Abstract
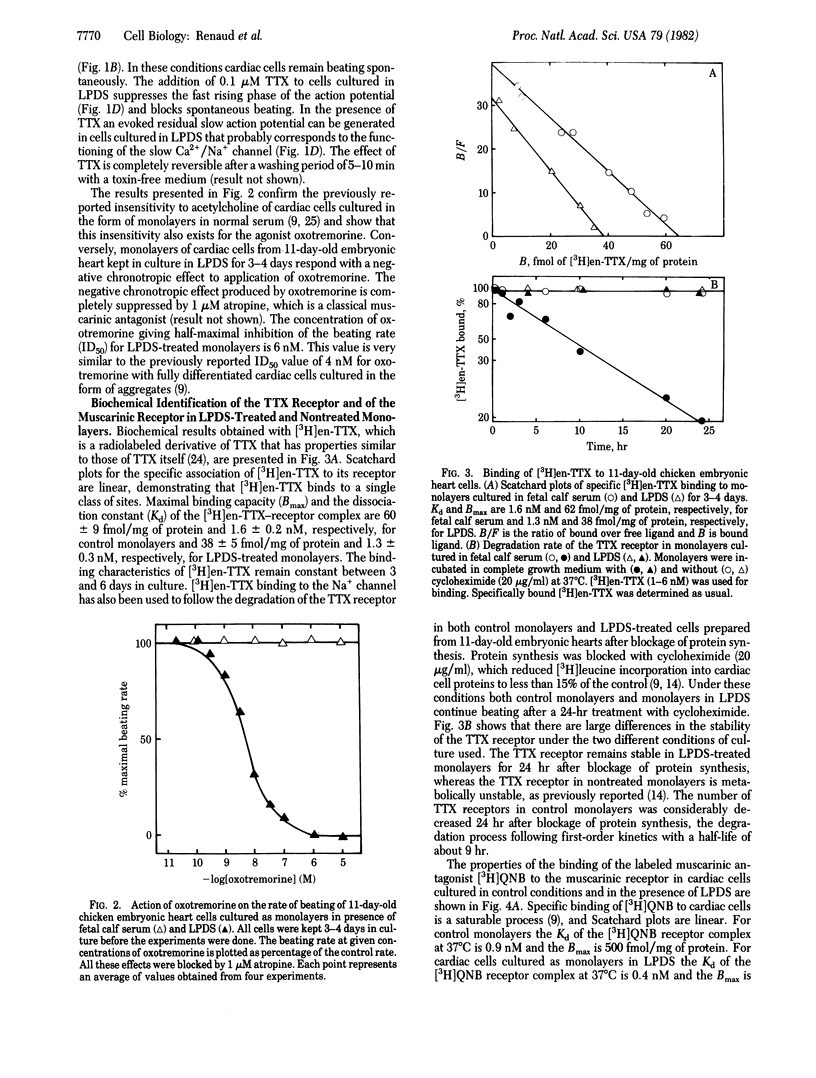
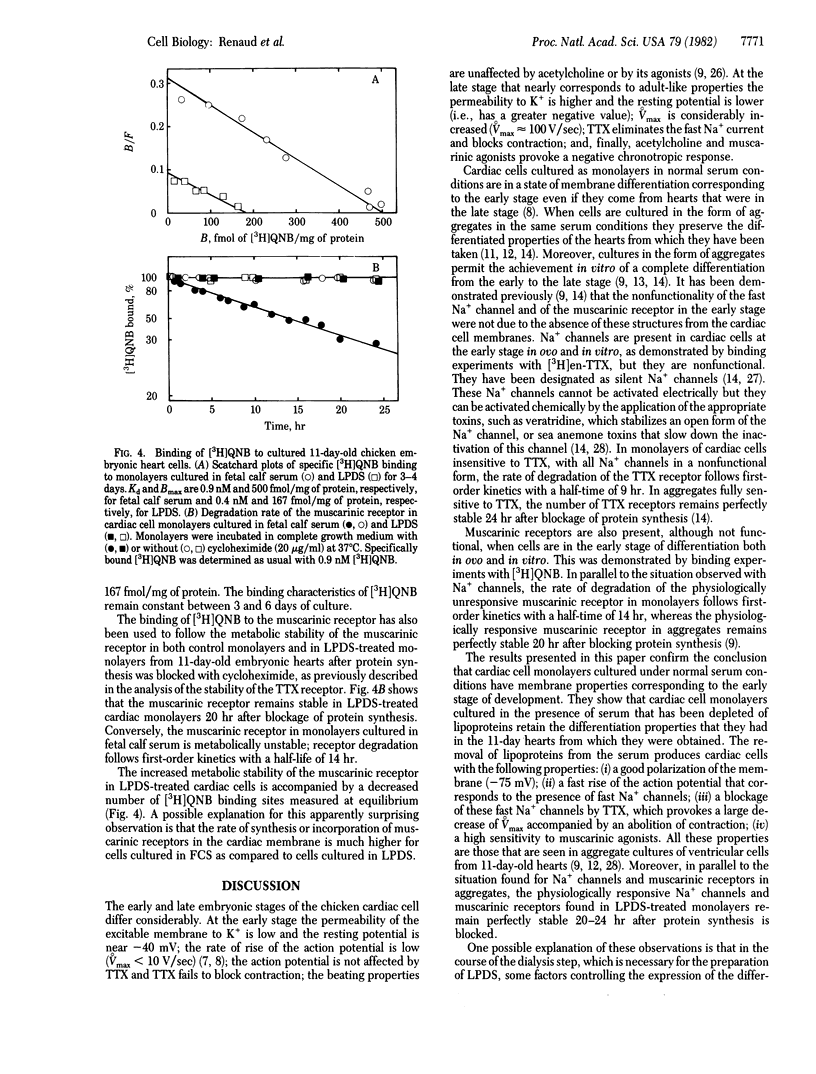
Monolayers of cardiac cells from 11-day-old chicken hearts have different properties when maintained in fetal calf serum or in a lipoprotein-deficient serum (LPDS). Cells in fetal calf serum have a resting potential near -60 mV; the rate of rise of the action potential is low (less than 10 V/sec); the action potential and the contraction are essentially unaffected by tetrodotoxin (TTX); and the beating properties are unaffected by muscarinic agents. Cells in LPDS have a resting potential near -75 mV, and a fast rise of the action potential (approximately equal to 100 V/sec) that is drastically decreased by TTX with a parallel abolition of contraction, and the beat is blocked by very low concentrations of muscarinic agonists. Cells that are physiologically fully responsive to TTX and to muscarinic agents have receptors that remain stable 24 hr after protein synthesis is blocked, whereas cells that are physiologically unresponsive to TTX and muscarinic agents have receptors that are rapidly degraded with half-lives between 9 hr (TTX receptor) and 14 hr (muscarinic receptor). Differences in the physiological and biochemical properties are accompanied by changes in the cholesterol contents of the cell membranes. The properties of cardiac cells cultured in normal serum are similar to those found for cells of chicken hearts in the very early embryonic stage, whereas those of cardiac cells cultured in LPDS correspond to the late embryonic stage.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Chicheportiche R., Balerna M., Lombet A., Romey G., Lazdunski M. Synthesis of new, highly radioactive tetrodotoxin derivatives and their binding properties to the sodium channel. Eur J Biochem. 1980 Mar;104(2):617–625. doi: 10.1111/j.1432-1033.1980.tb04466.x. [DOI] [PubMed] [Google Scholar]
- DUFOUR J. J., POSTERNAK J. M. [Chronotropic effects of acetylcholine on the chick embryo heart]. Helv Physiol Pharmacol Acta. 1960;18:563–580. [PubMed] [Google Scholar]
- DeHaan R. L. Differentiation of excitable membranes. Curr Top Dev Biol. 1980;16:117–164. doi: 10.1016/s0070-2153(08)60155-6. [DOI] [PubMed] [Google Scholar]
- Fayet G., Couraud F., Miranda F., Lissitzky S. Electro-optical system for monitoring activity of heart cells in culture: application to the study of several drugs and scorpion toxins. Eur J Pharmacol. 1974 Jul;27(2):165–174. doi: 10.1016/0014-2999(74)90142-3. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hasin Y., Shimoni Y., Stein O., Stein Y. Effect of cholesterol depletion on the electrical activity of rat heart myocytes in culture. J Mol Cell Cardiol. 1980 Jul;12(7):675–683. doi: 10.1016/0022-2828(80)90098-x. [DOI] [PubMed] [Google Scholar]
- Kutchai H., Barenholz Y., Ross T. F., Wermer D. E. Developmental changes in plasma membrane fluidity in chick embryo heart. Biochim Biophys Acta. 1976 Jun 4;436(1):101–112. doi: 10.1016/0005-2736(76)90223-6. [DOI] [PubMed] [Google Scholar]
- Kutchai H., King S. L., Martin M., Daves E. D. Glucose uptake by chicken embryo hearts at various stages of development. Dev Biol. 1977 Jan;55(1):92–102. doi: 10.1016/0012-1606(77)90322-0. [DOI] [PubMed] [Google Scholar]
- Lombet A., Renaud J. F., Chicheportiche R., Lazdunski M. A cardiac tetrodotoxin binding component: biochemical identification, characterization, and properties. Biochemistry. 1981 Mar 3;20(5):1279–1285. doi: 10.1021/bi00508a036. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Sachs H. G., DeHaan R. L. Development of sensitivity to tetrodotoxin in beating chick embryo hearts, single cells, and aggregates. Science. 1972 Jun 16;176(4040):1248–1250. doi: 10.1126/science.176.4040.1248. [DOI] [PubMed] [Google Scholar]
- McLean M. J., Sperelakis N. Retention of fully differentiated electrophysiological properties of chick embryonic heart cells in culture. Dev Biol. 1976 May;50(1):134–141. doi: 10.1016/0012-1606(76)90073-7. [DOI] [PubMed] [Google Scholar]
- Monard D., Rentsch M., Schuerch-Rathgeb Y., Lindsay R. M. Morphological differentiation of neuroblastoma cells in medium supplemented with delipidated serum. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3893–3897. doi: 10.1073/pnas.74.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan R. D., DeHaan R. L. In vitro differentiation of a fast Na+ conductance in embryonic heart cell aggregates. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2776–2780. doi: 10.1073/pnas.75.6.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott P., Binggeli Y., Brodbeck U. A rapid and sensitive assay for determination of cholesterol in membrane lipid extracts. Biochim Biophys Acta. 1982 Feb 23;685(2):211–213. doi: 10.1016/0005-2736(82)90101-8. [DOI] [PubMed] [Google Scholar]
- Pappano A. J. Ontogenetic development of autonomic neuroeffector transmission and transmitter reactivity in embryonic and fetal hearts. Pharmacol Rev. 1977 Mar;29(1):3–33. [PubMed] [Google Scholar]
- Paris S., Fosset M., Samuel D., Ailhaud G. Chick embryo plasma membrane from cardiac muscle and cultured heart cells: isolation procedure and absence of fatty acid-activating enzymes. J Mol Cell Cardiol. 1977 Feb;9(2):161–174. doi: 10.1016/0022-2828(77)90047-5. [DOI] [PubMed] [Google Scholar]
- Renaud J. F., Barhanin J., Cavey D., Fosset M., Lazdunski M. Comparative properties of the in ovo and in vitro differentiation of the muscarinic cholinergic receptor in embryonic heart cells. Dev Biol. 1980 Jul;78(1):184–200. doi: 10.1016/0012-1606(80)90328-0. [DOI] [PubMed] [Google Scholar]
- Renaud J. F., Romey G., Lombet A., Lazdunski M. Differentiation of the fast Na+ channel in embryonic heart cells: interaction of the channel with neurotoxins. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5348–5352. doi: 10.1073/pnas.78.9.5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romey G., Renaud J. F., Fosset M., Lazdunski M. Pharmacological properties of the interaction of a sea anemone polypeptide toxin with cardiac cells in culture. J Pharmacol Exp Ther. 1980 Jun;213(3):607–615. [PubMed] [Google Scholar]
- Sachs H. G., McDonald T. F., DeHaan R. L. Tetrodotoxin sensitivity of cultured embryonic heart cells depends on cell interactions. J Cell Biol. 1973 Jan;56(1):255–258. [PMC free article] [PubMed] [Google Scholar]
- Sperelakis N., Lehmkuhl D. Insensitivity of cultured chick heart cells to autonomic agents and tetrodotoxin. Am J Physiol. 1965 Oct;209(4):693–698. doi: 10.1152/ajplegacy.1965.209.4.693. [DOI] [PubMed] [Google Scholar]



