily based on theoretical risk. Several concerns were expressed as justification for excluding diabetic patients from LASIK surgery. Diabetes is known to affect the eye in multiple ways, and there was concern that the ocular abnormalities seen in diabetic patients could result in surgical complications or poor refractive outcomes.3 The depressed immune response that is characteristic of diabetic patients was also a topic of discussion, and fueled worries that patients might experience a high rate of postoperative infections and/or impaired wound healing.4
Despite the FDA recommendation, some diabetic patients do receive elective LASIK surgery each year. Research on the outcomes of LASIK in this patient population is limited; however, available studies indicate that LASIK may be safe in carefully screened diabetic patients with tight glycemic control and no systemic or ocular complications. In this review, the basis for the FDA recommendation against LASIK in the diabetic population is examined by reviewing the ocular and systemic complications of diabetes and examining the existing data on the outcomes of LASIK in diabetic patients. Guidelines, based on the available data, are suggested for the refractive surgeon performing LASIK in this patient population.
Epidemiology
In the United States, diabetes has a prevalence of 8.3%. There are currently 18.8 million diagnosed diabetic patients in the United States, another 7.0 million who are undiagnosed, and 79 million prediabetic patients.5 There are 1.9 million newly diagnosed patients each year.6
LASIK surgery is a popular treatment for refractive vision correction. At its peak in 2007, 1.4 million patients in the United States received elective LASIK in 1 year. The unfavorable economic conditions of the past 4 years have reduced this rate to 700,000–900,000 surgeries annually.7 Given the prevalence of diabetes in the United States, diabetic patients make up a significant percentage of the patients who desire elective LASIK. The number of diabetic patients seeking LASIK is expected to increase in the next 10 years, as the 79 million prediabetic patients develop outright diabetes.8
Ocular complications of diabetes
Diabetic retinopathy (DR)
DR is the most well-known ocular complication of diabetes. The pathophysiology of DR is strongly linked to chronic hyperglycemia. The Diabetes Control and Complications Trial discovered that diabetic patients who received insulin therapy to maintain hemoglobin A1c levels below 7.9% had a 76% decrease in the incidence of DR.9 Further, diabetic patients with A1c levels consistently below 7.5% were unlikely to develop retinopathy.10
There are several mechanisms by which chronic hyperglycemia is thought to cause DR (Figure 1). Normally, blood flow to the retina is autoregulated to ensure consistent flow, even with moderate elevations in systemic mean arterial pressure. In patients with chronic hyperglycemia, this regulatory mechanism is defective.11 High flow volume through the retinal vessels leads to shear stress and increased vascular permeability, which ultimately allows fluid to build up in the retina, causing macular edema and ischemia. Chronic hyperglycemia also produces a surplus of advanced glycosylation end products (AGE), which are created when excess glucose combines with amino acids and tissue proteins.12 Interaction between AGE and their receptors within the retina leads to the release of reactive oxygen species, which increases retinal vascular inflammation and damage.13 As the disease progresses, retinal tissue becomes increasingly ischemic, triggering the release of vascular endothelial growth factor from neighboring retinal tissue. Neovascularization ensues, at which point the disease process is classified as proliferative retintopathy.14 Proliferative DR is associated with significant visual compromise and is the leading cause of blindness in the United States in patients aged 25–75 years.
Figure 1.
Pathophysiology of ocular complications of diabetes.
Abbreviations: AGE, advanced glycosylation end products; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor.
Corneal complications
The corneal changes in diabetic patients may be less recognized than retinal complications, but they are equally important, especially in the context of LASIK surgery.15 Corneal complications include compromised corneal stability as well as corneal denervation, factors that may affect safety and outcomes in patients undergoing LASIK.
Diabetic keratopathy
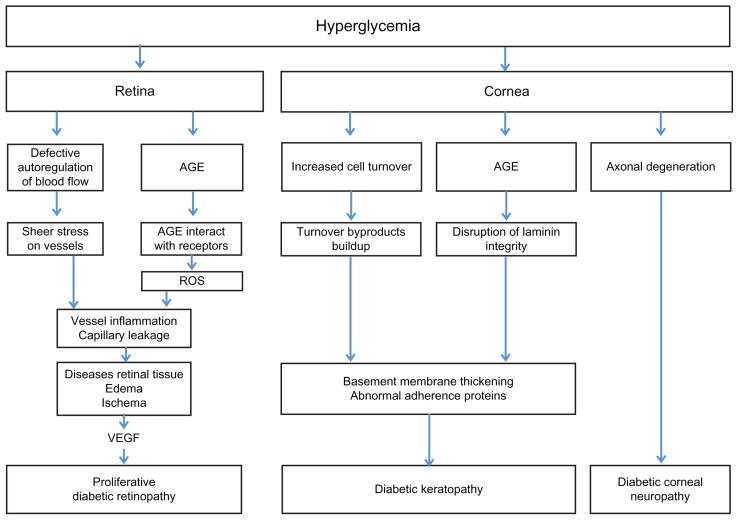
Diabetes induces several changes to the cornea; these abnormalities have been termed diabetic keratopathy.16 Keratopathy manifests clinically in a wide variety of findings including persistent epithelial defects and superficial punctate keratopathy (Table 1 and Figure 2).
Table 1.
Clinical manifestations of diabetic keratopathy
| Epithelial |
| Chronic epitheliitis |
| Delayed healing |
| Epithelial erosions |
| Epithelial fragility |
| Filamentary keratitis |
| Persistent epithelial defects |
| Microcystic edema and bleb formation |
| Superficial corneal ulcers |
| Superficial punctate keratopathy |
| Descemet’s membrane |
| Wrinkles |
| Endothelial |
| Beaten silver appearance |
| Pigmentation |
| Unpigmented precipitates |
Figure 2.
Confluent superficial punctate keratopathy in the eye of a diabetic patient.
Studies have shown that despite the cornea’s avascular nature, diabetic corneas are directly exposed to high serum glucose levels.17,18 Thus, the etiology behind diabetic keratopathy is likely hyperglycemia, similar to that of DR. Hyperglycemia accelerates the normal aging process of the basement membrane, increasing cell turnover and resulting in the accumulation of turnover byproducts.15 Buildup of byproducts in the cornea leads to an abnormally thickened and discontinuous basement membrane.19–25 Newer research indicates AGE may also play a role in the development of diabetic keratopathy. Kaji et al found evidence of AGE in eight of eight diabetic corneas versus one of eight nondiabetic corneas, and hypothesized that AGE buildup within the laminin of the basement membrane may compromise the connective integrity of the basement membrane, resulting in an abnormal, dysfunctional membrane.26 Abnormalities in the basement membrane anchoring complex (anchoring fibrils, anchoring plaques, basal lamina, and hemidesmosomes) are also associated with the abnormal basement membranes seen in diabetic corneas. The anchoring complex is critical in the normal adherence of the basement membrane to the corneal stroma, and altered adherence is linked to delayed epithelial healing rates and epithelial instability.15,27
Keratopathy is a common complication of diabetes, especially in those with evidence of retinopathy. Schultz et al found between 47%–64% of patients had evidence of corneal abnormalities on exam, and that this incidence increased with comorbid DR.28 Saini and Khandalavla demonstrated evidence of keratopathy in 84% of diabetic patients with a diagnosis of DR versus 41% in diabetic patients without retinal complications.29
Diabetic corneal neuropathy
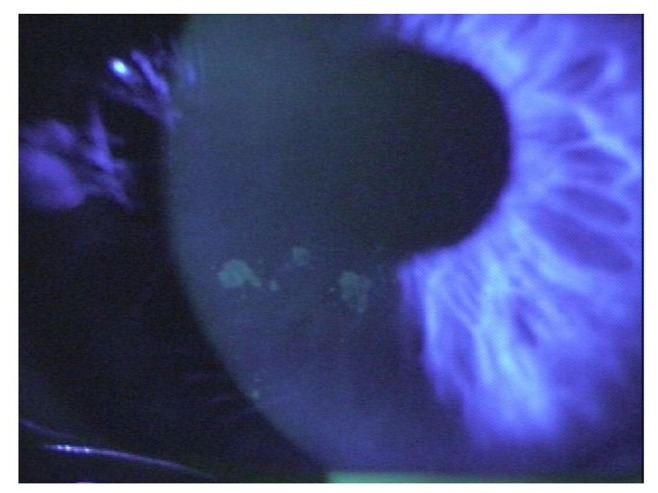
Corneal neuropathy is the ocular manifestation of diabetic peripheral neuropathy, the most common systemic complication of diabetes.28,30–34 Clinically, corneal neuropathy presents as diminished corneal sensation secondary to corneal denervation. Desensitization is thought to disrupt the cornea’s ability to maintain a normal epithelium, alter normal tear production (Figure 3), and is linked to a low rate of cell regeneration and impaired wound healing.35–40 Animal studies have shown a link between corneal denervation and slow-healing corneal ulcers.30
Figure 3.
Dry eye in a diabetic patient as a result of diabetic corneal neuropathy.
The pathophysiology of diabetic corneal neuropathy is chronic hyperglycemia. In chronic high glucose states, the unmyelinated nerves of the cornea experience axonal degeneration. Interestingly, corneal nerves seem to be the first nerves affected by neuropathy, and corneal desensitization frequently appears before other neuropathic symptoms are observed.15,19,28,33,34 As such, corneal esthesiometry has been proposed as an inexpensive screening test for diabetes.41 Patients with corneal neuropathy are more likely to have comorbid retinopathy. Rogell found that testing corneal sensation to screen for retinopathy had a sensitivity of 88.9% and a specificity of 77.8%.35
Hyperglycemia and the lens
A number of studies have shown that hyperglycemia, defined as serum glucose > 300 mg/dL, is a source of transient myopic refractive changes in diabetic patients.42 The pathophysiology to explain this phenomenon is linked to the production of the glucose breakdown product sorbitol, which is osmotically active. Sorbitol can accumulate in the lens of diabetic patients during hyperglycemic episodes, which in turn draws water into the lens, making it rounder and inducing a transient myopia. Studies have shown that when hyperglycemia is medically treated, these transient refractive changes abate, resulting in most patients becoming more hyperopic than during their transient hyperglycemic state.42,43 It is therefore recommended that refraction in diabetic patients be measured when blood glucose can be confirmed at a value below 200 mg/dL.
Diabetes, infection, and wound healing
It is widely accepted that diabetics are at increased risk for infection and poor wound healing. Like most other complications of diabetes, the etiology is linked to chronic hyperglycemia. Research has demonstrated neutrophil chemotaxis, phagocytosis, and bactericidal activity are all depressed in diabetic patients with poor glycemic control.44,45 Infection risk is compounded by vascular insufficiency, a common comorbidity in diabetic patients. This is because ischemic tissue can serve as a harbor for bacteria, and it also limits the oxygen-dependent bactericidal activity of leukocytes.4,11
Diabetes and surgical site infections
Due to the impaired immune response, diabetic patients are at risk for surgical-site infections.46,47 In a study of 1561 general and cardiothoracic surgery patients, glucose level was the most important risk factor for developing a surgical-site infection.46 The same study found a linear relationship between glucose level and infection risk; the higher the serum glucose at time of surgery, the greater the risk of infection.46 It follows that diabetic patients with a history of tight glycemic control and hemoglobin A1c levels at or below target levels do not appear to be at a greater risk than the general population.47
Diabetes and ocular infections
While diabetes is a risk factor for several types of infection (notably skin and foot ulcers and urinary tract infections), there is no direct evidence to show that diabetic patients have an increased risk of ocular infection. A correlational relationship between diabetes and eye infections was reported in a case series from Romania, which found patients presenting with serious ocular infections were significantly more likely to have diabetes than the general population.48 Reports of a global increase in the risk of infection in the diabetic population suggest that diabetic patients with poor glycemic control are at increased risk for eye infections; however, further investigation is needed to firmly establish this relationship.
Wound healing in diabetic patients
The combination of depressed immune system, neuropathy, and vascular insufficiency creates the ideal conditions for chronic wounds to develop.49 Neuropathic patients have a diminished ability to sense pain, meaning that tissue trauma, especially microtrauma, can go unrecognized.50 Tissue disruption allows bacteria access to the body, and without a robust immune response, infections may continue unchecked. Venous insufficiency compounds the problem by creating an avascular, relatively hypoxic environment for wounds to persist.51
Corneal wound healing in diabetic patients
Multiple animal models have determined that diabetic corneas heal more slowly than healthy corneas.52–55 Clinical and laboratory studies regarding the corneal wound healing process in human diabetic subjects are limited; however, there are several studies that validate the animal models. Chen et al found that 72.3% of diabetic eyes showed evidence of delayed wound healing at 1 month and 3 months after corneal epithelial debridement in diabetic vitrectomy. All defects resolved by 6 months postoperatively.27 Slow-healing neurotrophic corneal ulcerations were reported by Hyndiuk et al, who showed an increased incidence of persistent corneal ulcers in diabetic patients after vitrectomy cases in which the corneal epithelium was exposed, abraded, or removed.30 It is likely that the delayed healing seen in diabetic corneas is directly related to diminished corneal sensation resulting from diabetic corneal neuropathy. Rosenberg et al found a significant correlation between the diminished corneal sensation seen in diabetic corneal neuropathy and slow corneal wound healing. They also found that patients with diabetic corneal neuropathy eventually develop significant thinning of the epithelial layer, predisposing them to corneal damage.56 Sigelman and Friedenwald found that the corneal epithelial mitotic rate is significantly reduced in patients with reduced corneal innervation seen in diabetic corneal neuropathy, which further explains the impaired healing rate seen in these patients.57
Ocular surgery in diabetic patients
When the FDA made its recommendations about LASIK surgery in diabetic patients, the outcomes of diabetic patients during other ocular surgeries were cited for support. There is a significant collection of data in the literature documenting a correlation between diabetes and increased risk for postsurgical complications in these patients. In addition to the delayed corneal wound healing observed after diabetic vitrectomy noted in the two studies above, multiple studies have identified diabetes as a risk factor for corneal decompensation after argon laser photocoagulation.27,58–60 Diabetic patients tend to experience increased and extended inflammation after cataract extraction when compared to controls.61 Although rare, diabetic patients also have a higher incidence of both infectious and sterile endophthalmitis after cataract surgery when compared to the general population. The Endophthalmitis Vitrectomy Study found postsurgical infectious endophthalmitis in diabetic patients is more frequently associated with more virulent organisms and a higher percentage of Gram-negative isolates.62 Using data from the Endophthalmitis Vitrectomy Study, Doft et al discovered that although endophthalmitis in diabetic patients responds to aggressive treatment, postinfection outcomes in these patients tends to be worse, with only 56% of patients achieving 20/100 versus 77% of nondiabetic patients.63
LASIK in diabetic patients
Given that corneal complications, infection risk, and delayed wound healing are common among diabetic patients, understandable questions exist regarding the safety and efficacy of LASIK in this patient population. Unfortunately, limited data is currently available on this topic. To date, three retrospective analyses and a small selection of case reports of the outcomes of LASIK surgery in diabetic patients are available.
One of the earliest publications on this topic is a retrospective analysis of 30 diabetic eyes by Fraunfelder and Rich.3 They found that the rate of post-LASIK complications in diabetic patients was 47%, which was significantly higher than the rate of complications reported in control eyes (6.9%; P < 0.001). The most frequent complications were related to wound healing; nine of 17 eyes developed punctate epithelial erosions, and six of 17 developed persistent epithelial defects. Six eyes experienced delayed wound healing lasting longer than 1 month. The authors did not report any cases of postoperative infection. Refractive outcomes for the diabetic group were worse than in the control population. Postoperative uncorrected visual acuity (in logarithm of the minimal angle of resolution) in the diabetic population was 0.30 ± 0.11 compared to 0.17 ± 0.89 in the control group (P = 0.18). Unfortunately, this study did not offer any comment on the patients’ glycemic control, or whether the patients presented with comorbid ocular or systemic diabetic complications.3
Two more recent retrospective studies directly contradict Fraunfelder and Rich’s findings. In a study of 46 eyes, Halkiadakis et al reported a complication rate of 6.5% (three eyes). All three complications were epithelial defects. There was no incidence of postoperative infection in the diabetic population. Refractive outcomes for diabetic patients in this study were very good. The logarithm of the minimal angle of resolution of the diabetic cohort was 0.11 ± 0.16 and at last follow-up, 43 eyes (93.5%) were within 0.5 Diopters (D) of targeted spherical equivalent. Only patients with a history of well-controlled diabetes without systemic complications or evidence of keratopathy were included in this analysis. Two patients with mild, nonproliferative retinopathy were included; these eyes did not experience any complications or exacerbation of retinopathy.8
A study by Cobo-Soriano et al, of similar size (44 eyes) and patient characteristics to Halkiadakis et al, reported similar results. The complication rate in this study was slightly higher (9.1%). Reported complications included postoperative punctate keratopathy, mild epithelial ingrowth, and a peripheral interface reaction, all of which resolved without sequelae. Refractive outcomes were good, 92% of patients were within 0.5 D of intended spherical equivalent.64
An isolated case report by Ghanbari and Ahmadieh details the acute worsening of proliferative DR after LASIK. This patient had poorly controlled diabetes, with a hemoglobin A1c of 13.1% (upper limit of normal = 7.5%), and a long history of proliferative DR.65 Proposed theories on why LASIK aggravated the retinopathy in this patient include transient ischemia induced by increased intraocular pressure during the procedure and increased inflammatory response related to the patient’s uncontrolled hyperglycemia.65,66
Clearly, a significant difference exists between the complication rate reported by Fraunfelder and Rich and those reported by Cobo-Soriano et al and Halkiadakis et al (Table 2). The most obvious explanation for this discrepancy is that both Cobo-Soriano et al and Halkiadakis et al excluded diabetics with a history of poor glycemic control. In the two studies that eliminated patients with poor glucose control, the complication rate was minimal.8,28 Additionally, the sole case report of post-LASIK exacerbation of proliferative DR was in a patient with significantly elevated fasting glucose and glycosylated hemoglobin levels (glucose = 250 mg/dL and hemoglobin A1c = 13%).65 As noted earlier, the pathophysiology behind both ocular and systemic complications of diabetes is directly linked to hyperglycemia. The risk of developing ocular complications is significantly lower in diabetic patients with glucose levels within target range. Therefore, it may be reasonable to assume that diabetic patients with tight glycemic control may not be at increased risk for LASIK-related complications when compared to diabetic patients with poor disease control.
Table 2.
Comparison of results of three retrospective studies of laser-assisted in situ keratomileusis in diabetic patients
| Author | Year | Eyes | Complication rate | Complications |
|---|---|---|---|---|
| Fraunfelder and Rich3 | 2002 | 30 | 47% | Punctate epithelia erosions (9) Persistent epithelial defects (6) Flap edema (1) Flap stroma (1) Total = 17 |
| Halkiadakis et al8 | 2005 | 46 | 6.5% | Epithelial defects (3) Total = 3 |
| Cobo-Soriano et al64 | 2006 | 43 | 11% | Punctate epithelial erosions (2) Epithelial ingrowth (1) Intraoperative deepithelialization (1) Peripheral interface reaction (1) Total = 5 |
Further explanation of the difference in complication rates reported in these studies is the exclusion of patients with ocular complications of diabetes from two of the three studies. Diabetic keratopathy and corneal neuropathy were factors for exclusion from the two studies documenting favorable post-LASIK outcomes. As noted previously, corneal complications are an early complication of diabetes, and the presence of corneal disease is considered a marker for systemic complications and poor disease control. By excluding patients with evidence of diabetic keratopathy or corneal neuropathy, these studies likely screened out all diabetics with advanced disease, poor glycemic control, or systemic symptoms. As such, it appears that diabetic patients with no corneal or systemic complications may be suitable candidates for LASIK.
Halkiadakis et al did include two patients with stable, nonproliferative DR. These patients did not experience any postoperative complications or aggravation of their retinopathy.8 While this finding suggests that mild DR may not be a risk factor for complications, a sample size of two is not sufficient to support this assumption. DR is a clinical marker of systemic disease, and therefore still represents increased risk.
Femtosecond laser versus microkeratome
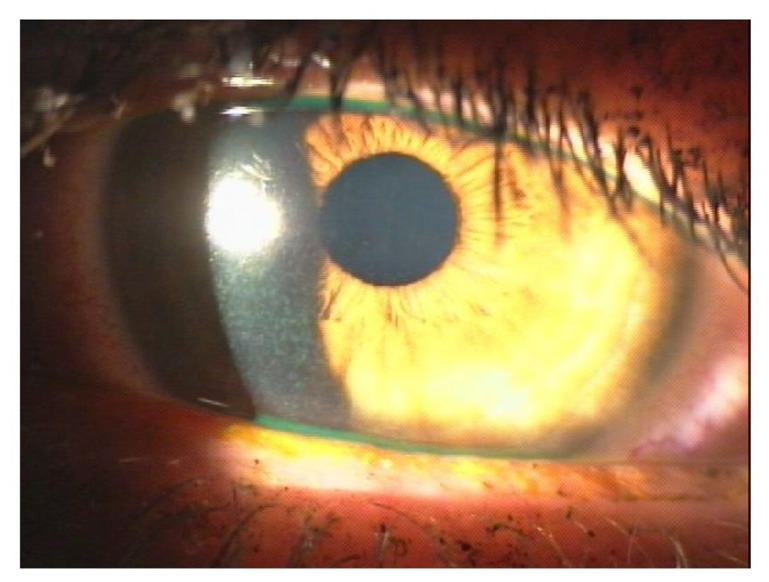
No studies are currently available on whether there is a difference in LASIK outcomes in diabetic patients between the femtosecond laser and the microkeratome. A major criticism of the microkeratome is the increased incidence of epithelial complications from the shear stress generated by the oscillating blade as it travels across the corneal stroma. The shearing force is believed to disrupt the adherence of the corneal epithelium to the basement membrane.67 The femtosecond laser eliminates this shear stress because the corneal flap is created with a laser, not a blade. A meta-analysis comparing outcomes of LASIK surgery with the microkeratome versus femtosecond laser found a statistically significant increase in the incidence of epithelial complications (P = 0.04) associated with the microkeratome.68 A study by Moshirfar et al comparing LASIK outcomes between the two techniques found that epithelial defects/sloughing were significantly more common in the microkeratome group (P = 0.006) compared to the femtosecond laser group.69 The previously cited studies report that diabetic patients tend to have both epithelial defects and sloughing (Figure 4). Some have theorized that since the femtosecond laser is associated with less epithelial complications, patients with preexisting epithelial abnormalities or irregularities of adhesion complexes may have better outcomes with the femtosecond laser.68 Further investigation into the potential benefits of using the femtosecond laser versus the microkeratome for flap creation in diabetic patients is warranted.
Figure 4.
Epithelial sloughing with mild dislocation of flap in the eye of a diabetic patient after laser-assisted in situ keratomileusis.
Type I versus type II diabetes
Only Halkiadakis et al examined the difference in complication rates between patients with type I and type II diabetes. Seven patients had type I diabetes and one of these patients experienced a complication, 17 patients were type II diabetics and two of these patients reported complications.8 The reported data is insufficient to determine a difference in complications between these two disease subtypes. Further investigation into the differences in outcomes between diabetes subtypes is necessary.
Limitations
There are significant limitations to this review. The current literature is devoid of prospective studies and only a small selection of retrospective studies is available on this topic. The three existing studies all have small sample sizes, making their results of questionable significance. However, as they represent the only available clinical data on this issue, they merit significant consideration. This review has identified a number of areas that require additional investigation including, but not limited to: prospective trials of LASIK in patients with well-controlled diabetic parameters, prospective or retrospective studies examining outcomes of LASIK surgery on diabetic patients with varying A1c levels, investigation into the use of microkeratome versus femtosecond laser, and the differences in complications between type I and type II diabetics.
Recommendations and conclusion
LASIK in patients with diabetes mellitus is currently a relative contraindication to refractive surgery. Reservations about performing refractive surgery in this population come from general observations on the pathologic changes identified in the diabetic cornea, the weakened immune response, and delayed wound healing seen in these patients. Investigation into the pathophysiology of the ocular and systemic complications of diabetes shows that these complications are tightly linked to hyperglycemia, and that diabetics with excellent glucose control are at significantly less risk for developing them. It is also clear that despite the recommendation against LASIK surgery in these patients, a significant number receive this procedure annually. Therefore, it is appropriate to offer recommendations on when LASIK is an acceptable procedure in these patients, despite the current paucity of clinical evidence that exists. Diabetic patients may be considered suitable candidates for LASIK only after a thorough preoperative assessment reveals evidence of excellent glucose control for at least 1 year prior to surgery, and confirms a lack of systemic complications (Table 3). Patients who do not satisfy these criteria should not be considered for LASIK.
Table 3.
Preoperative evaluation of the diabetic patient
| History | |
| Detailed diabetic history | |
| Onset and progression | |
| Medication regimen (insulin dependent?) | |
| History of diabetic ulcers or skin infection | |
| Loss of sensation or tingling in lower extremity? | |
| Consider obtaining records from internist | |
| Detailed ocular history | |
| Recent changes in visual acuity | |
| History of chronic or recurrent infections | |
| History of recurrent epithelial erosions | |
| History of morning dry eye (silent erosions) | |
| History of chronic itching or burning sensation? | |
| Full review of systems | |
| Physical exam | |
| Microfilament exam to rule out neuropathy | |
| Foot exam to rule out diabetic ulcer | |
| Slit lamp exam | |
| Normal external structures | |
| Normal tear function, no dry eye or stippling | |
| Intact cornea with no epithelial defects | |
| Corneal esthesiometry to rule out neuropathy* | |
| Thorough basement membrane exam | |
| Check for filaments | |
| Rule out subtle basement membrane changes | |
| No evidence of retinopathy | |
| Normal optic disk and vessels | |
| Laboratory | Value |
|
| |
| Fasting serum glucose | 90–130 mg/dL |
| Hemoglobin A1c | ≤7.9% |
| Urine analysis* | Absent glucose Microalbumin/creatinine ≤ 30 μg/mg |
Note:
Optional.
In addition to the preoperative evaluation recommended, the importance of informed consent should also be emphasized. A complete informed consent should include an explanation of the risks and benefits of the procedure and an explicit conversation about the current recommendations regarding diabetic patients and LASIK surgery. Patients should be aware that despite the recent evidence indicating that LASIK is likely safe in diabetics, there is still additional risk.
Based on the available evidence, diabetic patients with excellent glucose control and no ocular or systemic complications can receive LASIK surgery safely with little risk of complications or poor refractive outcomes. Further investigation to establish safety parameters, patient outcomes, and otherwise inform best surgical practice in this patient population is encouraged.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organization. Diabetes fact sheet. Aug, 2011. [Accessed July 11, 2012]. Available from: http://www.who.int/mediacentre/factsheets/fs312/en.
- 2.United States Food and Drug Administration. When is LASIK not for me? Dec 9, 2011. [Accessed July 11, 2012]. Available from: http://www.fda.gov/medicaldevices/productsandmedicalprocedures/surgeryandlifesupport/lasik/ucm061366.htm.
- 3.Fraunfelder FW, Rich LF. Laser-assisted in situ keratomileusis complications in diabetes mellitus. Cornea. 2002;21(3):246–248. doi: 10.1097/00003226-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Weintrob AC, Sexton DJ. UpToDate. Susceptibility to infections in persons with diabetes mellitus. 2012. [Accessed July 11, 2012]. Available from: http://www.uptodate.com/contents/susceptibility-to-infections-in-persons-with-diabetes-mellitus.
- 5.International Diabetes Federation. Diabetes Atlas. Fourth Edition. Oct, 2009. [Accessed July 11, 2012]. Available from: http://archive.diabetesatlas.org/
- 6.American Diabetes Association. Diabetes statistics. Jan, 2011. [Accessed July 11, 2012]. Available from: http://www.diabetes.org/diabetes-basics/diabetes-statistics.
- 7.Rossi L. A clear eyed view of LASIK. Durham Magazine. 2010 Feb-Mar;:26–29. [Google Scholar]
- 8.Halkiadakis I, Belfair N, Gimbel HV. Laser in situ keratomileusis in patients with diabetes. J Cataract Refract Surg. 2005;31(10):1895–1898. doi: 10.1016/j.jcrs.2005.03.075. [DOI] [PubMed] [Google Scholar]
- 9.Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350(1):48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 10.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 11.Kohner EM, Patel V, Rassam SM. Role of blood flow and impaired autoregulation in the pathogenesis of diabetic retinopathy. Diabetes. 1995;44(6):603–607. doi: 10.2337/diab.44.6.603. [DOI] [PubMed] [Google Scholar]
- 12.Brownlee M. Lilly Lecture 1993. Glycation and diabetic complications. Diabetes. 1994;43(6):836–841. doi: 10.2337/diab.43.6.836. [DOI] [PubMed] [Google Scholar]
- 13.Yeh PT, Yang CM, Huang JS, et al. Vitreous levels of reactive oxygen species in proliferative diabetic retinopathy. Ophthalmology. 2008;115(4):734–737. doi: 10.1016/j.ophtha.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 14.Boulton M, Foreman D, Williams G, McLeod D. VEGF localisation in diabetic retinopathy. Br J Ophthalmol. 1998;82(5):561–568. doi: 10.1136/bjo.82.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez-Thorin JC. The cornea in diabetes mellitus. Int Ophthalmol Clin. 1998;38(2):19–36. [PubMed] [Google Scholar]
- 16.Schultz RO, Van Horn DL, Peters MA, Klewin KM, Schutten WH. Diabetic keratopathy. Trans Am Ophthalmol Soc. 1981;79:180–199. [PMC free article] [PubMed] [Google Scholar]
- 17.Foulks GN, Thoft RA, Perry HD, Tolentino FI. Factors related to corneal epithelial complications after closed vitrectomy in diabetics. Arch Ophthalmol. 1979;97(6):1076–1078. doi: 10.1001/archopht.1979.01020010530002. [DOI] [PubMed] [Google Scholar]
- 18.Davies PD, Duncan G, Pynsent PB, Arber DL, Lucas VA. Aqueous humor glucose concentrations in cataract patients and its effect on the lens. Exp Eyes Res. 1984;39(5):605–609. doi: 10.1016/0014-4835(84)90060-5. [DOI] [PubMed] [Google Scholar]
- 19.Rao GN. Dr P Siva Reddy Oration: Diabetic keratopathy. Indian J Ophthalmol. 1987;35(5–6):16–36. [PubMed] [Google Scholar]
- 20.Azar DT, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Decreased penetration of anchoring fibrils into the diabetic stroma. A morphometric analysis. Arch Ophthalmol. 1989;107(10):1520–1523. doi: 10.1001/archopht.1989.01070020594047. [DOI] [PubMed] [Google Scholar]
- 21.Cobo LM, Hatchell DL. Treatment of severe diabetic keratopathy with a topical aldose reductase inhibitor: clinical response and electron microscopy [abstract] Invest Ophthalmol Vis Sci. 1980;26(Suppl):176. [Google Scholar]
- 22.Fukushi S, Merola LO, Tanaka M, Datiles M, Kinoshita JH. Reepithelialization of denuded corneas in diabetic rats. Exp Eye Res. 1980;31(5):611–621. doi: 10.1016/s0014-4835(80)80020-0. [DOI] [PubMed] [Google Scholar]
- 23.Kenyon K, Wafai Z, Michels R, et al. Corneal basement membrane abnormality in diabetes mellitus [abstract] Invest Ophthalmol Vis Sci. 1978;17(Suppl):245. [Google Scholar]
- 24.Taylor HR, Kimsey RA. Corneal epithelial basement membrane changes in diabetes. Invest Ophthalmol Vis Sci. 1981;20(4):548–553. [PubMed] [Google Scholar]
- 25.Hatchell DL, Magolan JJ, Jr, Besson MJ, Goldman AI, Pederson HJ, Schultz KJ. Damage to the epithelial basement membrane in the corneas of diabetic rabbits. Arch Ophthalmol. 1983;101(3):469–471. doi: 10.1001/archopht.1983.01040010469029. [DOI] [PubMed] [Google Scholar]
- 26.Kaji Y, Usui T, Oshika T, et al. Advanced glycation end products in diabetic corneas. Invest Ophthalmol Vis Sci. 2000;41(2):362–368. [PubMed] [Google Scholar]
- 27.Chen WL, Lin CT, Ko PS, et al. In vivo confocal microscopic findings of corneal wound healing after corneal epithelial debridement in diabetic vitrectomy. Ophthalmology. 2009;116(6):1038–1047. doi: 10.1016/j.ophtha.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Schultz RO, Peters MA, Sobocinski K, Nassif K, Schultz KJ. Diabetic corneal neuropathy. Trans Am Ophthalmol Soc. 1983;81:107–124. [PMC free article] [PubMed] [Google Scholar]
- 29.Saini JS, Khandalavla B. Corneal epithelial fragility in diabetes mellitus. Can J Ophthalmol. 1995;30(3):142–146. [PubMed] [Google Scholar]
- 30.Hyndiuk RA, Kazarian EL, Schultz RO, Seideman S. Neutrotrophic corneal ulcers in diabetes mellitus. Arch Ophthalmol. 1977;95(12):2193–2196. doi: 10.1001/archopht.1977.04450120099012. [DOI] [PubMed] [Google Scholar]
- 31.Martin XY, Safran AB. Corneal hypoesthesia. Surv Ophthalmol. 1988;33(1):28–40. doi: 10.1016/0039-6257(88)90070-7. [DOI] [PubMed] [Google Scholar]
- 32.Miller NR. Topical diagnosis in the trigeminal somatic sensory nerve. In: Miller NR, editor. Walsh and Hoyt’s Clinical Neuro-Ophthalmology. Baltimore, MD: Williams & Wilkins; 1985. pp. 1054–1070. [Google Scholar]
- 33.Schultz RO, Peters MA, Sobocinski K, Nassif K, Schultz KJ. Diabetic keratopathy as a manifestation of peripheral neuropathy. Am J Ophthalmol. 1983;96(3):368–371. doi: 10.1016/s0002-9394(14)77829-8. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz DE. Corneal sensitivity in diabetics. Arch Ophthalmol. 1974;91(3):174–178. doi: 10.1001/archopht.1974.03900060182003. [DOI] [PubMed] [Google Scholar]
- 35.Rogell GD. Corneal hypesthesia and retinopathy in diabetes mellitus. Ophthalmology. 1980;87(3):229–233. doi: 10.1016/s0161-6420(80)35257-3. [DOI] [PubMed] [Google Scholar]
- 36.Beuerman RW, Schimmelphennig B, Burstein N. Anatomy of the denervated corneal epithelium [abstract] Invest Ophthalmol Vis Sci. 1979;18(Suppl):126. [Google Scholar]
- 37.Paton L. The trigeminal and its ocular lesions. Br J Ophthalmol. 1926;10(6):305–342. doi: 10.1136/bjo.10.6.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parrish CM. The cornea in diabetes mellitus. In: Feman SS, editor. Ocular Problems in Diabetes Mellitus. Boston, MA: Blackwell Scientific; 1992. pp. 179–205. [Google Scholar]
- 39.Beuerman RW, Schimmelpfennig B. Sensory denervation of the rabbit cornea affects epithelial properties. Exp Neurol. 1980;69(1):196–201. doi: 10.1016/0014-4886(80)90154-5. [DOI] [PubMed] [Google Scholar]
- 40.Mishima S. The effects of the denervation and the stimulation of the sympathetic and the trigeminal nerve on the mitotic rate of the corneal epithelium in the rabbit. Jpn J Ophthalmol. 1957;1:65–73. [Google Scholar]
- 41.Scullica L, Proto F. Clinical and statistical findings on corneal sensitivity in diabetics. Boll Ocul. 1965;44(12):944–954. Italian. [PubMed] [Google Scholar]
- 42.Sonmez B, Bozkurt B, Atmaca A, Irkec M, Orhan M, Aslan U. Effect of glycemic control on refractive changes in diabetic patients with hyperglycemia. Cornea. 2005;24(5):531–537. doi: 10.1097/01.ico.0000151545.00489.12. [DOI] [PubMed] [Google Scholar]
- 43.Okamoto F, Sone H, Nonoyama T, Hommura S. Refractive changes in diabetic patients during intensive glycaemic control. Br J Ophthalmol. 2000;84(10):1097–1102. doi: 10.1136/bjo.84.10.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med. 1997;14(1):29–34. doi: 10.1002/(SICI)1096-9136(199701)14:1<29::AID-DIA300>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 45.Llorente L, De La Fuente H, Richaud-Patin Y, et al. Innate immune response mechanisms in non-insulin dependent diabetes mellitus patients assessed by flow cytoenzymology. Immunol Lett. 2000;74(3):239–244. doi: 10.1016/s0165-2478(00)00255-8. [DOI] [PubMed] [Google Scholar]
- 46.Ata A, Lee J, Bestle SL, Desemone J, Stain SC. Postoperative hyperglycemia and surgical site infection in general surgery patients. Arch Surg. 2010;145(9):858–864. doi: 10.1001/archsurg.2010.179. [DOI] [PubMed] [Google Scholar]
- 47.Lantham R, Lancaster AD, Covington JF, Pirolo JS, Thomas CS., Jr The association of diabetes and glucose control with surgical-site infections among cardiothoracic surgery patients. Infect Control Hosp Epidemiol. 2001;22(10):607–612. doi: 10.1086/501830. [DOI] [PubMed] [Google Scholar]
- 48.Ignat F, Mocanu C. Evolution of ocular infection in diabetes mellitus patients. Oftalmologia. 2001;53(3):74–77. Romanian. [PubMed] [Google Scholar]
- 49.Kurd SK, Hoffstad OJ, Bilker WB, Margolis DJ. Evaluation of the use of prognostic information for the care of individuals with venous leg ulcers or diabetic neuropathic foot ulcers. Wound Repair Regen. 2009;17(3):318–325. doi: 10.1111/j.1524-475X.2009.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bowering CK. Diabetic foot ulcers. Pathophysiology, assessment, and therapy. Can Fam Physician. 2001;47:1007–1016. [PMC free article] [PubMed] [Google Scholar]
- 51.Santilli JD, Santilli SM. Chronic critical limb ischemia: diagnosis, treatment and prognosis. Am Fam Physician. 1999;59(7):1899–1908. [PubMed] [Google Scholar]
- 52.Friend J, Ishii Y, Throft RA. Corneal epithelial changes in diabetic rats. Ophthalmic Res. 1982;14(4):269–278. doi: 10.1159/000265202. [DOI] [PubMed] [Google Scholar]
- 53.Hallberg CK, Trocme SD, Ansari NH. Acceleration of corneal wound healing in diabetic rats by the antioxidant trolox. Res Commun Mol Pathol Pharmacol. 1996;93(1):3–12. [PubMed] [Google Scholar]
- 54.Xu K, Yu FS. Impaired epithelial wound healing and EGFR signaling pathways in the corneas of diabetic rats. Invest Ophthalmol Vis Sci. 2011;52(6):3301–3308. doi: 10.1167/iovs.10-5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagai N, Murao T, Okamoto N, Ito Y. Kinetic analysis of the rate of corneal wound healing in Otsuka Long-Evans Tokushima Fatty rats, a model of type 2 diabetes mellitus. J Oleo Sci. 2010;59(8):441–449. doi: 10.5650/jos.59.441. [DOI] [PubMed] [Google Scholar]
- 56.Rosenberg ME, Tervo TM, Immonen IJ, Muller LJ, Gronhagen-Riska C, Vesaluoma MH. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci. 2000;41(10):2915–2921. [PubMed] [Google Scholar]
- 57.Sigelman S, Friedenwald JS. Mitotic and wound-healing activities of the corneal epithelium; effect of sensory denervation. AMA Arch Ophthalmol. 1954;52(1):46–57. doi: 10.1001/archopht.1954.00920050048005. [DOI] [PubMed] [Google Scholar]
- 58.Jeng S, Lee JS, Huang SC. Corneal decompensation after argon laser iridectomy – a delayed complication. Ophthalmic Surg. 1991;22(10):565–569. [PubMed] [Google Scholar]
- 59.Schwartz AL, Martin NF, Weber PA. Corneal decompensation after argon laser iridectomy. Arch Ophthalmol. 1988;106(11):1572–1574. doi: 10.1001/archopht.1988.01060140740047. [DOI] [PubMed] [Google Scholar]
- 60.Mackay CJ, Koester CJ, Campbell CJ. The corneal endothelium following photocoagulation: induced decompensation. Ann Ophthalmol. 1983;15(4):346–351. [PubMed] [Google Scholar]
- 61.Fintak DR, Ho AC. Perioperative and operative considerations in diabetes. Ophthalmol Clin North Am. 2006;19(4):427–434. doi: 10.1016/j.ohc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 62.Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study: a randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995;113(12):1479–1496. [PubMed] [Google Scholar]
- 63.Doft BH, Wisniewski SR, Kelsey SF, Fitzgerald SG. Diabetes and postoperative endophthalmitis in the Endophthalmitis Vitrectomy Study. Arch Ophthalmol. 2001;119(5):650–656. doi: 10.1001/archopht.119.5.650. [DOI] [PubMed] [Google Scholar]
- 64.Cobo-Soriano R, Beltran J, Baviera J. LASIK outcomes in patients with underlying systemic contraindications: a preliminary study. Ophthalmology. 2006;113(7):1118. e1–1118.e8. doi: 10.1016/j.ophtha.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 65.Ghanbari H, Ahmadieh H. Aggravation of proliferative diabetic retinopathy after laser in situ keratomileusis. J Cataract Refract Surg. 2003;29(11):2232–2233. doi: 10.1016/s0886-3350(03)00355-9. [DOI] [PubMed] [Google Scholar]
- 66.Ersanli D, Akin T, Karadayi K. Aggravation of proliferative diabetic retinopathy after LASIK. J Cataract Refract Surg. 2005;31(6):1086–1087. doi: 10.1016/j.jcrs.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 67.Polk EE, Wexler SA, Kymes S. Incidence of corneal epithelial defects with the standard and zero-compression Hansatome microkeratomes. J Refract Surg. 2005;21(4):359–364. doi: 10.3928/1081-597X-20050701-10. [DOI] [PubMed] [Google Scholar]
- 68.Chen S, Feng Y, Stojanovic A, Jankov MR, 2nd, Wang Q. IntraLase femtosecond laser vs mechanical microkeratomes in LASIK for myopia: a systematic review and meta-analysis. J Refract Surg. 2012;28(1):15–24. doi: 10.3928/1081597X-20111228-02. [DOI] [PubMed] [Google Scholar]
- 69.Moshirfar M, Gardiner JP, Schliesser JA, et al. Laser in situ keratomileusis flap complications using mechanical microkeratome versus femtosecond laser: retrospective comparison. J Cataract Refract Surg. 2010;36(11):1925–1933. doi: 10.1016/j.jcrs.2010.05.027. [DOI] [PubMed] [Google Scholar]