Abstract
Purpose
High-dose ketoconazole and docetaxel have shown activity as single agents against castration-resistant prostate cancer (CRPC). The goal of this phase I study was to determine the maximum tolerated doses, side effects, and pharmacokinetic interaction of coadministered docetaxel and ketoconazole.
Experimental Design
Patients with metastatic CRPC received weekly docetaxel for 3 of every 4 weeks, plus daily ketoconazole. Pharmacokinetic studies were performed on day 1 (docetaxel alone) and day 16 (after ketoconazole).
Results
The study enrolled 42 patients at 9 different dose levels. The combination regimens investigated included docetaxel weekly for three weeks out of four escalating from 5 to 43 mg/m2, with starting doses of ketoconazole of 600, 800, or 1200 mg/day. Declines in prostate-specific antigen of ≥ 50% were seen in 62% of patients. Of 25 patients with soft tissue disease, 7 (28%) had partial response. Median overall survival was 22.8 months, and was significantly greater in docetaxel-naïve patients than in patients pretreated with docetaxel (36.8 vs. 10.3 months; P = 0.0001). The most frequently observed adverse events were anemia, edema, fatigue, diarrhea, nausea, sensory neuropathy, and elevated liver function tests. The fractional change in docetaxel clearance correlated significantly with ketoconazole exposure (P < 0.01). Concomitant ketoconazole increased docetaxel exposure 2.6-fold with 1200 mg/day, 1.6-fold with 800 mg/day, and 1.3- to 1.5-fold with 600 mg/day.
Conclusions
Results suggest that the combination of weekly docetaxel and ketoconazole has significant antitumor activity in CRPC with manageable toxicities. The extremely long survival in the docetaxel-naïve cohort (36.8 months) warrants additional larger trials of docetaxel with ketoconazole or possibly CYP17A1 inhibitors such as abiraterone.
Keywords: castration-resistant prostate cancer, docetaxel, ketoconazole, drug-drug interaction, CYP3A4
Introduction
Given the expansion of an aging population in the United States, prostate cancer is expected to continue as the leading cause of cancer in men and a major public health issue for decades to come (1, 2). While localized disease is initially responsive to hormonal therapy, metastatic disease eventually becomes castration resistant, leading to death within a few years in the majority of patients (3). Severe morbidity is associated with advanced stages of prostate cancer, and treatment for castration-resistant prostate cancer (CRPC) is primarily palliative. Recent reviews of clinical trials evaluating different chemotherapeutic regimens for CRPC have consistently concluded that docetaxel-based chemotherapy regimens have a significant survival advantage in patients with metastatic CRPC (4, 5), leading to the investigation of new docetaxel combination regimens to further improve clinical response (6–8).
At high doses, the antifungal agent ketoconazole suppresses testicular and adrenal androgen production by interfering with the CYP450-dependent enzyme required for steroid synthesis. Androgen-deprivation therapy (ADT) with ketoconazole is used as a secondary hormonal treatment in prostate cancer. Ketoconazole has also shown direct cytotoxic effects on prostate tumor cells when used as a single agent (9) and synergistic effects preclinically and clinically when combined with chemotherapeutic agents (10, 11). Combination therapy with ketoconazole and doxorubicin in CRPC patients resulted in a 55% PSA response rate (11). We have previously shown that the addition of ketoconazole potentiates the antitumor effects of microtubule-active drugs such as paclitaxel and vinblastine on prostate cancer cell lines (10). Based on our observation of the synergistic antitumor activity of docetaxel plus ketoconazole against castration resistant prostate cancer cells, we designed a phase I clinical study of docetaxel plus ketoconazole.
Besides its antiandrogen effect, ketoconazole also strongly inhibits CYP3A-mediated metabolism and is a weak to modest inhibitor of ABCB1. CYP3A4/5 are major metabolic isoenzymes responsible for the inactivation of docetaxel in vivo. Docetaxel and its metabolites are primarily eliminated in feces. ABCB1 is believed to play a primary role in the fecal elimination of docetaxel and its metabolites via biliary excretion, as docetaxel is also a substrate for ABCB1. Given the pharmacokinetic properties of docetaxel and ketoconazole, potential pharmacokinetic interaction between the two agents was expected (12, 13).
This report describes a phase I study of weekly docetaxel with daily high-dose ketoconazole in metastatic CRPC to evaluate the combination’s safety, tolerability, clinical activity, and pharmacokinetic interactions. This trial was designed prior to the publication of TAX 327 which demonstrated that only docetaxel every 21 days was superior to mitoxantrone and prednisone. We also performed a correlative study to assess whether variants in the genes involved in docetaxel disposition are associated with docetaxel pharmacokinetics and ketoconazole-mediated drug interactions in these patients.
Patients and Methods
This was a single-arm phase I dose-escalation clinical trial of weekly docetaxel plus daily high-dose ketoconazole and hydrocortisone. The primary objective of the study was to determine the maximum tolerated dose (MTD) and dose-limiting toxicity (DLT) of this combination. Secondary objectives included assessment of the pharmacokinetic interaction and clinical activity of coadministered ketoconazole and docetaxel. The study was approved by the NCI Institutional Review Board and conducted in the Clinical Center of the NIH. All patients provided written informed consent.
Patient eligibility
Eligible patients had histopathological documentation of prostate cancer and evidence of progression of metastatic CRPC, and continued to receive ADT on study. Evidence of progression was documented by at least one new metastatic lesion on bone scintigraphy and/or progression of soft-tissue metastases, or PSA progression as defined by 2 consecutive increases in PSA to ≥ 5 ng/mL. Other criteria included ECOG performance status of 0 to 2, estimated life expectancy of > 3 months, serum testosterone < 50 ng/dL, and adequate hematologic, renal, and hepatic function. Patients who had clinically significant heart disease or brain metastasis were ineligible. Patients were excluded if they had received substances known to interact with CYP3A-mediated activity (e.g., substrate, inducer, or inhibitor) because of possible pharmacokinetic interactions with ketoconazole and docetaxel. Coadministration of drugs affecting gastric pH may decrease dissolution and absorption of ketoconazole, and thus was prohibited. Prior docetaxel or ketoconazole were not exclusion criteria.
Treatment plan
Treatment cycles, repeated every 28 days, consisted of weekly docetaxel for three consecutive weeks followed by a one-week rest period, and daily ketoconazole and hydrocortisone. The initial dose of docetaxel was administered alone for pharmacokinetic evaluation in the absence of ketoconazole; docetaxel was not given again for approximately 2 weeks. About 2 to 3 days before initiating weekly docetaxel, patients began receiving a fixed dose of oral ketoconazole 3 times/day (TID) and continued throughout the study. Docetaxel was administered i.v. over 30 min at doses of 5, 10, 16, 24, 32, and 43 mg/m2 (see Table 1 for dose-escalation and combination schema). Due to hepatic toxicity observed at the higher doses of ketoconazole, the study protocol was progressively amended to lower starting doses from 1200 mg/day (400 mg TID), to 800 mg/day (200 mg in the morning and afternoon and 400 mg at night), to 600 mg/day (200 mg TID). All patients received 8 mg of oral dexamethasone at 12 h and 1 h prior to docetaxel infusion, then 12 h after the infusion was completed. Administration of daily hydrocortisone (20 mg in the morning and 10 mg in the evening) commenced on a parallel schedule with ketoconazole administration.
Table 1.
Dose escalation schema
| Dose level | N | Docetaxel (Weekly; mg/m2) | Ketoconazole (TID; mg/day) |
|---|---|---|---|
| 1 | 6 | 5 | 1200 |
| 2 | 6 | 5 | 800 |
| 3 | 6 | 10 | 1200 |
| 4 | 4 | 10 | 800 |
| 5 | 4 | 10 | 600 |
| 6 | 3 | 16 | 600 |
| 7 | 4 | 24 | 600 |
| 8 | 6 | 32 | 600 |
| 9 | 3 | 43 | 600 |
The plan was to accrue 3 patients for each dose level. If 1 of 3 patients experienced a DLT at a particular dose level, that group was expanded to 6 patients. If 2 or more of these 6 demonstrated a DLT, the dose level below would be considered the MTD. DLT was defined as treatment-related, occurring within the first cycle of therapy that included ≥ grade 3 nonhematologic toxicity (excluding nausea and vomiting without symptomatic prophylactic treatment) or grade 4 hematologic toxicity defined as grade 4 neutropenia and thrombocytopenia of ≥ 3 days duration.
Assessment of toxicity and response
Toxicity was assessed according to the NCI Common Toxicity Criteria for Adverse Events (CTCAE) version 2. Patients with measurable disease were assessed according to Response Evaluation Criteria in Solid Tumors (RECIST). PSA, blood chemistry, and hematologic function were measured monthly. Computed tomography scans of the chest, abdomen, and pelvis, and technetium-99m bone scintigraphy were performed at baseline and every 3 months during treatment.
Pharmacokinetic interaction studies
Pharmacokinetic studies were performed following docetaxel infusion on day 1 (docetaxel alone) and day 16 (2 to 3 days after initiating daily ketoconazole). Blood samples were obtained at pre, 0.5, 1, 1.5, 2, 3, 4, 5, 7, 24, 32, 48, and 56 h after the start of docetaxel infusion. The plasma was separated immediately and stored at −70°C until analysis. All samples were analyzed using an assay validated for the simultaneous measurement of docetaxel and ketoconazole. Briefly, 100 μL of plasma was transferred to a glass centrifuge tube and 1mL of methyl tert-butyl ether containing the internal standard, paclitaxel, was added. After vortex mixing and centrifuging, supernatant layer was collected and dried down. Then, the residue was reconstituted with a mixture of methanol/0.1% formic acid (v:v, 60:40), out of which 5 μL solution was injected into the Acquity UPLC (Waters Corp, Milford, MA). Mass analysis was achieved by a Quattro Premier Triple Quadrupole Mass Spectrometer (Waters Corp, Milford, MA) using electrospray ionization method. The compounds were separated on a Symmetry Shield Rp18 column (2.1x 50 mm, 3.5 μm) using mobile phase consisting of methanol (B)/0.1% formic acid (A) at a flow rate of 0.2 mL/min. Initial condition, 40% B was gradually increased to 65% within the first 4 min of gradient run, then held for 3 min before it was set to the initial condition. The total run time was 8 min. Three ion transitions were monitored: docetaxel, 808.5→527.3 m/z; ketoconazole, 531.2→209.0, m/z; paclitaxel, 854.4→569.1, m/z. Assay range was 1 to 1000 ng/mL for docetaxel and 1 to 15 μg/mL for ketoconazole. Accuracy and precision of three concentrations of quality control samples ranged from 98% to 104.3% and 0 to 3.2% for docetaxel and 99.3 to 104.3% and 1.2 to 4.2% for ketoconazole.
Individual plasma concentration-time profiles were analyzed by noncompartmental pharmacokinetic methods using WinNonlin (version 5.2, Pharsight Corporation, Mountain View, CA). For docetaxel, pharmacokinetic parameters included the area under the plasma curve (AUC) extrapolated to infinity, clearance (CL), steady-state volume of distribution (Vss), and terminal half-life. For ketoconazole, values of Cmin and Cmax at steady-state were obtained by inspection; AUC7hr,ss was calculated based on 6 to 8 samples serially collected up to 7 h over one dosing interval.
Genotyping analysis
Genomic DNA was extracted from plasma using a QiaBlood DNA extraction kit (Qiagen, Valencia, CA). Direct nucleotide sequencing polymerase chain reaction (PCR) was conducted using the Big Dye Terminator Cycle Sequencing Ready Reaction kit V1.1 on an ABI Prism 3130 xl Genetic Analyzer (Applied BioSystems, Foster City, CA). Genotyping was performed at 3 ABCB1 loci (1236C>T, 2677G>T/A, and 3435C>T) (14), CYP3A4*1B (15), CYP3A5*3C (15), and SLCO1B3 334T>G (16), as previously described. The most likely ABCB1 diplotype was computed only in the Caucasian population using an EM algorithm implemented in Helix Tree® (Golden Helix Inc., Bozeman, MT). ABCB1 diplotypes were constituted from ABCB1 1236C-2677G-3435C for a correlative study using a previously reported method (14), and further categorized into two groups, carrier or noncarrier, based on whether or not individual carries ABCB1 TTT/TTT diplotype.
Statistical analysis
The values of docetaxcel AUC were natural log-transformed and compared with and without concomitant ketoconazole using a linear mixed-effects model, with ketoconazole treatment as a fixed factor and subject as a random factor. The least square mean and 90% confidence intervals for the mean difference between ketoconazole treatments were estimated from the model and exponentiated to obtain geometric mean ratio and confidence intervals on the original scale. A two-tailed Mann-Whitney test was used for 2 group comparisons. A P value < 0.05 was considered statistically significant. PSA response was analyzed with Chi-squared test. Progression-free survival (PFS) and overall survival (OS) were analyzed using the Kaplan-Meier method, and comparison among treatment groups was performed using the log-rank test. All P-values are two-tailed.
Results
This clinical trial enrolled 42 patients between April 2002 and June 2009. A summary of patient characteristics is reported in Table 2. The median age was 66 and a majority of patients had an ECOG status of 1. Median PSA at baseline was 76.5 ng/mL (range, 1.4 to 4677 ng/mL). Prior to enrollment, 15 patients (35%) had received docetaxel therapy and 2 had received ketoconazole therapy. Other prior therapies included thalidomide, estramustine, sorafenib, and radiotherapy.
Table 2.
Patient characteristics
| Characteristics | Values |
|---|---|
| Age (years) | |
| Median | 66 |
| Range | 44 – 84 |
|
| |
| Race | |
| White | 38 |
| Black | 4 |
|
| |
| Gleason score | |
| ≤ 6 | 2 |
| 7 | 13 |
| 8 | 10 |
| 9 | 14 |
| 10 | 3 |
|
| |
| ECOG Performance | |
| 0 | 5 |
| 1 | 29 |
| 2 | 4 |
|
| |
| Prior therapy | |
| Median number of prior therapies | 2 |
| Docetaxel | 15 |
| Thalidomide | 9 |
| Estramustine | 5 |
| Sorafenib | 5 |
| Bevacizumab | 4 |
| Mitoxantrone | 3 |
| Immunotherapy | 12 |
| Radiotherapy | 29 |
|
| |
| PSA at enrollment | |
| Median | 76.5 |
| Range | 1.4 – 4677 |
Patients received 9 different combination regimens of docetaxel and ketoconazole (Table 1) within a median of 6 treatment cycles (range, 1 to 64). Patients who began with 1200 mg of ketoconazole reached the MTD of docetaxel at 5 mg/m2 due to hepatic toxicity. The starting dose of ketoconazole was subsequently lowered to 800 mg/day. At this dose of ketoconazole and 10 mg/m2 of docetaxel, 1 of 3 patients had reversible grade 3 hepatic toxicity; therefore, 3 additional patients were added at this dose level, one of whom experienced a grade 3 elevation in liver enzymes. No grade 3 or 4 hematologic or docetaxel-related toxicities were observed at the 10 mg/m2 dose of docetaxel. Subsequently, the initial dose of daily ketoconazole was reduced to 600 mg/day (200 mg TID) combined with an initial dose of 10 mg/m2 docetaxel. This dose of ketoconazole was well tolerated and allowed for the escalation of docetaxel, as called for in the study protocol (Table 1). The MTD of docetaxel was 43 mg/m2 administered weekly for 3 consecutive weeks.
Toxicity
Observed toxicities in the 42 study patients are summarized in Table 3. Grade 1 to 2 anemia was observed in 50% of patients, and 3 patients (7%) experienced grade 3 anemia. The incidence of grade 3/4 neutropenia (n = 1) or febrile neutropenia (n = 1) was very low (2%). Low-grade nonhematologic toxicities that occurred at least of 15% of patients included fatigue, insomnia, anorexia, diarrhea, dyspepsia, muscle weakness, dizziness, tearing, dyspnea, and abdominal pain. Other common grade 1/2 toxicities attributed to docetaxel included edema, nail changes, taste disturbances, nausea, vomiting, and sensory neuropathy. However, the majority of these events did not require dose reduction or treatment of symptoms. Grade 3 fatigue was noted in 12% of patients, and 10% experienced grade 3 diarrhea. Toxicities attributed to ketoconazole included transient increases in serum AST and/or ALT (56%), bilirubin, and/or alkaline phosphatase, especially at the higher doses (800 and 1200 mg/day). For most patients with grade 2/3 AST/ALT elevation, the dose of ketoconazole was reduced and/or temporarily discontinued. Of the 7 patients with grade 3 hepatic toxicities, 5 had received an initial dose of ≥ 800 mg/day of ketoconazole; 2 patients were taken off study due to elevated liver enzymes.
Table 3.
Incidence of treatment-related adverse events
| Adverse events | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Hematologic | ||||
| Anemia | 12 | 9 | 3 | |
| Leukopenia | 1 | 2 | ||
| Neutropenia | 1 | 1 | 1 | |
| Febrile neutropenia | 1 | |||
| Lymphopenia | 7 | |||
| Thrombocytopenia | 3 | |||
|
| ||||
| Non-hematologic | ||||
| Supraventricular arrhythmia | 1 | |||
| Edema | 15 | 2 | ||
| Pericardial effusion | 1 | |||
| Thrombosis/embolism | 2 | |||
| Fatigue | 17 | 8 | 5 | |
| Nail changes | 8 | 4 | ||
| Anorexia | 7 | 2 | ||
| Constipation | 4 | 2 | 2 | |
| Diarrhea | 9 | 3 | 4 | |
| Change in taste | 6 | |||
| Melena/GI bleeding | 1 | 1 | ||
| Nausea | 12 | 2 | 1 | |
| Vomiting | 10 | 2 | ||
| Infection | 1 | 4 | 6 | |
| Muscle weakness | 10 | 4 | ||
| Dyspnea | 8 | |||
| Sensory neuropathy | 12 | 4 | ||
|
| ||||
| Metabolic/laboratory abnormalities | ||||
| Elevated AST/ALT | 9 | 7 | 7 | |
| Elevated total bilirubin | 6 | 3 | 2 | |
| Elevated alkaline phosphatase | 6 | 2 | 3 | |
| Hypoalbuminemia | 8 | 11 | ||
| Hyperglycemia | 7 | 12 | 10 | |
Response to therapy
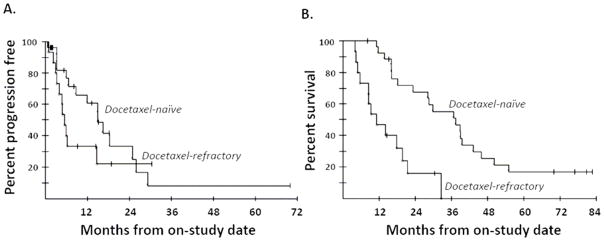
Overall, 62% (26 of 42) of patients had PSA declines of ≥ 50%. More than half demonstrated a ≥ 50% PSA decline in all combination regimens of docetaxel and ketoconazole, except for the combination of docetaxel 10 mg/m2 and ketoconazole 600 mg/day, at which only 25% showed the PSA decline. PSA declines of ≥ 50% were slightly higher in patients without prior docetaxel therapy (18/27; 67%) than in those who had received docetaxel before (8/15; 53%; P = 0.50). Of patients with soft tissue disease, 7 of 25 (28%) had a partial response (PR), with tumor reduction of 31% to 72% by RECIST. Of those 7 patients with PR, 5 had a PSA decline of ≥ 50%, 2 had rises in PSA, and 3 had previous docetaxel-based treatment. The median PFS was 11.1 months for all patients, 15 months for patients without prior docetaxel therapy, and 5.2 months for patients with prior docetaxel treatment (P = 0.19, Figure 1A). After a median potential follow-up of 65.8 months, 33 of 42 patients have died. The median OS was 22.8 months for all patients, which exceeded the Halabi predicted survival of 14 months (17). The median survival was 36.8 months for docetaxel-naïve patients and 10.3 months for those who had received docetaxel prior to enrollment (P = 0.0001, Figure 1B).
Figure 1.
Progression-free survival (A) and overall survival (B) for patients with and without prior docetaxel therapy.
Ketoconazole pharmacokinetics
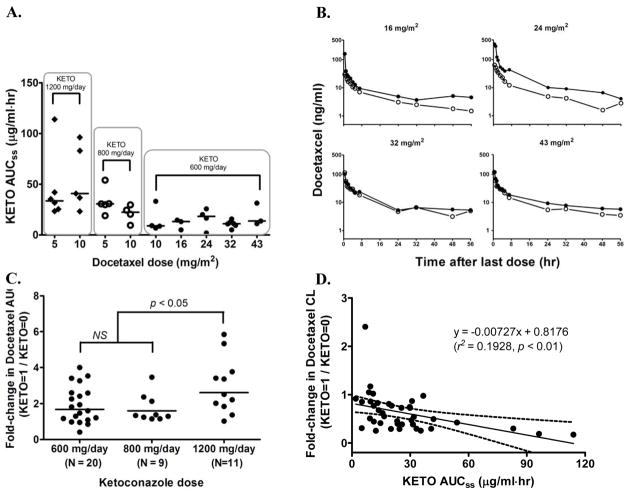
Ketoconazole pharmacokinetics in the presence of docetaxel was assessed 2 to 3 days after initiating daily ketoconazole. Given the half-life of ketoconazole (~ 8 h), the drug was expected to be in steady-state by the time of the pharmacokinetic evaluation. Ketoconazole at 200 mg TID yielded mean concentrations between 1.24 and 2.79 μg/mL during steady-state (Table 4). The increase in ketoconazole exposure measured in Cmax, Cmin, and AUC7hr,ss was more than proportional to the dose given. This is consistent with the previous observation that ketoconazole follows a nonlinear kinetic, presumably due to temporary saturation of first-pass metabolism in the liver (18, 19). The estimated mean AUC7hr,ss over one dosing interval in our study was 13.15 μg·h/mL at 200 mg and 52.08 μg·h/mL at 400 mg, which was comparable to previously reported values for single-agent ketoconazole at 200 mg (7.80 to 17.55 μg·h/mL) (12, 18, 20, 21) and 400 mg (23.11 to 70.95 μg·h/mL) (20, 22–24). Although we did not assess ketoconazole pharmacokinetics alone, comparison with historical data suggests that coadministration of docetaxel has little or no effect on ketoconazole pharmacokinetics. We also found no noticeable effects of concomitant docetaxel on ketoconazole exposure (Figure 2A).
Table 4.
Mean ketoconazole pharmacokinetic parameters calculated during one dosing interval following daily oral ketoconazole three times a day (TID).
| Parameter (Mean ± SD) |
Ketoconazole Dose
|
||
|---|---|---|---|
| 600 mg/day (200 mg, TID) | 800 mg/day (200/200/400 mg, TID) | 1200 mg/day (400 mg, TID) | |
| N | 19 | 9 | 11 |
| Cmin (μg/mL) | 1.24 ± 0.95 | 2.88 ±1.81 | 5.69 ± 4.84 |
| Cmax (μg/mL) | 2.79 ±1.39 | 5.51 ±1.99 | 9.77 ± 5.83 |
| AUC7hr,ss (μg·hr/mL) | 13.15 ± 7.51 | 28.86 ±12.70 | 52.08 ± 33.13 |
Figure 2.
Pharmacokinetic interaction between docetaxel and ketoconazole. (A) Effects of docetaxel on exposure to ketoconazole in patients receiving 1200 mg/day, 800 mg/day, or 600 mg/day; (B) mean plasma concentration of docetaxel vs. time profiles at doses of 16 to 43 mg/m2 in the absence (open circles) and presence (closed circles) of ketoconazole 600 mg/day; (C) fold-changes in docetaxel AUC by ketoconazole coadministration; (D) correlation between ketoconazole AUC and fold-changes in docetaxel CL. Solid line is generated from a linear regression and broken lines represent 95% confidence intervals.
Docetaxel pharmacokinetics and interaction analysis
All 42 patients completed the initial first cycle for evaluation of docetaxel pharmacokinetics in the absence of ketoconazole and subsequent interaction with ketoconazole. Two patient samples were not available for interaction analysis and were thus excluded. At various doses of docetaxel (16 to 43 mg/m2), mean plasma concentrations were slightly higher in the presence of ketoconazole 600 mg/day (Figure 2B). A distinct change in plasma concentration was seen with lower doses of docetaxel (5 to 10 mg/m2) plus ketoconazole at 800 or 1200 mg/day (data not shown). The fold-change in docetaxel AUC was ketoconazole dose-dependent (Table 5). Exposure to docetaxel 5 to 10 mg/m2 increased 2.6-fold with coadministration of ketoconazole 1200 mg/day and 1.6-fold with 800 mg/day. Concomitant ketoconazole 600 mg/day doubled docetaxel AUC at doses of 16 and 24 mg/m2, but in general, fractional changes in this ketoconazole regimen were 1.3- to 1.5-fold for all docetaxel doses studied. Due to the small number of patients in each cohort (n = 2 to 4), the effects of ketoconazole on docetaxel pharmacokinetics were compared based on ketoconazole dose levels regardless of docetaxel doses (Figure 2C). The mean geometric ratio at ketoconazole 600 mg/day was 1.68 (90% CI, 1.34 to 2.10), which was statistically significant (P < 0.01) in comparison to a ratio of 1 (i.e., no change in AUC). Ketoconazole 800 mg/day yielded a 1.6-fold increase (90% CI, 1.26 to 2.04; P < 0.01) in docetaxel AUC, but was not statistically different from ketoconazole 600 mg/day. Exposure to docetaxel with daily ketoconazole at 1200 mg/day was 2.62 times higher than docetaxel alone (1.95 to 3.52; P < 0.001) and significantly higher than 2 lower ketoconazole dosing regimens. There were no consistent changes in docetaxel Cmax values by ketoconazole dose, as previously reported (13).
Table 5.
Pharmacokinetic comparison of docetaxel with and without multiple oral doses of ketoconazole
| Docetaxel (mg/m2) | KETOa (mg/day) | n | AUC (ng/ml·hr)b
|
CL (L/hr)
|
Vss (L)
|
t1/2 (hr)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
KETO =
|
GMRc | 90% CI |
KETO =
|
Ratiod |
KETO =
|
Ratiod |
KETO =
|
Ratiod | |||||||
| 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | ||||||||
| 5 | 1200 | 6 | 105 | 269 | 2.56** | 1.66 – 3.95 | 104.40 | 39.78 | 0.43 | 2449 | 1395 | 0.76 | 17.0 | 25.8 | 1.52 |
| 800 | 5 | 233 | 369 | 1.59* | 1.16 – 2.17 | 44.89 | 27.91 | 0.66 | 909 | 1161 | 1.83 | 18.1 | 28.9 | 1.60 | |
|
| |||||||||||||||
| 10 | 1200 | 5 | 348 | 933 | 2.68* | 1.48 – 4.85 | 59.22 | 24.23 | 0.44 | 1562 | 1034 | 0.64 | 19.0 | 24.9 | 1.31 |
| 800 | 4 | 305 | 496 | 1.62 | 0.89 – 2.95 | 71.70 | 49.06 | 0.67 | 1566 | 1333 | 0.83 | 16.0 | 20.0 | 1.25 | |
| 600 | 4 | 499 | 635 | 1.27 | 0.44 – 3.70 | 63.21 | 36.22 | 1.06 | 1331 | 870 | 1.39 | 16.1 | 17.9 | 1.11 | |
|
| |||||||||||||||
| 16 | 600 | 3 | 343 | 727 | 2.12 | 0.90 – 4.99 | 109.68 | 47.65 | 0.51 | 3182 | 1700 | 0.53 | 22.4 | 24.2 | 1.08 |
|
| |||||||||||||||
| 24 | 600 | 4 | 510 | 1052 | 2.06 | 1.14 – 3.73 | 101.23 | 64.08 | 0.54 | 2513 | 1351 | 0.45 | 20.8 | 17.6 | 0.85 |
|
| |||||||||||||||
| 32 | 600 | 6 | 706 | 1100 | 1.56 | 0.97 – 2.51 | 105.42 | 75.94 | 0.73 | 2847 | 2318 | 0.87 | 18.5 | 21.5 | 1.16 |
|
| |||||||||||||||
| 43 | 600 | 2 | 666 | 921 | 1.38 | 0.94 – 2.03 | 132.16 | 95.17 | 0.72 | 3553 | 2954 | 0.85 | 21.7 | 22.7 | 1.05 |
KETO, ketoconazole; KETO = 0, docetaxel alone; KETO = 1, docetaxel with ketoconazole; GMR, geometric mean ratio; CI, confidence interval.
Total daily dose of ketoconazole in mg: 1200 mg/day (400 mg TID), 800 mg/day (200/200/400 mg TID), 600 mg/day (200 mg TID).
AUC values are geometric means and the remaining variables are means.
GMR, geometric mean ratio of KETO = 1/KETO = 0 calculated from least square mean estimate from the linear mixed model based on the log-transformed AUC.
Ratio, ratio of KETO = 1/KETO = 0.
p < 0.01,
p < 0.05
Docetaxel CL was reduced 56% with ketoconazole 1200 mg/day, 34% with 800 mg/day, and 18% with 600 mg/day. Docetaxel Vss was 15% to 50% lower with coadministration of ketoconazole, compared to docetaxel alone. The half-life of docetaxel was minimally affected although prolonged 1.3- to 1.5-fold when low doses were coadministered with higher doses of ketoconazole. Fractional changes in docetaxel AUC (P < 0.0001, r2 = 0.48) and CL (P < 0.01, r2 = 0.2) (Figure 2D) were significantly correlated with ketoconazole exposure, albeit in a weak to moderate manner, but not with liver function (AST/ALT, alkaline phophatase, total bilirubin), age, serum creatinine, or albumin.
Genotypes and pharmacokinetic interaction
Genotypes and allele frequencies for CYP3A4/5, ABCB1, and SLCO1B3 in the Caucasian population are shown in Table 6. All genotype frequencies were in the Hardy-Weinberg equilibrium, except for CYP3A4*1B (P = 0.019), probably due to the low sample size. The small number of patients within either wild-type or variant genotypes of CYP3A isoenzyme prevented further analysis. In addition, no patient in this study carried both the CYP3A4*1B and CYP3A5*1A alleles, which are associated with an increase in docetaxel CL (25). The genetic polymorphism in SLCO1B3 was not associated with docetaxel pharmacokinetics.
Table 6.
Genotype information for Caucasian patients.
| Polymorphism | Genotype frequencies
|
||
|---|---|---|---|
| Wild type | Heterozygous | Variant | |
| CYP3A4*1Ba | 33 | 2 | 1 |
| CYP3A5*3Cb | 0 | 4 | 30 |
| SLCO1B3 S112Ac | 3 | 11 | 21 |
| ABCB1 1236C>Tc | 11 | 15 | 9 |
| 2677G>A/Tc | 10 | 16/1 (GT/GA) | 8/0 (TT/AA) |
| 3435C>Tc | 7 | 17 | 11 |
(n=1),
(n=3),
(n=2) unable to genotype
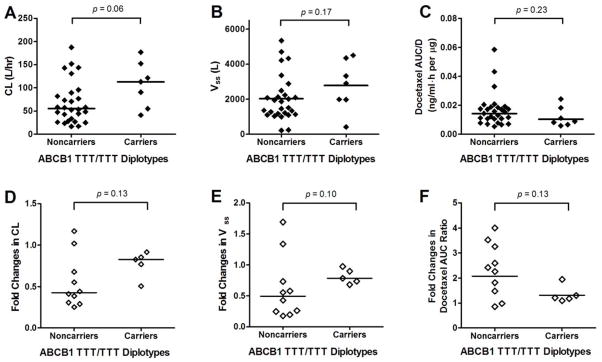
Seven of 35 patients carried a fully variant form of all 3 ABCB1 SNPs. Figure 3 presents docetaxel pharmacokinetic parameters as a function of ABCB1 diplotypes. The median value of docetaxel CL in patients carrying the ABCB1 TTT/TTT diplotype was twice that of noncarriers (P = 0.06, Figure 3A). Among patients who received ketoconazole 600 mg/day, docetaxel CL and Vss appeared to be less influenced by ketoconazole in carriers of ABCB1 TTT/TTT diplotype (e.g., near the ratio of 1; Figure 3D–E) than noncarriers, which, in turn, led to less change in total docetaxel AUC (Figure 3F), although this was statistically insignificant.
Figure 3.
Effects of ABCB1 polymorphism on pharmacokinetics of docetaxel alone (A–C) and in the presence of ketoconazole 600 mg/day (D–F).
Discussion
Ketoconazole is used as a second-line hormonal agent in patients with CRPC, based on its ability to reduce the biosynthesis of androgens. At the standard single-agent dose of 1200 mg/day, 30% to 80% of patients have a PSA decline of ≥ 50% (22, 26–29). Ketoconazole also appears to enhance the antitumor activity of chemotherapeutic agents, including doxorubicin (11) and mitoxantrone (30). Improved response rates have been observed with an adriamycin-ketoconazole regimen (55%) compared to adriamycin alone (33%). In a preclinical study, we found that the antitumor activity of microtubule agents against prostate cancer was significantly enhanced when combined with ketoconazole (10). Ketoconazole also potentiates the cytotoxic activity of docetaxel, the standard of care for metastatic CRPC. To further evaluate this synergistic effect, we designed a phase I clinical trial of weekly docetaxel combined with high-dose ketoconazole in metastatic CRPC patients.
In solid tumors, docetaxel is normally administered weekly or every 21 days. Toxicities associated with docetaxel appear to be schedule-dependent. Grade 3/4 neutropenia is significantly more common in patients on a 21-day schedule (32%) than in patients receiving weekly docetaxel (2%) (5). With weekly docetaxel, however, myelosuppression is not a significant DLT, while fatigue is the most commonly reported DLT. In our study, rates of grade 3/4 anemia (7%) and grade 3/4 neutropenia (2%) were similar to those reported by Tannock et al. (5). Patients in our combination regimens experienced more grade 3/4 fatigue (12%) than has been reported in patients receiving weekly docetaxel alone (5%) (5). Rates of gastrointestinal side effects including nausea and/or vomiting (50%) and diarrhea (38%) were slightly higher in our combination regimens compared to the TAX327 study of weekly docetaxel (5). Rates of fluid retention characterized by peripheral edema were also higher in this study (40%) than those previously reported (12%) (5).
We observed the limited tolerability of 1200 mg/day and 800 mg/day of ketoconazole when combined with weekly docetaxel, which led to a significant reduction in the dose of ketoconazole to 600 mg/day. A total of 35 instances of ≥ grade 2 elevated liver enzymes were reported in 14 patients. Treatment interruption and/or dose reduction of ketoconazole was indicated for 57% of these patients, and treatment was discontinued for 2 patients. Higher grades of ketoconazole-related hepatic toxicity were seen in this study compared to other studies evaluating the same doses of ketoconazole. In a phase I clinical trial using a fixed dose (55 mg/m2) of docetaxel every 21 days combined with escalating doses of ketoconazole in CRPC patients, the MTD of ketoconazole was 800 mg twice daily (BID) (13). Two thirds of patients receiving ketoconazole 1200 mg/day BID had DLTs of both febrile neutropenia and fatigue; however, no liver toxicity was reported. Our findings on ketoconazole pharmacokinetics were similar to previous studies in healthy subjects (18, 20, 21, 23). We also found that docetaxel did not appear to alter ketoconazole exposure. However, the possibility cannot be completely ruled out because, in the current study, systemic exposure to ketoconazole 1200 mg/day was almost twice what was previously reported by our group in prostate cancer patients receiving the same dose. Nevertheless, the difference in toxicity profiles appears to be attributable to different dosing schedules of both docetaxel (weekly vs. every 21 days) and ketoconazole (BID vs. TID) as well as different study populations.
In this phase I study, docetaxel combined with ketoconazole resulted in significant PSA response and OS in CRPC patients. A 50% PSA decline was seen in 67% of docetaxel-naïve patients, with a median OS of 36.8 months compared to the Halabi predicted median OS of 15.8 months (17). These results compare favorably to the clinical benefits observed with ketoconazole in combination with antiandrogen withdrawal in the CALGB 9583 trial (≥ 50% PSA decline in 27% of patients) (31) and weekly docetaxel alone in the TAX 327 trial (≥ 50% PSA decline in 48% of patients and 17.8 months OS) (5, 32). Our results suggest that the combination of ketoconazole and docetaxel has significant additive and possibly synergistic antitumor activity in CRPC. The underlying mechanism of ketoconazole/docetaxel interaction is not fully understood. Although concomitant ketoconazole increased the total exposure of docetaxel, the clinical benefits observed with this combination cannot be explained solely in terms of pharmacokinetic interaction, because similar results cannot be achieved by increasing docetaxel concentrations through doubling of the dose. A previous in vitro study demonstrated that ketoconazole abrogated the recovery of PC3 cells following removal of paclitaxel from the cell culture media, mimicking systemic elimination of the drug (10).
The docetaxel elimination pathway is characterized by complex processes involving hepatic metabolizing enzymes and uptake and efflux drug transporters (25). Docetaxel is predominantly metabolized by CYP3A4/5 into 4 pharmacologically inactive metabolites. Preclinical and clinical studies using radio-labeled docetaxel showed that > 80% of an administered dose was excreted in feces and about 5% in urine (33, 34). ABCB1 is responsible for the fecal elimination of docetaxel and its metabolites, and OATP1B3 is an influx transporter for docetaxel. Ketoconazole inhibits 95% of docetaxel metabolism in human hepatocytes in primary culture and 99% in human liver microsomes (35). A preclinical study in mice showed that coadministration of ketoconazole significantly altered docetaxel metabolism (36). Given the in vitro Ki (0.0037 to 0.015 μM/L) of ketoconazole for CYP3A4 inhibition (37, 38), the mean ketoconazole concentrations achieved at 600 to 1200 mg/day were several orders of magnitude higher than Ki value, enough to effectively inhibit CYP3A-mediated docetaxel metabolism. Previous studies have reported that ketoconazole AUC or Cmax were correlated with a fold-change in docetaxel CL (12, 39). Some studies, however, observed little or no relationship between the fractional change in docetaxel CL and ketoconazole AUC (12, 13, 40), presumably owing to the small number of patients (n = 7) with a limited range of ketoconazole AUC for comparison. In our study, ketoconazole exposure resulting from 600 to 1200 mg/day significantly correlated with changes in docetaxel CL. Notably, the estimated slope from our study is identical to that from a previously reported study (12).
Bosch et al. (41) reported that docetaxel CL was reduced by 25% in patients with the ABCB11236TT genotype and suggested that these patients may therefore require dose reductions. A more comprehensive analysis of the docetaxel disposition pathway by Baker et al. (25) suggest that docetaxel CL is not significantly dependent on previously known reduced-function alleles in the ABCB1 genes. Our study also found no association between any of the variant ABCB1 genotypes or diplotypes and docetaxel CL. In addition to the pharmacokinetics of docetaxel alone, we also examined whether genetic polymorphisms of ABCB1 or CYP3A4/5 would affect ketoconazole/docetaxel interaction. Patients carrying fully variant forms of all 3 ABCB1 SNPs seemed less affected by the interaction than patients carrying alternative genotypes, although this finding was based on a too small number of patients to be statistically confirmed.
In summary, concomitant ketoconazole significantly increased exposure to docetaxel in a dose-dependent manner. Genetic polymorphisms on metabolic enzymes and transporters responsible for docetaxel disposition did not appear to play a significant role in overall systemic exposure to docetaxel in the presence of ketoconazole. Combination regimens employing 600 mg/day of ketoconazole were fairly well tolerated. The MTD of docetaxel was 43 mg/m2 administered once weekly for 3 consecutive weeks. Ketoconazole at 1200 and 800 mg/day with weekly docetaxel showed limited tolerability with frequent liver function abnormalities. The combination therapy was active and led to significantly longer OS in docetaxel-naïve patients than patients in prior studies of weekly docetaxel. There has been recent excitement about drugs targeting either androgen receptor or androgen synthesis in patients with CRPC. Our study demonstrates that combinations of chemotherapy and drugs targeting androgen synthesis in CRPC are highly active and warrant further exploration in larger randomized trials.
Translational Relevance.
Demonstration of improved survival with docetaxel-based chemotherapy in patient with metastatic castration-resistant prostate cancer (CRPC) has opened a new era of investigation for new docetaxel combination regimens to further improve clinical response. Based on the preclinical work that demonstrated potential synergy, the combination of docetaxel and ketoconazole was studied in men with metastatic CRPC. This regimen led to significant prostate-specific antigen declines and an overall survival of 36.8 months in the docetaxel naïve strata. Although the underlying mechanism of ketoconazole-docetaxel interaction is not fully understood, effects of ketoconazole could be three fold: inhibiting androgen synthesis, potentiating antitumor activity of docetaxel, and increasing concentration of docetaxel via CYP3A4 inhibition. Ketoconazole exerts its androgen deprivation effect by interfering with the CYP450-dependent enzyme required for steroid synthesis. As such, this study may provide a rationale for investigation of abiraterone, an inhibitor of CYP17A1, in combination with docetaxel in metastatic CRPC.
Acknowledgments
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, Nation institutes of Health. The results and discussions presented herein do not represent the views of these federal agencies. We would like to thank the nursing staff of NCI, the research nurses (David Draper, R.N.), and the fellow of the Medical Oncology Branch at NCI for their assistance on the clinical trial.
References
- 1.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–65. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Damber JE, Aus G. Prostate cancer. Lancet. 2008;371:1710–21. doi: 10.1016/S0140-6736(08)60729-1. [DOI] [PubMed] [Google Scholar]
- 4.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 5.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 6.Hadaschik BA, Gleave ME. Therapeutic options for hormone-refractory prostate cancer in 2007. Urol Oncol. 2007;25:413–9. doi: 10.1016/j.urolonc.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Mike S, Harrison C, Coles B, Staffurth J, Wilt TJ, Mason MD. Chemotherapy for hormone-refractory prostate cancer. Cochrane Database Syst Rev. 2006:CD005247. doi: 10.1002/14651858.CD005247.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrylak DP. Future directions in the treatment of androgen-independent prostate cancer. Urology. 2005;65:8–12. doi: 10.1016/j.urology.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Dixon SC, Zalles A, Giordano C, et al. In vitro effect of gallium nitrate when combined with ketoconazole in the prostate cancer cell line PC-3. Cancer Lett. 1997;113:111–6. doi: 10.1016/s0304-3835(97)04603-x. [DOI] [PubMed] [Google Scholar]
- 10.Blagosklonny MV, Dixon SC, Figg WD. Efficacy of microtubule-active drugs followed by ketoconazole in human metastatic prostate cancer cell lines. J Urol. 2000;163:1022–6. [PubMed] [Google Scholar]
- 11.Sella A, Kilbourn R, Amato R, et al. Phase II study of ketoconazole combined with weekly doxorubicin in patients with androgen-independent prostate cancer. J Clin Oncol. 1994;12:683–8. doi: 10.1200/JCO.1994.12.4.683. [DOI] [PubMed] [Google Scholar]
- 12.Engels FK, Ten Tije AJ, Baker SD, et al. Effect of cytochrome P450 3A4 inhibition on the pharmacokinetics of docetaxel. Clin Pharmacol Ther. 2004;75:448–54. doi: 10.1016/j.clpt.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Van Veldhuizen PJ, Reed G, Aggarwal A, Baranda J, Zulfiqar M, Williamson S. Docetaxel and ketoconazole in advanced hormone-refractory prostate carcinoma: a phase I and pharmacokinetic study. Cancer. 2003;98:1855–62. doi: 10.1002/cncr.11733. [DOI] [PubMed] [Google Scholar]
- 14.Sissung TM, Baum CE, Deeken J, et al. ABCB1 genetic variation influences the toxicity and clinical outcome of patients with androgen-independent prostate cancer treated with docetaxel. Clin Cancer Res. 2008;14:4543–9. doi: 10.1158/1078-0432.CCR-07-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lepper ER, Baker SD, Permenter M, et al. Effect of common CYP3A4 and CYP3A5 variants on the pharmacokinetics of the cytochrome P450 3A phenotyping probe midazolam in cancer patients. Clin Cancer Res. 2005;11:7398–404. doi: 10.1158/1078-0432.CCR-05-0520. [DOI] [PubMed] [Google Scholar]
- 16.Hamada A, Sissung T, Price DK, et al. Effect of SLCO1B3 haplotype on testosterone transport and clinical outcome in caucasian patients with androgen-independent prostatic cancer. Clin Cancer Res. 2008;14:3312–8. doi: 10.1158/1078-0432.CCR-07-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–7. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 18.Huang YC, Colaizzi JL, Bierman RH, Woestenborghs R, Heykants J. Pharmacokinetics and dose proportionality of ketoconazole in normal volunteers. Antimicrob Agents Chemother. 1986;30:206–10. doi: 10.1128/aac.30.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daneshmend TK, Warnock DW, Ene MD, et al. Multiple dose pharmacokinetics of ketoconazole and their effects on antipyrine kinetics in man. J Antimicrob Chemother. 1983;12:185–8. doi: 10.1093/jac/12.2.185. [DOI] [PubMed] [Google Scholar]
- 20.Daneshmend TK, Warnock DW, Turner A, Roberts CJ. Pharmacokinetics of ketoconazole in normal subjects. J Antimicrob Chemother. 1981;8:299–304. doi: 10.1093/jac/8.4.299. [DOI] [PubMed] [Google Scholar]
- 21.Wire MB, Ballow CH, Borland J, et al. Fosamprenavir plus ritonavir increases plasma ketoconazole and ritonavir exposure, while amprenavir exposure remains unchanged. Antimicrob Agents Chemother. 2007;51:2982–4. doi: 10.1128/AAC.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Figg WD, Liu Y, Arlen P, et al. A randomized, phase II trial of ketoconazole plus alendronate versus ketoconazole alone in patients with androgen independent prostate cancer and bone metastases. J Urol. 2005;173:790–6. doi: 10.1097/01.ju.0000147013.09157.8e. [DOI] [PubMed] [Google Scholar]
- 23.Marcelin-Jimenez G, Hernandez J, Angeles AP, et al. Bioequivalence evaluation of two brands of ketoconazole tablets (Onofin-K and Nizoral) in a healthy female Mexican population. Biopharm Drug Dispos. 2004;25:203–9. doi: 10.1002/bdd.399. [DOI] [PubMed] [Google Scholar]
- 24.Sriwiriyajan S, Mahatthanatrakul W, Ridtitid W, Jaruratanasirikul S. Effect of efavirenz on the pharmacokinetics of ketoconazole in HIV-infected patients. Eur J Clin Pharmacol. 2007;63:479–83. doi: 10.1007/s00228-007-0282-8. [DOI] [PubMed] [Google Scholar]
- 25.Baker SD, Verweij J, Cusatis GA, et al. Pharmacogenetic pathway analysis of docetaxel elimination. Clin Pharmacol Ther. 2009;85:155–63. doi: 10.1038/clpt.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trump DL, Havlin KH, Messing EM, Cummings KB, Lange PH, Jordan VC. High-dose ketoconazole in advanced hormone-refractory prostate cancer: endocrinologic and clinical effects. J Clin Oncol. 1989;7:1093–8. doi: 10.1200/JCO.1989.7.8.1093. [DOI] [PubMed] [Google Scholar]
- 27.Johnson DE, Babaian RJ, von Eschenbach AC, Wishnow KI, Tenney D. Ketoconazole therapy for hormonally refractive metastatic prostate cancer. Urology. 1988;31:132–4. doi: 10.1016/0090-4295(88)90036-2. [DOI] [PubMed] [Google Scholar]
- 28.Vanuytsel L, Ang KK, Vantongelen K, Drochmans A, Baert L, van der Schueren E. Ketoconazole therapy for advanced prostatic cancer: feasibility and treatment results. J Urol. 1987;137:905–8. doi: 10.1016/s0022-5347(17)44291-1. [DOI] [PubMed] [Google Scholar]
- 29.Oh WK. Secondary hormonal therapies in the treatment of prostate cancer. Urology. 2002;60:87–92. doi: 10.1016/s0090-4295(02)01581-9. discussion 3. [DOI] [PubMed] [Google Scholar]
- 30.Eklund J, Kozloff M, Vlamakis J, et al. Phase II study of mitoxantrone and ketoconazole for hormone-refractory prostate cancer. Cancer. 2006;106:2459–65. doi: 10.1002/cncr.21880. [DOI] [PubMed] [Google Scholar]
- 31.Small EJ, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583) J Clin Oncol. 2004;22:1025–33. doi: 10.1200/JCO.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 32.Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–5. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 33.Bruno R, Sanderink GJ. Pharmacokinetics and metabolism of Taxotere (docetaxel) Cancer Surv. 1993;17:305–13. [PubMed] [Google Scholar]
- 34.Engels FK, Loos WJ, Mathot RA, van Schaik RH, Verweij J. Influence of ketoconazole on the fecal and urinary disposition of docetaxel. Cancer Chemother Pharmacol. 2007;60:569–79. doi: 10.1007/s00280-006-0412-5. [DOI] [PubMed] [Google Scholar]
- 35.Marre F, Sanderink GJ, de Sousa G, Gaillard C, Martinet M, Rahmani R. Hepatic biotransformation of docetaxel (Taxotere) in vitro: involvement of the CYP3A subfamily in humans. Cancer Res. 1996;56:1296–302. [PubMed] [Google Scholar]
- 36.Kamataki T, Yokoi T, Fujita K, Ando Y. Preclinical approach for identifying drug interactions. Cancer Chemother Pharmacol. 1998;42 (Suppl):S50–3. doi: 10.1007/s002800051079. [DOI] [PubMed] [Google Scholar]
- 37.Bourrie M, Meunier V, Berger Y, Fabre G. Cytochrome P450 isoform inhibitors as a tool for the investigation of metabolic reactions catalyzed by human liver microsomes. J Pharmacol Exp Ther. 1996;277:321–32. [PubMed] [Google Scholar]
- 38.Gibbs MA, Thummel KE, Shen DD, Kunze KL. Inhibition of cytochrome P-450 3A (CYP3A) in human intestinal and liver microsomes: comparison of Ki values and impact of CYP3A5 expression. Drug Metab Dispos. 1999;27:180–7. [PubMed] [Google Scholar]
- 39.Yong WP, Wang LZ, Tham LS, et al. A phase I study of docetaxel with ketoconazole modulation in patients with advanced cancers. Cancer Chemother Pharmacol. 2008;62:243–51. doi: 10.1007/s00280-007-0598-1. [DOI] [PubMed] [Google Scholar]
- 40.Engels FK, Mathot RA, Loos WJ, van Schaik RH, Verweij J. Influence of high-dose ketoconazole on the pharmacokinetics of docetaxel. Cancer Biol Ther. 2006;5:833–9. doi: 10.4161/cbt.5.7.2839. [DOI] [PubMed] [Google Scholar]
- 41.Bosch TM, Huitema AD, Doodeman VD, et al. Pharmacogenetic screening of CYP3A and ABCB1 in relation to population pharmacokinetics of docetaxel. Clin Cancer Res. 2006;12:5786–93. doi: 10.1158/1078-0432.CCR-05-2649. [DOI] [PubMed] [Google Scholar]