Abstract
Glycoconjugates play various roles in biological processes. In particular, oligosaccharides on the surface of animal cells are involved in virus infection and cell-cell communication. Inhibitors of carbohydrate-protein interactions are potential antiviral drugs. Several anti-influenza drugs such as oseltamivir and zanamivir are derivatives of sialic acid, which inhibits neuraminidase. However, it is very difficult to prepare a diverse range of sugar derivatives by chemical synthesis or by the isolation of natural products. In addition, the pathogenic capsular polysaccharides of bacteria are carbohydrate antigens, for which a safe and efficacious method of vaccination is required. Phage-display technology has been improved to enable the identification of peptides that bind to carbohydrate-binding proteins, such as lectins and antibodies, from a large repertoire of peptide sequences. These peptides are known as “carbohydrate-mimetic peptides (CMPs)” because they mimic carbohydrate structures. Compared to carbohydrate derivatives, it is easy to prepare mono- and multivalent peptides and then to modify them to create various derivatives. Such mimetic peptides are available as peptide inhibitors of carbohydrate-protein interactions and peptide mimotopes that are conjugated with adjuvant for vaccination.
1. Introduction
A variety of glycoconjugate carbohydrate structures on the cell surface are important for biological events [1]. Carbohydrate structures on the cell surface change according to cell status, for example, during development, differentiation, and malignant alteration. Several glycoconjugates, including stage-specific embryonic antigen (SSEA)-3, SSEA-4, and tumor-rejection antigen (TRA)-1-60, are used as molecular makers of pluripotency to control the quality of induced pluripotent stem (iPS) cells [2]. Carbohydrate-protein interactions are the first cell surface events in cell-cell communication, following which processes such as infection and signal transduction occur. However, the reasons for the changes in carbohydrate structures on the cell surface are not clear. In addition, most receptors for glycoconjugates have not been identified. To investigate the biological roles of carbohydrates, sets of carbohydrates and their corresponding carbohydrate-binding proteins are required.
Carbohydrate-binding proteins such as plant lectins, bacterial toxins, and anticarbohydrate antibodies are available for studying carbohydrate-protein interactions [3, 4]. However, the repertoire of carbohydrate structures recognized by these proteins is limited and insufficient to cover the majority of structures. In addition, because carbohydrates are ubiquitous components of cell membranes and bio(macro)molecules, the immune response stimulated by glycoconjugates is negligible [5, 6], that is, high affinity carbohydrate-specific IgG-isotype antibodies are not easily obtained. Even if anticarbohydrate antibodies are generated, IgG comprises no more than 28% of the antibodies (74 IgGs in a total of 268 antibodies, with the remainder being IgMs) [7]. Therefore, while anticarbohydrate antibodies of the IgG isotype are preferred for carbohydrate research, IgM-antibodies with low affinity have been often used. Moreover, obtaining pure and homogeneous carbohydrates (or glycoconjugates) is very difficult. This is because regioselective protection of the hydroxy groups of the monosaccharide is required. Programmable one-pot oligosaccharide synthesis is widely performed using protected monosaccharides and/or oligosaccharides [8–10]. Enzyme-catalyzed oligosaccharide synthesis has been also developed [10–12]. Several oligosaccharides such as KH-1 antigen (nonasaccharide of LeY-LeX), globo-H hexasaccharide, and the core pentamannosides have been prepared by automated solid-phase oligosaccharide synthesis [8]. However, due to the complicated procedures of carbohydrate preparation, a general methodology for their chemical synthesis is not yet established.
To compensate for the lack of synthetic carbohydrates and to overcome their inherent weak immunogenicity, short peptides that bind to carbohydrate-binding proteins have been identified from phage-display libraries (Figure 1). These peptides mimic carbohydrate structures [13] and are called “carbohydrate-mimetic peptides (CMPs)” or “peptide mimotopes.” It is predicted that CMPs, as well as carbohydrates, are recognized by carbohydrate-binding proteins. Small molecules such as biotin and carbohydrate mimotope (Glycotope) mimicking peptides have been frequently identified, and a number of reviews focusing on different aspects of their properties and uses have been published [14–16]. In this paper, recent studies on the selection and application of CMPs are surveyed and summarized according to the classification of target carbohydrate-binding proteins.
Figure 1.
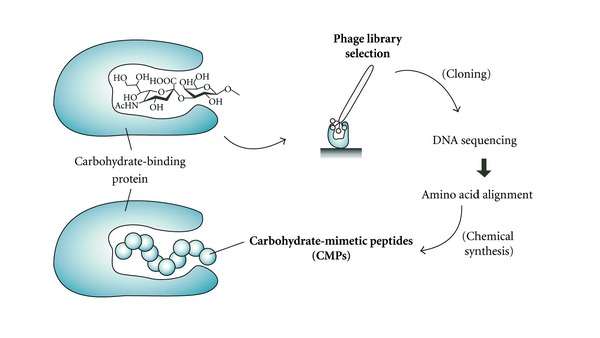
Identification of carbohydrate-mimetic peptides (CMPs) by affinity selection from a phage-display library. Selection is performed against carbohydrate-binding proteins. The peptides identified are chemically synthesized and recognized by the carbohydrate-binding protein.
2. Peptide Selection from Phage Display Libraries
Phage display is an efficient selection (and screening) system for the identification of target-specific peptides and proteins from a large number of candidates [20–22]. A filamentous virus (M13 and fd, etc.) that infects E. coli is frequently used in phage display technology. When DNA encoding foreign sequences is inserted into the coat protein (pIII or pVIII) region in the virus genome (M13 phage vector, etc.), the corresponding sequence is fused with the coat protein of the viral particle (Figure 2(a)) [20]. The foreign sequence is “displayed” on the viral particle and is able to interact with various types of target molecules.
Figure 2.
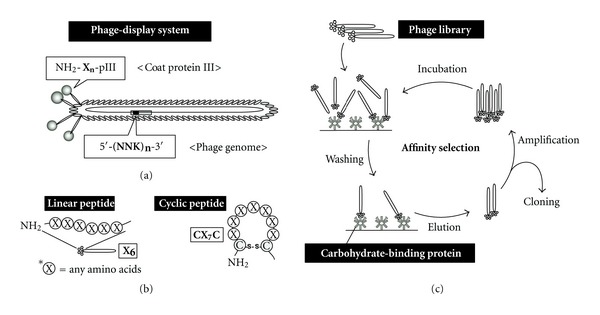
Phage-display system for affinity selection. (a) A typical filamentous phage carrying a peptide library. Foreign peptides (Xn) are displayed on the N-terminus of coat protein III (pIII) (type 3; M13 or fd phage). An oligonucleotide coding peptide library [-(NNK)n-] is inserted into the phage genome. X = any amino acid; N = A, C, G, or T; K = G or T. (b) Linear (hexamer, left) and cyclic (heptamer, right) peptide libraries. (c) Schematic representation of the procedure for affinity selection (biopanning). The phage library is incubated with target receptors (carbohydrate-binding proteins), and unbound phages are removed by washing. Bound phages are eluted, amplified in E. coli, and subjected to the next cycle of biopanning. The cycle is repeated several times to enrich target-specific phages. Individual enriched phages are isolated and used for DNA sequencing.
In the case of peptide libraries, the length of the peptides is often 5–20 amino acids. There are two types of peptide library: linear peptide libraries and cyclic peptide libraries (Figure 2(b)). The randomized region of cyclic peptide libraries is surrounded by two cysteines (e.g., CX7C) to restrict the peptide conformation via disulfide bonds. The diversity of a peptide library is often 108-109, which is sufficient to cover a combination of hexapeptide libraries (X6; 206 = 6.4 × 107). Several kinds of peptide libraries (e.g., Ph.D. Phage Display Peptide Library Kits, New England Biolabs) and customizable phage vectors (Ph.D. Peptide Display Cloning System) are commercially available.
To isolate phage clones that have high affinity for a target molecule, a set of procedures called “affinity selection (biopanning)” is performed (Figure 2(c)). First, the target molecule is incubated with the phage library in order to bind to specific peptide sequences. After removal of excess phages by washing, the bound phages are eluted by incubation with a known ligand for the target or an acidic buffer. The phages are amplified by infection of hosts (E. coli), and the phage pool is subjected to another round of biopanning. By repeating these steps, target-binding phages are enriched, and, finally, phage clones are obtained. The peptides with high affinity for the target molecule are identified by DNA sequencing of individual phage clones. Huang and coworkers established a mimotope database MimoDB (http://immunet.cn/mimodb/) that contains the results of biopanning experiments including the phage libraries used and the peptide sequences identified [23, 24]. This database will help in the development of therapeutic molecules and the identification of superior peptide mimotopes for vaccination.
3. CMPs against Lectins
3.1. Monosaccharide-Mimetic Peptides
Most lectins recognize monosaccharides and disaccharides [4]. Concanavalin A (ConA) is a lectin from jack-bean (Canavalia ensiformis) that binds to α-mannose (α-Man) and α-glucose (α-Glc). ConA is a famous lectin that is commercially available for the biological investigation of glycoconjugates. The first CMPs were selected from a random peptide library against ConA simultaneously by Oldenburg et al. (octapeptide library) [25] and Scott et al. (hexapeptide library) [13] (Table 1). Peptides containing the consensus sequence, Tyr-Pro-Tyr (YPY), showed high affinity for ConA with a dissociation constant (K d) of 46 μM, and the K d for methyl α-Man was 89 μM. The peptides are considered to mimic the structure of carbohydrates because the ConA-peptide interaction was inhibited by α-Man.
Table 1.
Summary of the selection of CMPs with lectins.
| Target lectins (abbreviations) | Peptide library | Peptide motif or representative sequences (peptide name) | Lectin-binding carbohydrate structures | References | Notes* |
|---|---|---|---|---|---|
| Concanavalin A (ConA) | X8, X6 | YPY motif | Man; Glc | [13, 25, 26] | Inhibition of Man binding |
| Griffonia simplicifolia I-B4 isolectin (GS-I-B4) | X6 | SSLRGF | Galα1-3Gal | [27] | Inhibition of RBC agglutination |
| Bandeiraea simplicifolia I-B4 isolectin (BS-I-B4) | XCX15 | NCVSPYWCEPLAPSARA | Galα1-3Gal | [28] | Inhibition of RBC agglutination |
| E-selectin | X12 | DITWDQLWDLMK | Sialyl LewisX [Neu5Acα2-3Galβ1-4(Fucα1–3)GlcNAc] | [29] | Inhibition of cell adhesion, reduction of neutrophil rolling, and so forth |
| X7 | IELLQAR | [30] | Octameric MAP, inhibition of HL-60, and B16 cell adhesion | ||
| Concanavalin A (ConA); Lens culinaris agglutinin (LCA); Pisum sativum agglutinin (PSA) | X12, CX7C | CNTPLTSRC; CSRILTAAC | Man; Glc | [31] | Inhibition of Man binding; docking study |
| Lectin from Helix pomatia (HPA) | X12 | VQATQSNQHTPRGGGS | O-linked α-GalNAc; Galβ1-3GalNAc; α-GlcNAc | [32] | Tetrameric dendrimer, stimulation of IL-8, and IL-21 secretion |
| Lipopolysaccharide (LPS) binding protein (LBP); CD14 | X12 | FHRWPTWPLPSP (MP12) | Lipopolysaccharide | [33] | Inhibition of LPS-induced INF-α expression |
| Influenza virus hemagglutinin (HA) | X15 | ARLPRTMVHPKPAQP (s2); ARLPR [s2(1–5)] | Neu5Acα2-3Gal | [17] | N-stearoyl peptide; inhibition of flu infection |
*RBC: red blood cell; IL: interleukin; INF: interferon.
To obtain Man/Glc-mimetic peptides, Yu et al. used three lectins, including ConA, Lens culinaris agglutinin (LCA) from lentil, and Pisum sativum agglutinin (PSA) from pea [31]. Two cyclic peptides, CNTPLTSRC and CSRILTAAC, were selected from a cyclic heptapeptide library, but these peptides did not contain the YPY motif. Docking simulation of the peptide-lectin interaction suggested that the cyclic peptides bound to an alternative binding site, not to the sugar-binding site that is recognized by YPY-containing peptides. In another screen using monosaccharide-binding lectins, Eggink and Hoober identified a GalNAc/Gal-mimetic dodecapeptide, VQATQSNQHTPR, that was selected against Helix pomatia (HPA) lectin [32]. A tetrameric dendrimer of the peptide, [(VQATQSNQHTPR)2 K]2 K, was synthesized chemically (Figure 3), which was shown to stimulate the secretion of interleukin (IL)-8 and IL-21 from human peripheral blood mononuclear cells (PBMCs).
Figure 3.
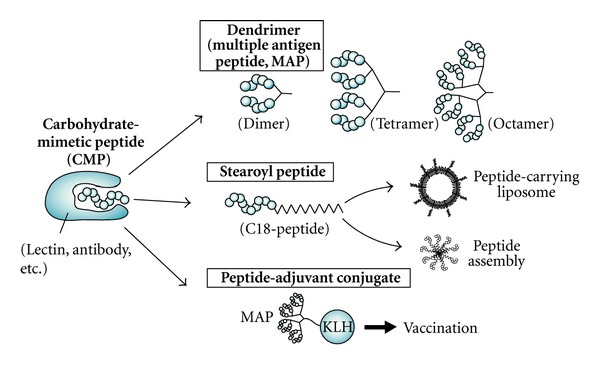
Representative chemical modifications of CMPs. To enhance the binding affinity, multiple CMPs are synthesized to give dimeric, tetrameric, and octameric dendrimers (multiple antigen peptide; MAP) (upper). The dendrimers are further conjugated with biotin, fluorescence groups, or adjuvants for vaccination. The peptide is modified with an alkyl group (stearic acid), enabling the peptide lipid to be incorporated into liposomes or to undergo self-assembly (middle). Monomeric CMP or CMP dendrimers are conjugated with adjuvants such as keyhole limpet hemocyanin (KLH), QS-21, and so forth (lower). The peptide-adjuvant conjugate is vaccinated into animals.
3.2. Disaccharide-Mimetic Peptides
The Galα1-3Gal disaccharide is recognized by Griffonia simplicifolia I-B4 (GS-I-B4) and Bandeiraea simplicifolia isolectin B4 (BS-I-B4) (Figure 4). The Galα1-3Gal structure is a major carbohydrate antigen recognized by human anti-pig antibodies, and inhibitors of human natural antibodies may be useful in pig-to-human xenotransplantation. Kooyman et al. identified a peptide sequence, SSLRGF, that binds to GS-I-B4 lectin from a hexapeptide library [27]. Zhan et al. identified a peptide, NCVSPYWCEPLAPSARA, by selection with BS-I-B4 lectin [28]. These peptides, SSLRGF and NCVSPYWCEPLAPSARA, inhibited the agglutination of pig red blood cells (RBCs) by human serum. Two peptides, FHENWPS and FHEFWPT, that inhibit the agglutination of RBCs were identified by selection against anti-Gal antibody by Lang et al. [42]. However, the peptides identified from three selections contained no obvious consensus sequence.
Figure 4.
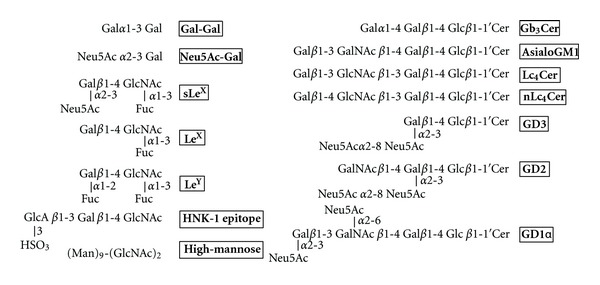
Oligosaccharide structures of carbohydrate antigens that are mimicked by peptides.
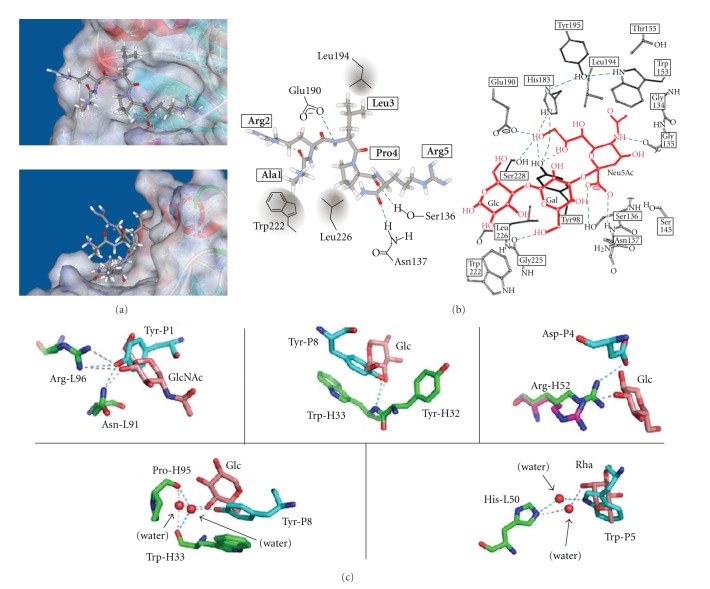
Influenza virus hemagglutinin (HA) recognizes sialylgalactose structures (Neu5Ac-Gal) in glycoproteins and glycolipids on the cell surface in the initial stage of the infection process (Figure 4). Matsubara et al. identified CMPs from a pentadecapeptide library by selection with HAs of the H1 and H3 subtypes [17]. A HA-binding peptide, ARLSPTMVHPNGAQP, was identified from the first selection, and mutational sublibraries were prepared. A secondary selection was performed to improve the binding affinity for HAs, and the peptide was matured to peptide s2, ARLPRTMVHPKPAQP. The peptide was modified with a stearoyl group, and a molecular assembly of the alkylated peptides inhibited the infection of Madin-Darby canine kidney cells by influenza virus (Figure 3). Finally, a pentapeptide fragment from the N-terminal of s2, ARLPR [s2(1–5)], was found to show the highest inhibitory activity. A docking study of the interaction between the peptide s2(1–5) and HA suggested that the peptide is recognized by the Neu5Ac-Gal receptor-binding pocket (Figure 5(a)). The figure indicates that three side chains of H3HA (Ser 136, Asn137, and Glu190) have the potential to interact with the peptide instead of Neu5Ac, and hydrophobic residues (Leu194, Leu226, and Trp222) are close to the peptide (Figure 5(b)).
Figure 5.
(a) Computer simulation of the interaction between peptide s2(1–5) and HA. A docking pose of the s2(1–5)-HA complex (left) and schematic diagram of the binding site of HA (right). The peptide is thought to be recognized by the Neu5Ac-Gal receptor-binding pocket. The peptide is shown as a stick model. Three potential hydrogen bonds (green dotted lines) between H3 and s2(1–5) are proposed (Glu190-Leu3, Ser136-Pro4, and Asn137-Arg5), which are similar to those in H3-Neu5Ac. Adapted from reference [17]. (b) Schematic diagram of the binding site of H3HA (Protein Data Bank entry, 1HGG). Neu5Acα2–3Gal-Glc (sialyllactose) is shown in red. Modified from [18]. (c) Comparison of the polar interactions shown in the oligosaccharide (O-antigen of S. flexneri serotype 2a) and peptide B1 (YLEDWIKYNNQK) complexes of monoclonal antibody F22-4. The peptide and oligosaccharide ligands are distinguished by carbon atoms shown in cyan and pink, respectively (P, peptide; Rha, rhamnose). The carbon atoms of the F22-4 residues are shown in green (H, heavy chain; L, light chain). Adapted from [19].
4. CMPs against Oligosaccharide-Binding Antibodies
4.1. Oligosaccharide-Mimetic Peptides for Inhibition
Glycoproteins and glycosphingolipids have unique oligosaccharide structures at their nonreducing termini [1]. Cell-cell communication is performed by oligosaccharides that are recognized by families of cell adhesion proteins such as selectins and sialic acid-binding immunoglobulin- (Ig-) like lectins (siglecs). Pathogenic viruses, toxins, and bacteria also recognize oligosaccharide structures [3]. Because an abundant variety of oligosaccharide structures relates to many carbohydrate-protein interactions, oligosaccharide-mimetic peptides mediate many kinds of inhibitory activities.
The sialyl-LewisX (sLeX) structure, Neu5Acα2-3Galβ1-4(Fucα1-3)GlcNAc, is recognized by E-selectin and is a famous carbohydrate antigen (Figure 4). sLeX-mimetic peptides were identified by selection against E-selectin [29, 30] and anti-sLeX antibody [36] (Tables 1 and 2). Martens et al. identified the HITWDQLWNVMN peptide and further optimized the sequence as DITWDQLWDLMK using a mutagenesis library [29]. The binding affinity of the synthetic peptide for E-selectin was improved 100-fold by this optimization (IC50 for sLeX binding to E-selectin; from 420 nM to 4 nM). The DITWDQLWDLMK peptide inhibited the adhesion of HL-60 cells and reduced neutrophil rolling on lipopolysaccharide- (LPS-) stimulated human umbilical vein endothelial cells. Qiu et al. designed WRY-containing peptides from the sLeX-mimetic peptide sequences, but these peptides cross-reacted with anti-LewisY antibody. Octameric multiple antigen peptides (MAPs) were conjugated with QS-21 adjuvant, which resulted in cytotoxic IgM and IgG antibodies (Figures 3 and 6). MAPs, in which peptides are attached to an octabranched amino acid backbone, are used to generate antibodies against a synthetic peptide, which is useful for the design of vaccines [94]. Katagihallimath et al. selected a cyclic CSRLNYLHC peptide against anti-LeX antibody [37]. The trisaccharide LeX structure is known as CD15 or SSEA-1, and this structure is expressed in the developing and adult murine central nervous system. The LeX mimetic peptide inhibited CD24-induced neurite outgrowth.
Table 2.
Summary of the selection of CMPs with oligosaccharide-binding antibodies.
| Target antibodies (abbreviations) |
Name of antibody | Peptide library | Peptide motif or representative sequences (peptide name) | Carbohydrate antigen | References | Notes* |
|---|---|---|---|---|---|---|
| Anti-LewisY (LeY) | B3 | X8 | APWLYGPA | Fucα1-2Galβ1-4(Fucα1-3)GlcNAc | [34, 35] | Induction of anti-LeY immune responses |
| BR55-2; 15-6A | X15 | WRY-containing peptide | [36] | Cross-reaction with anti-LeX | ||
| Anti-sialyl LewisX (sLeX) | FH-6 | X15 | WRY-containing peptide | Neu5Acα2-3Galβ1-4(Fucα1-3)GlcNAc | [36] | Cross-reaction with anti-LeY; octameric MAP-QS21 |
| Anti-LewisX (LeX) | L5 | X12, CX7C | CSRLNYLHC | Galβ1-4(Fucα1-3)GlcNAc | [37] | Inhibition of CD24-induced neurite outgrowth |
| Antilipooligosaccharide (LOS) | — | X7 | SMYGSYN, APARQLP | LOS of group B Neisseria meningitidis | [38] | Peptide-DT |
| Antilipooligosaccharide (LOS) | — | X12 | NMMRFTSQPPNN and so forth | LOS of nontypeable Haemophilus influenzae | [39] | Peptide-KLH |
| Anti-β-1,2-oligomannoside | DJ2.8 | X7 | FHENWPS | β-1,2-oligomannoside | [40] | Peptide-KLH |
| Anti-L2/HNK-1 | L2-412 | X15 | FLHTRLFVSDWYHTP, FLHTRLFV | SO4-3GlcAβ1-3Galβ1-4GlcNAc | [41] | Promotion of neurite outgrowth |
| Anti-Gal | B | X7, CX7C | FHENWPS, FHEFWPT | Xenoreactive α-Gal antigenic epitope | [42] | Inhibition of RBC agglutination |
| Anti-GMDP | E6/1.2 | X15 | RVPPRYHAKISPMVN | N-acetylglucosaminyl-β1,4-N-acetylmuramyl-alanyl-d-isoglutamine (GMDP) | [43] | Peptide-OVA |
| Antiglucitollysine | 41; 226 | X12, CX9C | CTSRXC motif | Glc-Lys | [44] | Inhibition of Glu-Lys binding |
| Antihigh-mannose oligosaccharides | 2G12 | X15CX | ACPPSHVLDMRSGTCLAAEGK (2G12.1) | Man9GlcNAc2 (HIV-1 gp120) | [45] | X-ray analysis (no structural mimic) |
*DT: diphtheria toxoid; KLH: keyhole limpet hemocyanin; OVA: ovalbumin.
Figure 6.
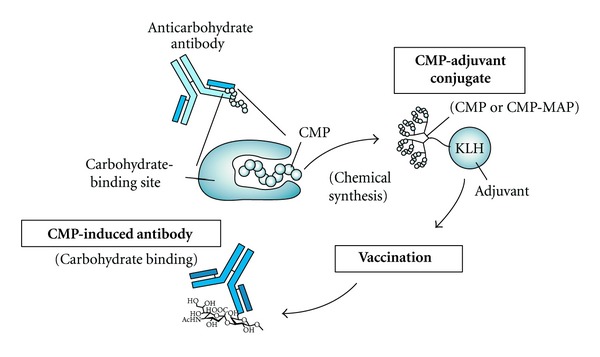
Procedure for obtaining CMP-induced antibodies by vaccination. A peptide mimotope (CMP) is conjugated with an adjuvant such as KLH and used for vaccination.
Neutral glycosphingolipid Lc4Cer-mimetic peptides showed unique activity [46] (Table 3). Lc4Cer contains Galβ1-3GlcNAcβ1-3Galβ1-4Glc tetrasaccharide that is linked to ceramide (Figure 4), and Jack bean β-galactosidase digests a nonreducing terminus β-Gal to give Lc3Cer. The Lc4Cer-mimetic peptides inhibited digestion by β-galactosidase at a high concentration of enzyme, whereas the peptides enhanced the digestion of Lc4Cer at lower concentration of enzyme. This unique activity of the peptides was also shown in the digestion of nLc4Cer. This group also identified WHW-containing peptides such as WHWRHRIPLQLAAGR by selection with anti-GD1α antibody [47]. The ganglioside GD1α is cell adhesion molecule of murine metastatic large cell lymphoma (RAW117-H10 cells) that binds to endothelial cells. GD1α-mimetic peptides inhibited the adhesion between RAW117-H10 cells and hepatic sinusoidal endothelial (HSE) cells. Furthermore, the metastasis of RAW117-H10 cells to lung and spleen was completely inhibited by the intravenous injection of the peptide. Subsequently, WHW was found to be a minimal sequence that mimics the GD1α structure [48]. To modify the liposome surface with the WHW peptide, the WHW tripeptide was conjugated to alkyl groups such as palmitoyl or stearoyl groups (Figure 3). Coating of liposomes with peptides is often performed in drug delivery systems. The WHW-modified liposomes inhibited the adhesion between RAW117-H10 cells and HSE cells.
Table 3.
Summary of the selection of CMPs with glycolipid-binding antibodies.
| Target antibodies | Name of antibody | Peptide library | Peptide motif or representative sequences (peptide name) | Glycolipid structures | References | Notes* |
|---|---|---|---|---|---|---|
| Anti-Lc4Cer; anti-nLc4Cer | AD117m; H11 | X15 | VPPXFXXXY | Galβ1-3GlcNAcβ1-3Galβ1-4Glcβ1-1′Cer; Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ1-1′Cer | [46] | Modulation of β-galactosidase activity |
| Anti-GD1α | KA17 | X15 | WHWRHRIPLQLAAGR | Neu5Acα2-3Galβ1-3(Neu5Acα2-6)GalNAcβ1-4Galβ1-4Glcβ1-1′Cer | [47, 48] | Inhibition of metastasis; peptide-liposome |
| Anti-asialo GM1 | clone 10 | X7, CX7C | KL/VWQXXX | Galβ1-3GalNAcβ1-4Galβ1-4Glcβ1-1′Cer | [49] | (Phage ELISA only) |
| Anti-GD3/GD2 | ME36.1 | X15 | WRY-containing peptide and so forth |
Neu5Acα2-8Neu5Acα2-3Galβ1-4Glcβ1-1′Cer; GalNAcβ1-4(Neu5Acα2-8Neu5Acα2-3)Galβ1-4Glcβ1-1′Cer | [36, 50, 51] | Octameric MAP-QS21/KLH |
| Anti-GD2 | 14.18 | CX10C | CDGGWLSKGSWC; CGRLKMVPDLEC | GalNAcβ1-4(Neu5Acα2-8Neu5Acα2-3)Galβ1-4Glcβ1-1′Cer | [52–54] | Docking study; peptide-KLH |
| 14G2a | X15 | EDPSHSLGLDVALFM | [55, 56] | Molecular modeling; DNA vaccine; induction of CD8+ T cell response | ||
| 14G2a | XCX8CX | RCNPNMEPPRCF | [57, 58] | Inhibition of antibody binding to IMR-32 cells | ||
| Anti-GD3 | 4F6 | X15 | LAPPRPRSELVFLSV (GD3P4) | Neu5Acα2-8Neu5Acα2-3Galβ1-4Glcβ1-1′Cer | [59] | Peptide-VSSP |
| Anti-Gb3Cer | — | X12 | WHWTWLSEY | Galα1-4Galβ1-4Glcβ1-1′Cer | [60] | Neutralization of Shiga toxin |
| Anti-Neu5Gc-containing ganglioside (Neu5Gc-GM3) | 1E10; chimeric P3; 1E10 | X9, X12 | KPPR, RRPR/K; LEICSYTPDEGC; KCGHHYCRQVDL | Neu5Gcα2-3Galβ1-4Glcβ1-1′Cer | [61, 62] | Inhibition of 1E10 binding to P3 |
| Anti-phenolic glycolipid-I (PGL-I) | III603.8 | X7 | W(T/R)LGPY(V/M) | Mycobacterium leprae-specific antigen | [63] | Does not bind to antibodies in serum |
*MAP: multiple antigen peptide; VSSP: very small size proteoliposomes.
Tryptophan/tyrosine-containing tripeptides (YPY for ConA, WRY for sLeX(Y), and WHW for GD1α) may comprise a key sequence that mimics oligosaccharide structure. Although Gb3 (Galα1-4Galβ1-4Glc trisaccharide) is dissimilar to the disaccharide (Galβ1-3GlcNAcβ) structure of Lc4 at the nonreducing terminus, Miura et al. identified a WHW-containing peptide (WHWTWLSEY) that mimics the Gb3 structure [60]. Gb3 is well known as a receptor for Shiga toxin (Stx). The Gb3-mimetic peptide showed neutralization activity against Stxs (Stx-1 and Stx-2) in a HeLa cell cytotoxicity assay. The binding affinity of the Gb3-mimetic peptide for Stx-1 was also investigated by surface plasmon resonance analysis (K d = 1.4 nM).
4.2. Oligosaccharide-Mimetic Peptides for Vaccination
The immunogenicity of oligosaccharides is weak because oligosaccharides are ubiquitous components of cell membranes in tissues throughout the human body. When antioligosaccharide antibodies are generated, they attack these tissues and cause the risk of autoimmune disease. For example, lipopolysaccharides of Campylobacter jejuni isolated from GBS patients contain ganglioside-like epitopes such as GM1, GM1b, GD1a, and GalNAc-GDla, and these epitopes induce Guillain-Barre syndrome [95]. However, this low immunogenicity interferes with the preparation of antioligosaccharide antibodies that are useful for the investigation of glycoconjugate function.
To improve the binding affinity, specificity and cytotoxicity of antibodies, oligosaccharide-mimetic peptides are applied as peptide mimotopes of carbohydrate antigens for vaccination (Figure 6). Oligosaccharide-mimetic peptides were identified by selection against LeX(Y) [34, 35, 37], sLeX(Y) [36, 50], GD2 [36, 50–56], GD3 [36, 50, 59], lipooligosaccharide (LOS) [38, 39], β-1,2-oligomannoside [40], N-acetylglucosaminyl-β1,4-N-acetylmuramyl-alanyl-d-isoglutamine (GMDP) [43], and high-mannose oligosaccharide (Man9GlcNAc2 for HIV-1 gp120) [45]. The oligosaccharide-mimetic peptides were chemically synthesized and conjugated with adjuvant. To enhance the immunogenicity of the peptides, MAPs were prepared and resulted in dimeric, tetrameric, and octameric dendrimers (Figure 3). The peptide-adjuvant conjugates were vaccinated, with the adjuvants used being keyhole limpet hemocyanin (KLH) [39, 40, 53, 54], QS-21 [36, 50, 54], diphtheria toxoid (DT) [38], ovalbumin (OVA) [43], or very small size proteoliposomes (VSSP) [59] (Figure 6, Tables 2 and 3). In some cases, DNA vaccination was also performed [55, 56]. The CMP-induced antibodies are able to bind to peptide mimotopes and carbohydrate antigens.
5. CMPs against Polysaccharide-Binding Antibodies
Most polysaccharide-mimetic peptides to be applied for vaccination are identified as peptide mimotopes of carbohydrate antigens (Figure 6). Capsular polysaccharides of microorganisms are carbohydrate antigens, and it is known that these polysaccharides cause meningoencephalitis in immunocompromised patients, particularly those with AIDS (polysaccharide from Cryptococcus neoformans), pneumonia and bacteremia (Streptococcus pneumoniae), bacterial meningitis (Neisseria meningitidis), cholera (Vibrio cholerae), tuberculosis (Mycobacterium tuberculosis), and so forth (Table 4). These peptide mimotopes are potential antigens for safe vaccination and are expected to produce highly cytotoxic antibodies.
Table 4.
Summary of the selection of CMPs with polysaccharide-binding antibodies.
| Species | Carbohydrate antigen | Name of antibody | Peptide library | Peptide motif or representative sequences (peptide name) | References | Notes* |
|---|---|---|---|---|---|---|
|
Cryptococcus
neoformans |
Polysaccharide (glucuronoxylomannan; GXM) | 2H1 | X6, X10, ADVA X6 TPXW [M/L][M/L] X6 AAG | (E)TPXWM/LM/L, W/YXWM/LY; GLQYTPSWMLVG (PA1); SYSWMYE (P601E); FGGETFTPDWMMEVAIDNE (P206.1) | [64–67] | Four motifs; X-ray analysis (PA1); peptide evolution (P206.1); peptide-KLH/TT |
| Streptococcus species | Capsular polysaccharide (type 3, group B) | S9 | X9 | WENWMMGNA; FDTGAFDPDWPA | [68] | Group B streptococci (GBS); peptide-KLH/BSA/OVA |
|
Streptococcus
pneumoniae |
Capsular polysaccharide (serotype 4) | mAb4 | X15 | SGQARVLYSEFINAL (pep4) | [69] | DNA vaccine |
| (serotype 8) | (human mAb IgA) | X12 | FHLPYNHNWFAL (PUB1) | [70] | Peptide-TT | |
| (serotype 6B, 9V) | 206; F-5; Db3G9 | X12, X15 | MP7, 12, 55, 58 | [71] | Peptide-KLH | |
| Streptococcus pyogenes | Cell-wall polysaccharide (group A) | SA-3; Strep9; HGAC39; HGAC47; HGAC101 | X6, XCX8CX, X8CX8, X15CX, X15 | DRPVPY | [72] | Basis of peptide-carbohydrate cross-reactivity |
| Streptococcus agalactiae | (serotype A, B, C) | (IgG2, Ig polyclonal) | X12 | NPDHPRVPTFMA (2–8); LIPFHKHPHHRG (3-2) | [73] | DNA vaccine; MAP-CFA/IFA |
| Neisseria meningitidis | Capsular polysaccharide (serogroup A) | 9C10 | X15 | GEASGLCCRWSSLKGC | [74] | Peptide-proteasome |
| (serogroup B) | HmenB3 | X12 | NKVIWDRDWMYP | [75] | Peptide-BSA-CFA/IFA | |
| (serogroup B) | 9-2-L3, 7, 9 | CX6C, CX9C | CGAVIDDC | [76] | Peptide-KLH | |
| (serogroup B) [poly-α2–8 sialic acid (PSA)] | 30H12 | CX9C | CSSVTAWTTGCG | [77, 78] | Enhanced migration of grafted neuroblasts in mouse brain | |
| (serogroup B) | Seam3 | X9, X12 | DYAWDQTHQDPAK (9M) | [79] | Peptide-KLH, DNA vaccine | |
| (serogroup B) | 13D9 | X15 | RGDKSRPPVWYVEGE | [80] | Phage vaccine | |
| (serogroups B and C) | (IgG2, Ig polyclonal) | X12 | EQEIFTNITDRV (G3) | [73] | DNA vaccine; MAP-CFA/IFA | |
| (serogroup C) | 1E4 | X15 | GFSYYRPPWIL (Pep2C) | [81] | Peptide-proteasome | |
| [N-propionyl derivative of CPS] | — | — | — | [82] | ||
| Vibrio cholerae | Capsular polysaccharide (serogroup O139) | Vc1; Vc2; ICL12 | six libraries (X9, X12, X28 etc.) | AEGEFSPGVWKAAFQGDKLPDPAK and so forth | [83] | Peptide-KLH/BSA |
| (serogroup O1) Ogawa serotype | S-20-4; A-20-6 | X12, X7, CX7C | NHNYPPLSLLTF (4P-8) | [84] | Peptide-KLH/BSA; docking study | |
| (serogroup O1) Ogawa and Inab serotypes | 72.1 | X12, X7, CX7C | ECLLLSKYCMPS (3ME-1); SMCMHGGAYCFP (3ME-2) | [85] | Peptide-KLH/BSA-CFA/IFA | |
| Shigella flexneri | O-antigen of lipopolysaccharide (serotype 5a) | mIgAs C5; I3 | X9 | YKPLGALTH; KVPPWARTA | [86] | Phage vaccine |
| (serotype 2a) | F22-4 | X12 | YLEDWIKYNNQK (B1) | [19] | X-ray analysis | |
| — | Melanoma-associated chondroitin sulfate proteoglycan (MCSP) | 763.74 | X6 | VHINAH | [87] | Inhibition of MCSP binding |
| Entamoeba histolytica | GPI-linked proteophosphoglycan antigens | EH5 | six libraries (X9, X12, X28 etc.) | GTHPXL | [88] | Glycosylphosphatidylinositol (GPI); phage vaccine |
| Mycobacterium tuberculosis | Neutral polysaccharides | — | X12 | QEPLMGTVPIRAGGGS (P1) | [89] | Peptide oligomer vaccine |
| Mycobacterium tuberculosis | Mannosylated lipoarabinomannan | CS40 | X12 | ISLTEWSMWYRH (B11) | [90] | Peptide-KLH-adjuvants |
| Burkholderia pseudomallei | Exopolysaccharide (EPS) | 3VIE5; 4VA5 | X12, X7, CX7C | CYLPFQLSC; CHPLFDARC | [91] | Peptide-thyroglobulin |
| Brucella melitensis; Brucella abortus | Lipopolysaccharide | 4F9; 11B2 | X9, X12, CX9CX, X15 |
WTEIHDWEAAME | [92] | DNA vaccine |
| Staphylococcus aureus | Peptidoglycan | — | X12 | Sp-31 | [93] | MAP vaccine |
*BSA: bovine serum albumin; TT: tetanus toxoid; CFA: complete Freund's adjuvant; IFA: incomplete Freund's adjuvant.
The typical methodology for vaccination uses a CMP-conjugated adjuvant. Valadon et al. identified CMPs that bind to anticryptococcal polysaccharide (glucuronoxylomannan, GXM) monoclonal antibody 2H1 [64]. The CMPs shared four motifs, for example, (E)TPXWM/LM/L and W/YXWM/LYE, and the dodecapeptide, GLQYTPSWMLVG (PA1) was found to bind 2H1 with a K d of 295 nM [64]. The three-dimensional structure of 2H1 has been solved in a complex with PA1 [65]. The peptide PA1 was improved by selection from a PA1-based sublibrary, which identified the peptide P206-1 (FGGETFTPDWMMEVAIDNE) [66]. The affinity of peptide 206-1 for 2H1 was 80-fold higher than that of PA1 (K d of 3.7 nM). Immunization of mice with P206-1-tetanus toxoid (TT), but not PA1 or P601E (DGASYSWMYEA), induced an anti-GXM response [66, 67].
Although antibodies against the capsular polysaccharide of the same species (e.g., Neisseria meningitidis serogroup B) were used, the CMPs identified were different and shared no consensus motif [73, 75–80] (Table 4). This may be due to the different antibodies used (HmenB3, 9-2-L3, 30H12, Seam3, or 13D9), different primary peptide libraries (CX6C, X9, CX9C, X12, or X15), or different selection conditions. Harris et al. also concluded that the CMPs identified by each antibody possessed distinct consensus motifs [72]. A variety of peptide-conjugating adjuvants such as KLH, TT, BSA, OVA, proteasome, and thyroglobulin have been used. In some cases, phage particles were directly used for vaccination [80, 86, 88], and a high level of the IgG2a subtype in the response against CMPs was shown [80].
Theillet et al. clarified the structural mimicry of O-antigen oligosaccharide by CMPs [19]. Figure 5(c) shows a structural representation of the antibody-peptide complex in which the sugar chains were replaced by amino acids. Glc and GlcNAc were replaced by Tyr or Asp, and one or more hydrogen bonds are indicated. On the other hand, high-mannose oligosaccharide-mimic peptide (2G12-1 peptide) binds to a neighboring pocket of the oligosaccharide (Table 2) [45]. The binding site for the DVFYPYPYASGS peptide, which was selected against ConA, was different from the mannose/trimannose-binding site [26]. However, the peptide inhibits α-mannopyranoside binding to ConA [25], indicating that this peptide shows functional mimicry rather than structural mimicry.
6. Conclusion
Anticarbohydrate antibodies are necessary for clarifying the biological functions of carbohydrates, the detection of carbohydrates during etiological diagnosis, and therapy for carbohydrate-related diseases [7, 96]. Due to the difficulty in obtaining homogeneous glycoconjugates and carbohydrate-binding proteins, phage display libraries have been applied for the identification of peptide mimotopes. In this paper, the selection of CMPs was classified according to the types of target carbohydrates. The first selection was performed against lectins, and then the selections were performed against anticarbohydrate antibodies. To apply the peptide mimotopes for vaccination, this methodology is becoming more widespread.
Acknowledgment
The preparation of this paper was supported in part by the Keio Gijuku Fukuzawa Memorial Fund for the Advancement of Education and Research.
References
- 1.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Lis H, Sharon N. Lectins: carbohydrate-specific proteins that mediate cellular recognition. Chemical Reviews. 1998;98(2):637–674. doi: 10.1021/cr940413g. [DOI] [PubMed] [Google Scholar]
- 4.Weis WI, Drickamer K. Structural basis of lectin-carbohydrate recognition. Annual Review of Biochemistry. 1996;65:441–473. doi: 10.1146/annurev.bi.65.070196.002301. [DOI] [PubMed] [Google Scholar]
- 5.Nores GA, Lardone RD, Comín R, Alaniz ME, Moyano AL, Irazoqui FJ. Anti-GM1 antibodies as a model of the immune response to self-glycans. Biochimica et Biophysica Acta. 2008;1780(3):538–545. doi: 10.1016/j.bbagen.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Slovin SF, Keding SJ, Ragupathi G. Carbohydrate vaccines as immunotherapy for cancer. Immunology and Cell Biology. 2005;83(4):418–428. doi: 10.1111/j.1440-1711.2005.01350.x. [DOI] [PubMed] [Google Scholar]
- 7.Kannagi R, Hakomori S. A guide to monoclonal antibodies directed to glycotopes. Advances in Experimental Medicine and Biology. 2001;491:587–630. doi: 10.1007/978-1-4615-1267-7_38. [DOI] [PubMed] [Google Scholar]
- 8.Hsu C-H, Hung S-C, Wu C-Y, Wong C-H. Toward automated oligosaccharide synthesis. Angewandte Chemie. 2011;50(50):11872–11923. doi: 10.1002/anie.201100125. [DOI] [PubMed] [Google Scholar]
- 9.Boltje TJ, Buskas T, Boons GJ. Opportunities and challenges in synthetic oligosaccharide and glycoconjugate research. Nature Chemistry. 2009;1(8):611–622. doi: 10.1038/nchem.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimura SI. Combinatorial syntheses of sugar derivatives. Current Opinion in Chemical Biology. 2001;5(3):325–335. doi: 10.1016/s1367-5931(00)00209-x. [DOI] [PubMed] [Google Scholar]
- 11.Hancock SM, Vaughan MD, Withers SG. Engineering of glycosidases and glycosyltransferases. Current Opinion in Chemical Biology. 2006;10(5):509–519. doi: 10.1016/j.cbpa.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Daines AM, Maltman BA, Flitsch SL. Synthesis and modifications of carbohydrates, using biotransformations. Current Opinion in Chemical Biology. 2004;8(2):106–113. doi: 10.1016/j.cbpa.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Scott JK, Loganathan D, Easley RB, Gong X, Goldstein IJ. A family of concanavalin A-binding peptides from a hexapeptide epitope library. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(12):5398–5402. doi: 10.1073/pnas.89.12.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuda MN. Peptide-displaying phage technology in glycobiology. Glycobiology. 2012;22(3):318–325. doi: 10.1093/glycob/cwr140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudak FC, Boyaci IH, Orner BP. The discovery of small-molecule mimicking peptides through phage display. Molecules. 2011;16(1):774–789. doi: 10.3390/molecules16010774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortese R, Felici F, Galfre G, Luzzago A, Monaci P, Nicosia A. Epitope discovery using peptide libraries displayed on phage. Trends in Biotechnology. 1994;12(7):262–267. doi: 10.1016/0167-7799(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 17.Matsubara T, Onishi A, Saito T, et al. Sialic acid-mimic peptides as hemagglutinin inhibitors for anti-influenza therapy. Journal of Medicinal Chemistry. 2010;53(11):4441–4449. doi: 10.1021/jm1002183. [DOI] [PubMed] [Google Scholar]
- 18.Sauter NK, Hanson JE, Glick GD, et al. Binding of influenza virus hemagglutinin to analogs of its cell-surface receptor, sialic acid: analysis by proton nuclear magnetic resonance spectroscopy and X-ray crystallography. Biochemistry. 1992;31(40):9609–9621. doi: 10.1021/bi00155a013. [DOI] [PubMed] [Google Scholar]
- 19.Theillet FX, Saul FA, Vulliez-Le Normand B, et al. Structural mimicry of O-antigen by a peptide revealed in a complex with an antibody raised against Shigella flexneri serotype 2a. Journal of Molecular Biology. 2009;388(4):839–850. doi: 10.1016/j.jmb.2009.03.057. [DOI] [PubMed] [Google Scholar]
- 20.Smith GP, Petrenko VA. Phage display. Chemical Reviews. 1997;97(2):391–410. doi: 10.1021/cr960065d. [DOI] [PubMed] [Google Scholar]
- 21.Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228(4705):1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 22.Scott JK, Smith GP. Searching for peptide ligands with an epitope library. Science. 1990;249(4967):386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- 23.Ru B, Huang J, Dai P, et al. MimoDB: a new repository for mimotope data derived from phage display technology. Molecules. 2010;15(11):8279–8288. doi: 10.3390/molecules15118279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Ru B, Zhu P, et al. MimoDB 2.0: a mimotope database and beyond. Nucleic Acids Research. 2012;40(D1):D271–D277. doi: 10.1093/nar/gkr922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oldenburg KR, Loganathan D, Goldstein IJ, Schultz PG, Gallop MA. Peptide ligands for a sugar-binding protein isolated from a random peptide library. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(12):5393–5397. doi: 10.1073/pnas.89.12.5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain D, Kaur K, Sundaravadivel B, Salunke DM. Structural and functional consequences of peptide-carbohydrate mimicry: crystal structure of a carbohydrate-mimicking peptide bound to concanavalin A. Journal of Biological Chemistry. 2000;275(21):16098–16102. doi: 10.1074/jbc.275.21.16098. [DOI] [PubMed] [Google Scholar]
- 27.Kooyman DL, Mcclellan SB, Parker W, et al. Identification and characterization of a galactosyl peptide mimetic. Implications for use in removing xenoreactive anti-a gal antibodies. Transplantation. 1996;61(6):851–855. doi: 10.1097/00007890-199603270-00001. [DOI] [PubMed] [Google Scholar]
- 28.Zhan J, Xia Z, Xu L, Yan Z, Wang K. A peptide mimetic of Gal-α1,3-Gal is able to block human natural antibodies. Biochemical and Biophysical Research Communications. 2003;308(1):19–22. doi: 10.1016/s0006-291x(03)01312-3. [DOI] [PubMed] [Google Scholar]
- 29.Martens CL, Cwirla SE, Lee RYW, et al. Peptides which bind to E-selectin and block neutrophil adhesion. Journal of Biological Chemistry. 1995;270(36):21129–21136. doi: 10.1074/jbc.270.36.21129. [DOI] [PubMed] [Google Scholar]
- 30.Fukuda MN, Ohyama C, Lowitz K, et al. A peptide mimic of E-selectin ligand inhibits sialyl Lewis X-dependent lung colonization of tumor cells. Cancer Research. 2000;60(2):450–456. [PubMed] [Google Scholar]
- 31.Yu L, Yu PS, Yee Yen Mui E, et al. Phage display screening against a set of targets to establish peptide-based sugar mimetics and molecular docking to predict binding site. Bioorganic and Medicinal Chemistry. 2009;17(13):4825–4832. doi: 10.1016/j.bmc.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 32.Eggink LL, Hoober JK. A biologically active peptide mimetic of N-acetylgalactosamine/galactose. BMC Research Notes. 2009;2, article 23 doi: 10.1186/1756-0500-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Z, Qian GS, Li Q, Feng QJ, Wu GM, Li KL. Screening of mimetic peptides for CD14 binding site with LBP and antiendotoxin activity of mimetic peptide in vivo and in vitro. Inflammation Research. 2009;58(1):45–53. doi: 10.1007/s00011-008-8178-3. [DOI] [PubMed] [Google Scholar]
- 34.Hoess R, Brinkmann U, Handel T, Pastan I. Identification of a peptide which binds to the carbohydrate-specific monoclonal antibody B3. Gene. 1993;128(1):43–49. doi: 10.1016/0378-1119(93)90151-r. [DOI] [PubMed] [Google Scholar]
- 35.Lou Q, Pastan I. A Lewis(y) epitope mimicking peptide induces anti-Lewis(y) immune responses in rabbits and mice. Journal of Peptide Research. 1999;53(3):252–260. doi: 10.1034/j.1399-3011.1999.00025.x. [DOI] [PubMed] [Google Scholar]
- 36.Qiu J, Luo P, Wasmund K, Steplewski Z, Kieber-Emmons T. Towards the development of peptide mimotopes of carbohydrate antigens as cancer vaccines. Hybridoma. 1999;18(1):103–112. doi: 10.1089/hyb.1999.18.103. [DOI] [PubMed] [Google Scholar]
- 37.Katagihallimath N, Mehanna A, Guseva D, Kleene R, Schachner M. Identification and validation of a Lewisx glycomimetic peptide. European Journal of Cell Biology. 2010;89(1):77–86. doi: 10.1016/j.ejcb.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Charalambous BM, Feavers IM. Peptide mimics elicit antibody responses against the outer-membrane lipooligosaccharide of group B Neisseria meningitidis . FEMS Microbiology Letters. 2000;191(1):45–50. doi: 10.1111/j.1574-6968.2000.tb09317.x. [DOI] [PubMed] [Google Scholar]
- 39.Hou Y, Gu XX. Development of peptide mimotopes of lipooligosaccharide from nontypeable Haemophilus influenzae as vaccine candidates. Journal of Immunology. 2003;170(8):4373–4379. doi: 10.4049/jimmunol.170.8.4373. [DOI] [PubMed] [Google Scholar]
- 40.Jouault T, Fradin C, Dzierszinski F, et al. Peptides that mimic Candida albicans-derived β-1,2-linked mannosides. Glycobiology. 2001;11(8):693–701. doi: 10.1093/glycob/11.8.693. [DOI] [PubMed] [Google Scholar]
- 41.Simon-Haldi M, Mantei N, Franke J, Voshol H, Schachner M. Identification of a peptide mimic of the L2/HNK-1 carbohydrate epitope. Journal of Neurochemistry. 2002;83(6):1380–1388. doi: 10.1046/j.1471-4159.2002.01247.x. [DOI] [PubMed] [Google Scholar]
- 42.Lang J, Zhan J, Xu L, Yan Z. Identification of peptide mimetics of xenoreactive α-Gal antigenic epitope by phage display. Biochemical and Biophysical Research Communications. 2006;344(1):214–220. doi: 10.1016/j.bbrc.2006.03.112. [DOI] [PubMed] [Google Scholar]
- 43.Laman AG, Shepelyakovskaya AO, Berezin IA, et al. Identification of pentadecapeptide mimicking muramyl peptide. Vaccine. 2007;25(15):2900–2906. doi: 10.1016/j.vaccine.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 44.Rojas G, Pupo A, Del Rosario Aleman M, Santiago Vispo N. Preferential selection of Cys-constrained peptides from a random phage-displayed library by anti-glucitollysine antibodies. Journal of Peptide Science. 2008;14(11):1216–1221. doi: 10.1002/psc.1061. [DOI] [PubMed] [Google Scholar]
- 45.Menendez A, Calarese DA, Stanfield RL, et al. A peptide inhibitor of HIV-1 neutralizing antibody 2G12 is not a structural mimic of the natural carbohydrate epitope on gp120. FASEB Journal. 2008;22(5):1380–1392. doi: 10.1096/fj.07-8983com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taki T, Ishikawa D, Hamasaki H, Handa S. Preparation of peptides which mimic glycosphingolipids by using phage peptide library and their modulation on β-galactosidase activity. FEBS Letters. 1997;418(1-2):219–223. doi: 10.1016/s0014-5793(97)01386-0. [DOI] [PubMed] [Google Scholar]
- 47.Ishikawa D, Kikkawa H, Ogino K, Hirabayashi Y, Oku N, Taki T. GD1α-replica peptides functionally mimic GD1α, an adhesion molecule of metastatic tumor cells, and suppress the tumor metastasis. FEBS Letters. 1998;441(1):20–24. doi: 10.1016/s0014-5793(98)01511-7. [DOI] [PubMed] [Google Scholar]
- 48.Takikawa M, Kikkawa H, Asai T, et al. Suppression of GD1α ganglioside-mediated tumor metastasis by liposomalized WHW-peptide. FEBS Letters. 2000;466(2-3):381–384. doi: 10.1016/s0014-5793(00)01110-8. [DOI] [PubMed] [Google Scholar]
- 49.Qiu JX, Marcus DM. Use of peptide ligands to analyze the fine specificity of antibodies against asialo GM1. Journal of Neuroimmunology. 1999;100(1-2):58–63. doi: 10.1016/s0165-5728(99)00199-x. [DOI] [PubMed] [Google Scholar]
- 50.Kieber-Emmons T, Luo P, Qiu J, Chang TY, Blaszczyk-Thurin M, Steplewski Z. Vaccination with carbohydrate peptide mimotopes promotes anti-tumor responses. Nature Biotechnology. 1999;17(7):660–665. doi: 10.1038/10870. [DOI] [PubMed] [Google Scholar]
- 51.Wondimu A, Zhang T, Kieber-Emmons T, et al. Peptides mimicking GD2 ganglioside elicit cellular, humoral and tumor-protective immune responses in mice. Cancer Immunology, Immunotherapy. 2008;57(7):1079–1089. doi: 10.1007/s00262-007-0439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Förster-Waldl E, Riemer AB, Dehof AK, et al. Isolation and structural analysis of peptide mimotopes for the disialoganglioside GD2, a neuroblastoma tumor antigen. Molecular Immunology. 2005;42(3):319–325. doi: 10.1016/j.molimm.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 53.Riemer AB, Förster-Waldl E, Brämswig KH, et al. Induction of IgG antibodies against the GD2 carbohydrate tumor antigen by vaccination with peptide mimotopes. European Journal of Immunology. 2006;36(5):1267–1274. doi: 10.1002/eji.200535279. [DOI] [PubMed] [Google Scholar]
- 54.Bleeke M, Fest S, Huebener N, et al. Systematic amino acid substitutions improved efficiency of GD2-peptide mimotope vaccination against neuroblastoma. European Journal of Cancer. 2009;45(16):2915–2921. doi: 10.1016/j.ejca.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 55.Bolesta E, Kowalczyk A, Wierzbicki A, et al. DNA vaccine expressing the mimotope of GD2 ganglioside induces protective GD2 cross-reactive antibody responses. Cancer Research. 2005;65(8):3410–3418. doi: 10.1158/0008-5472.CAN-04-2164. [DOI] [PubMed] [Google Scholar]
- 56.Wierzbicki A, Gil M, Ciesielski M, et al. Immunization with a mimotope of GD2 ganglioside induces CD8+ T cells that recognize cell adhesion molecules on tumor cells. Journal of Immunology. 2008;181(9):6644–6653. doi: 10.4049/jimmunol.181.9.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horwacik I, Czaplicki D, Talarek K, et al. Selection of novel peptide mimics of the GD2 ganglioside from a constrained phage-displayed peptide library. International Journal of Molecular Medicine. 2007;19(5):829–839. [PubMed] [Google Scholar]
- 58.Horwacik I, Kurciński M, Bzowska M, et al. Analysis and optimization of interactions between peptides mimicking the GD2 ganglioside and the monoclonal antibody 14G2a. International Journal of Molecular Medicine. 2011;28(1):47–57. doi: 10.3892/ijmm.2011.655. [DOI] [PubMed] [Google Scholar]
- 59.Popa I, Ishikawa D, Tanaka M, Ogino K, Portoukalian J, Taki T. GD3-replica peptides selected from a phage peptide library induce a GD3 ganglioside antibody response. FEBS Letters. 2006;580(5):1398–1404. doi: 10.1016/j.febslet.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 60.Miura Y, Sakaki A, Kamihira M, Iijima S, Kobayashi K. A globotriaosylceramide (Gb3Cer) mimic peptide isolated from phage display library expressed strong neutralization to Shiga toxins. Biochimica et Biophysica Acta. 2006;1760(6):883–889. doi: 10.1016/j.bbagen.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 61.Perez A, Mier ES, Vispo NS, Vazquez AM, Rodríguez RP. A monoclonal antibody against NeuGc-containing gangliosides contains a regulatory idiotope involved in the interaction with B and T cells. Molecular Immunology. 2002;39(1-2):103–112. doi: 10.1016/s0161-5890(02)00041-x. [DOI] [PubMed] [Google Scholar]
- 62.López-Requena A, De Acosta CM, Moreno E, et al. Gangliosides, Ab1 and Ab2 antibodies. I. Towards a molecular dissection of an idiotype-anti-idiotype system. Molecular Immunology. 2007;44(4):423–433. doi: 10.1016/j.molimm.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 63.Youn JH, Myung HJ, Liav A, et al. Production and characterization of peptide mimotopes of phenolic glycolipid-I of Mycobacterium leprae . FEMS Immunology and Medical Microbiology. 2004;41(1):51–57. doi: 10.1016/j.femsim.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 64.Valadon P, Nussbaum G, Boyd LF, Margulies DH, Scharff MD. Peptide libraries define the fine specificity of anti-polysaccharide antibodies to Cryptococcus neoformans . Journal of Molecular Biology. 1996;261(1):11–22. doi: 10.1006/jmbi.1996.0438. [DOI] [PubMed] [Google Scholar]
- 65.Young ACM, Valadon P, Casadevall A, Scharff MD, Sacchettini JC. The three-dimensional structures of a polysaccharide binding antibody to Cryptococcus neoformans and its complex with a peptide from a phage display library: implications for the identification of peptide mimotopes. Journal of Molecular Biology. 1997;274(4):622–634. doi: 10.1006/jmbi.1997.1407. [DOI] [PubMed] [Google Scholar]
- 66.Beenhouwer DO, May RJ, Valadon P, Scharff MD. High affinity mimotope of the polysaccharide capsule of Cryptococcus neoformans identified from an evolutionary phage peptide library. Journal of Immunology. 2002;169(12):6992–6999. doi: 10.4049/jimmunol.169.12.6992. [DOI] [PubMed] [Google Scholar]
- 67.Valadon P, Nussbaum G, Oh J, Scharff MD. Aspects of antigen mimicry revealed by immunization with a peptide mimetic of Cryptococcus neoformans polysaccharide. Journal of Immunology. 1998;161(4):1829–1836. [PubMed] [Google Scholar]
- 68.Pincus SH, Smith MJ, Jennings HJ, Burritt JB, Glee PM. Peptides that mimic the group B streptococcal type III capsular polysaccharide antigen. Journal of Immunology. 1998;160(1):293–298. [PubMed] [Google Scholar]
- 69.Lesinski GB, Smithson SL, Srivastava N, Chen D, Widera G, Westerink MAJ. A DNA vaccine encoding a peptide mimic of Streptococcus pneumoniae serotype 4 capsular polysaccharide induces specific anti-carbohydrate antibodies in Balb/c mice. Vaccine. 2001;19(13-14):1717–1726. doi: 10.1016/s0264-410x(00)00397-2. [DOI] [PubMed] [Google Scholar]
- 70.Buchwald UK, Lees A, Steinitz M, Pirofski LA. A peptide mimotope of type 8 pneumococcal capsular polysaccharide induces a protective immune response in mice. Infection and Immunity. 2005;73(1):325–333. doi: 10.1128/IAI.73.1.325-333.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith CM, Passo CL, Scuderi A, et al. Peptide mimics of two pneumococcal capsular polysaccharide serotypes (6B and 9V) protect mice from a lethal challenge with Streptococcus pneumoniae . European Journal of Immunology. 2009;39(6):1527–1535. doi: 10.1002/eji.200839091. [DOI] [PubMed] [Google Scholar]
- 72.Harris SL, Craig L, Mehroke JS, et al. Exploring the basis of peptide-carbohydrate crossreactivity: evidence for discrimination by peptides between closely related anti-carbohydrate antibodies. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(6):2454–2459. doi: 10.1073/pnas.94.6.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu Y, Zhang Q, Sales D, Bianco AE, Craig A. Vaccination with peptide mimotopes produces antibodies recognizing bacterial capsular polysaccharides. Vaccine. 2010;28(39):6425–6435. doi: 10.1016/j.vaccine.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 74.Grothaus MC, Srivastava N, Smithson SL, et al. Selection of an immunogenic peptide mimic of the capsular polysaccharide of Neisseria meningitidis serogroup A using a peptide display library. Vaccine. 2000;18(13):1253–1263. doi: 10.1016/s0264-410x(99)00390-4. [DOI] [PubMed] [Google Scholar]
- 75.Park I, Choi IH, Kim SJ, Shin JS. Peptide mimotopes of Neisseria meningitidis group B capsular polysaccharide. Yonsei Medical Journal. 2004;45(4):755–758. doi: 10.3349/ymj.2004.45.4.755. [DOI] [PubMed] [Google Scholar]
- 76.Lauvrak V, Berntzen G, Heggelund U, et al. Selection and characterization of cyclic peptides that bind to a monoclonal antibody against meningococcal L3,7,9 lipopolysaccharides. Scandinavian Journal of Immunology. 2004;59(4):373–384. doi: 10.1111/j.1365-3083.2004.01400.x. [DOI] [PubMed] [Google Scholar]
- 77.Torregrossa P, Buhl L, Bancila M, et al. Selection of poly-α 2,8-sialic acid mimotopes from a random phage peptide library and analysis of their bioactivity. Journal of Biological Chemistry. 2004;279(29):30707–30714. doi: 10.1074/jbc.M403935200. [DOI] [PubMed] [Google Scholar]
- 78.Marino P, Norreel JC, Schachner M, Rougon G, Amoureux MC. A polysialic acid mimetic peptide promotes functional recovery in a mouse model of spinal cord injury. Experimental Neurology. 2009;219(1):163–174. doi: 10.1016/j.expneurol.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 79.Lo Passo C, Romeo A, Pernice I, et al. Peptide mimics of the group B meningococcal capsule induce bactericidal and protective antibodies after immunization. Journal of Immunology. 2007;178(7):4417–4423. doi: 10.4049/jimmunol.178.7.4417. [DOI] [PubMed] [Google Scholar]
- 80.Menéndez T, Santiago-Vispo NF, Cruz-Leal Y, et al. Identification and characterization of phage-displayed peptide mimetics of Neisseria meningitidis serogroup B capsular polysaccharide. International Journal of Medical Microbiology. 2011;301(1):16–25. doi: 10.1016/j.ijmm.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 81.Prinz DM, Smithson SL, Westerink MAJ. Two different methods result in the selection of peptides that induce a protective antibody response to Neisseria meningitidis serogroup C. Journal of Immunological Methods. 2004;285(1):1–14. doi: 10.1016/j.jim.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 82.Moe GR, Granoff DM. Molecular mimetics of Neisseria meningitidis serogroup B polysaccharide. International Reviews of Immunology. 2001;20(2):201–220. doi: 10.3109/08830180109043034. [DOI] [PubMed] [Google Scholar]
- 83.Falklind-Jerkérus S, Felici F, Cavalieri C, et al. Peptides mimicking Vibrio cholerae O139 capsular polysaccharide elicit protective antibody response. Microbes and Infection. 2005;7(15):1453–1460. doi: 10.1016/j.micinf.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 84.Dharmasena MN, Jewell DA, Taylor RK. Development of peptide mimics of a protective epitope of Vibrio cholerae Ogawa O-antigen and investigation of the structural basis of peptide mimicry. Journal of Biological Chemistry. 2007;282(46):33805–33816. doi: 10.1074/jbc.M707314200. [DOI] [PubMed] [Google Scholar]
- 85.Dharmasena MN, Krebs SJ, Taylor RK. Characterization of a novel protective monoclonal antibody that recognizes an epitope common to Vibrio cholerae Ogawa and Inaba serotypes. Microbiology. 2009;155(7):2353–2364. doi: 10.1099/mic.0.025726-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Phalipon A, Folgori A, Arondel J, et al. Induction of anti-carbohydrate antibodies by phage library-selected peptide mimics. European Journal of Immunology. 1997;27(10):2620–2625. doi: 10.1002/eji.1830271022. [DOI] [PubMed] [Google Scholar]
- 87.Geiser M, Schultz D, Le Cardinal A, Voshol H, García-Echeverría C. Identification of the human melanoma-associated chondroitin sulfate proteoglycan antigen epitope recognized by the antitumor monoclonal antibody 763.74 from a peptide phage library. Cancer Research. 1999;59(4):905–910. [PubMed] [Google Scholar]
- 88.Melzer H, Fortugno P, Mansouri E, et al. Antigenicity and immunogenicity of phage library-selected peptide mimics of the major surface proteophosphoglycan antigens of Entamoeba histolytica . Parasite Immunology. 2002;24(6):321–328. doi: 10.1046/j.1365-3024.2002.00463.x. [DOI] [PubMed] [Google Scholar]
- 89.Gevorkian G, Segura E, Acero G, et al. Peptide mimotopes of Mycobacterium tubercolosis carbohydrate immunodeterminants. Biochemical Journal. 2005;387(2):411–417. doi: 10.1042/BJ20041139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barenholz A, Hovav AH, Fishman Y, Rahav G, Gershoni JM, Bercovier H. A peptide mimetic of the mycobacterial mannosylated lipoarabinomannan: characterization and potential applications. Journal of Medical Microbiology. 2007;56(5):579–586. doi: 10.1099/jmm.0.46920-0. [DOI] [PubMed] [Google Scholar]
- 91.Legutki JB, Nelson M, Titball R, Galloway DR, Mateczun A, Baillie LW. Analysis of peptide mimotopes of Burkholderia pseudomallei exopolysaccharide. Vaccine. 2007;25(45):7796–7805. doi: 10.1016/j.vaccine.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 92.Beninati C, Garibaldi M, Passo CL, et al. Immunogenic mimics of Brucella lipopolysaccharide epitopes. Peptides. 2009;30(10):1936–1939. doi: 10.1016/j.peptides.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 93.Chen Y, Liu B, Yang D, et al. Peptide mimics of peptidoglycan are vaccine candidates and protect mice from infection with Staphylococcus aureus . Journal of Medical Microbiology. 2011;60(7):995–1002. doi: 10.1099/jmm.0.028647-0. [DOI] [PubMed] [Google Scholar]
- 94.Tam JP. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(15):5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yuki N. Infectious origins of, and molecular mimicry in, Guillain-Barré and Fisher syndromes. Lancet Infectious Diseases. 2001;1(1):29–37. doi: 10.1016/S1473-3099(01)00019-6. [DOI] [PubMed] [Google Scholar]
- 96.Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985;314(6006):53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]