Abstract
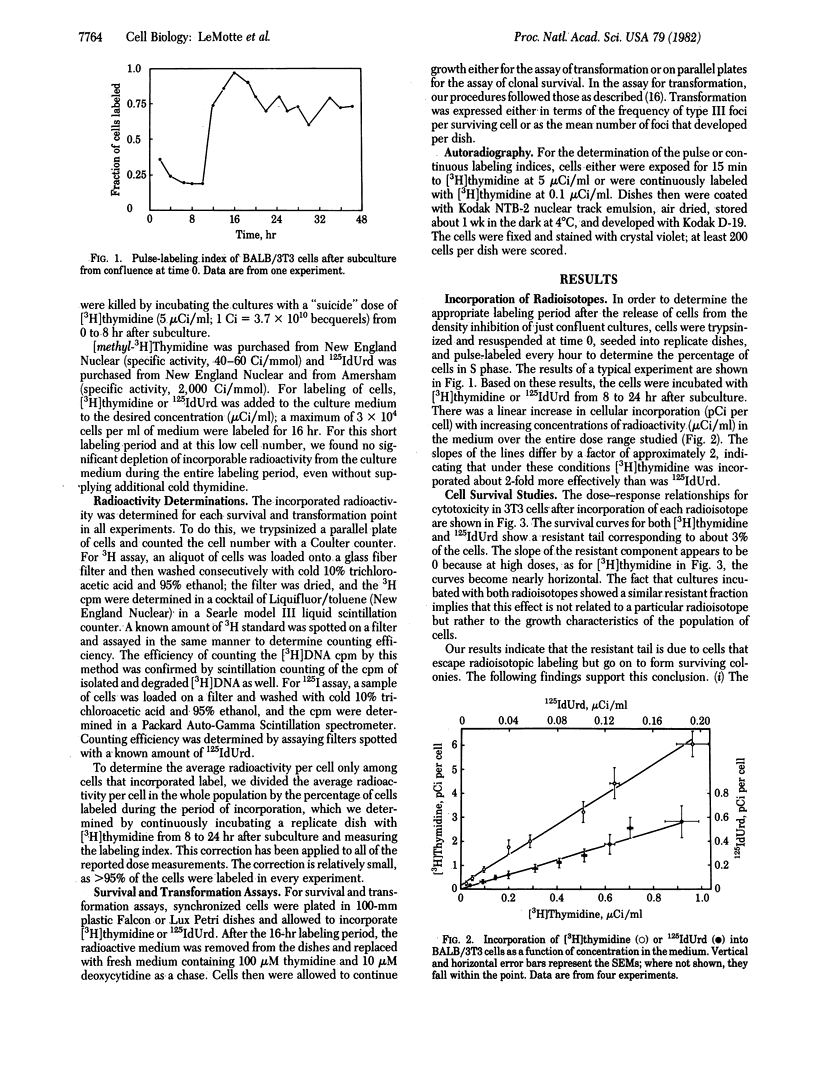
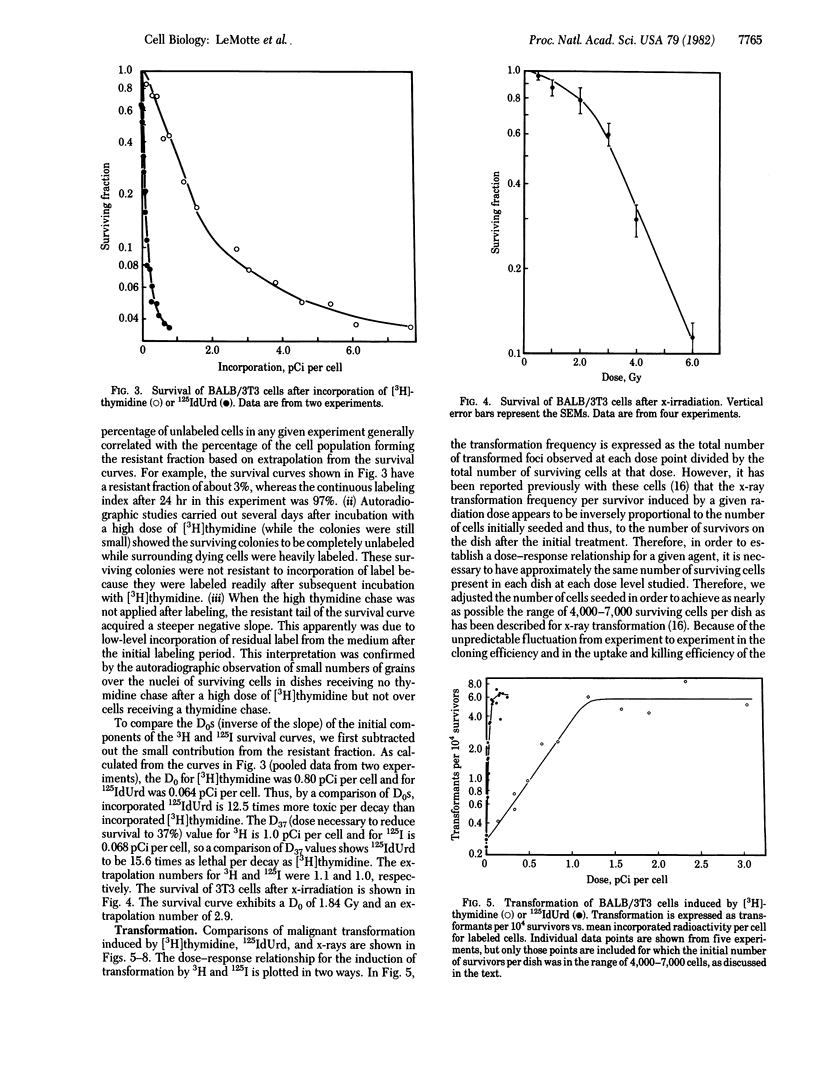
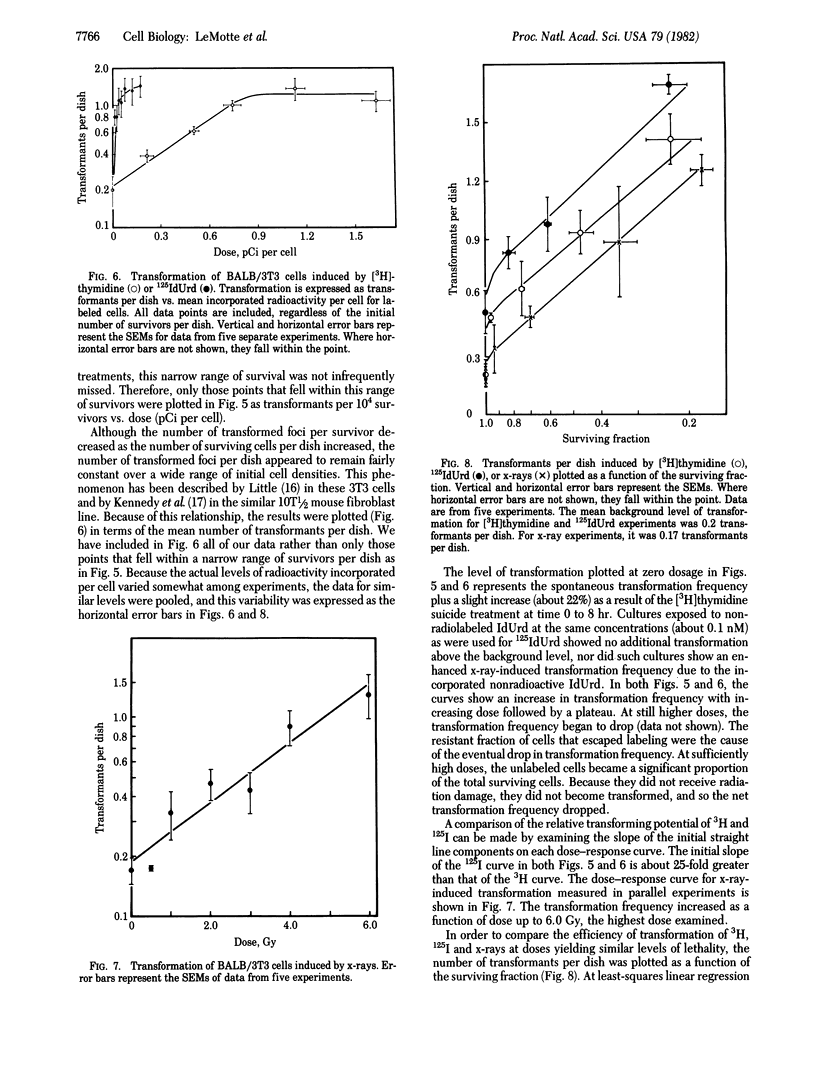
The induction of lethality and malignant transformation by 5-[125I]iododeoxyuridine and [3H]thymidine incorporated into cellular DNA and by x-irradiation was studied in vitro in BALB/3T3 cells. Under these conditions, 125I radiation is highly localized to small regions of the DNA at the site of each decay and produces DNA double-strand breaks with high efficiency. Incorporated 125I was found to be 12-16 times as lethal per decay as incorporated 3H. For the induction of malignant transformation, however, 125I was approximately 25 times as effective per decay as 3H. When the frequencies of transformation induced at various levels of survival by 125I, 3H, and x-rays were compared, lethality was found to correlate closely with transformation at doses that yielded significant cell killing. An exception occurred at low doses, where 125I appeared much more efficient than x-irradiation in inducing transformation; transformation frequencies equal to those induced by 3-5 Gy of x-rays resulted from 125I exposures that yielded little or no cell killing.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett J. C., Ts'o P. O. Evidence for the progressive nature of neoplastic transformation in vitro. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3761–3765. doi: 10.1073/pnas.75.8.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. C., Tsutsui T., Ts'o P. O. Neoplastic transformation induced by a direct perturbation of DNA. Nature. 1978 Jul 20;274(5668):229–232. doi: 10.1038/274229a0. [DOI] [PubMed] [Google Scholar]
- Bond V. P., Feinendegen L. E. Intranuclear 3H thymidine: dosimetric, radiobiological and radiation protection aspects. Health Phys. 1966 Aug;12(8):1007–1020. doi: 10.1097/00004032-196608000-00002. [DOI] [PubMed] [Google Scholar]
- Borek C., Sachs L. In vitro cell transformation by x-irradiation. Nature. 1966 Apr 16;210(5033):276–278. doi: 10.1038/210276a0. [DOI] [PubMed] [Google Scholar]
- Burki H. J., Roots R., Feinendegen L. E., Bond V. P. Inactivation of mammalian cells after disintegration of 3H or 125I in cell DNA at -196 degrees C. Int J Radiat Biol Relat Stud Phys Chem Med. 1973 Oct;24(4):363–375. doi: 10.1080/09553007314551221. [DOI] [PubMed] [Google Scholar]
- Chan P. C., Lisco E., Lisco H., Adelstein S. J. The radiotoxicity of iodine-125 in mammalian cells II. A comparative study on cell survival and cytogenetic responses to 125IUdR, 131TUdR, and 3HTdR. Radiat Res. 1976 Aug;67(2):332–343. [PubMed] [Google Scholar]
- Charlton D. E., Booz J. A Monte Carlo treatment of the decay of 125I. Radiat Res. 1981 Jul;87(1):10–23. [PubMed] [Google Scholar]
- Ertl H. H., Feinendegen L. E., Heiniger H. J. Iodine-125, a tracer in cell biology: physical properties and biological aspects. Phys Med Biol. 1970 Jul;15(3):447–456. doi: 10.1088/0031-9155/15/3/005. [DOI] [PubMed] [Google Scholar]
- Hofer K. G., Harris C. R., Smith J. M. Rdiotoxicity of intracellular 67Ga, 125I and 3H. Nuclear versus cytoplasmic radiation effects in murine L1210 leukaemia. Int J Radiat Biol Relat Stud Phys Chem Med. 1975 Sep;28(3):225–241. doi: 10.1080/09553007514550991. [DOI] [PubMed] [Google Scholar]
- Kakunaga T. A quantitative system for assay of malignant transformation by chemical carcinogens using a clone derived from BALB-3T3. Int J Cancer. 1973 Sep 15;12(2):463–473. doi: 10.1002/ijc.2910120217. [DOI] [PubMed] [Google Scholar]
- Kennedy A. R., Fox M., Murphy G., Little J. B. Relationship between x-ray exposure and malignant transformation in C3H 10T1/2 cells. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7262–7266. doi: 10.1073/pnas.77.12.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisch R. E., Sauri C. J. Further studies of DNA damage and lethality from the decay of iodine-125 in bacteriophages. Int J Radiat Biol Relat Stud Phys Chem Med. 1975 Jun;27(6):553–560. doi: 10.1080/09553007514550581. [DOI] [PubMed] [Google Scholar]
- LISCO H., BASERGA R., KISIELESKI W. E. Induction of tumours in mice with tritiated thymidine. Nature. 1961 Nov 11;192:571–572. doi: 10.1038/192571a0. [DOI] [PubMed] [Google Scholar]
- Little J. B. Quantitative studies of radiation transformation with the A31-11 mouse BALB/3T3 cell line. Cancer Res. 1979 May;39(5):1474–1480. [PubMed] [Google Scholar]
- Martin R. F., Haseltine W. A. Range of radiochemical damage to DNA with decay of iodine-125. Science. 1981 Aug 21;213(4510):896–898. doi: 10.1126/science.7256283. [DOI] [PubMed] [Google Scholar]
- Pienta R. J., Poiley J. A., Lebherz W. B., 3rd Morphological transformation of early passage golden Syrian hamster embryo cells derived from cryopreserved primary cultures as a reliable in vitro bioassay for identifying diverse carcinogens. Int J Cancer. 1977 May 15;19(5):642–655. doi: 10.1002/ijc.2910190508. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Terzaghi M., Little J. B. X-radiation-induced transformation in a C3H mouse embryo-derived cell line. Cancer Res. 1976 Apr;36(4):1367–1374. [PubMed] [Google Scholar]



