Over the past decade there has been a rapid evolution in our ability to noninvasively image the living human retina. Of particular interest is adaptive optics (AO), a technique that corrects for the eye’s monochromatic aberrations and allows nearly diffraction-limited imaging of the retina.1 There is increasing clinical application of AO imaging2–5 owing to the ability to resolve retinal pathological changes on a cellular level, although the future of AO imaging for clinical diagnosis is not clear. Of particular value in determining the potential diagnostic role of AO are cases in which the standard clinical picture is unclear. Here we describe a patient with bilateral progressive vision loss where AO imaging and optical coherence tomography (OCT) contributed to our understanding of the structural abnormalities associated with the visual dysfunction.
Report of a Case
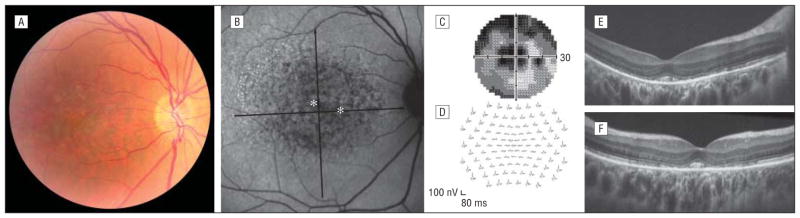
A 47-year-old woman noted slightly reduced visual acuity in both eyes, progressing over the past 2 years. Family history of retinal disease was negative. There was a history of anxiety and depression, for which she took sertraline hydrochloride for 7 years but stopped 2 years prior to the onset of her visual symptoms. Best-corrected visual acuity with low myopic correction was 20/40 OD and 20/30 OS. Anterior segment examination results were normal. Fundus examination (Figure 1A) revealed symmetric macular pigment irregularity with small drusenlike deposits. Imaging with the Spectralis HRA (Heidelberg Engineering, Heidelberg, Germany) showed nearly confluent hypofluorescence centrally in both eyes (Figure 1B). Visual fields had bilateral symmetric central and paracentral scotoma (Figure 1C). Multifocal electroretinography showed central depression in both eyes (Figure 1D). Macular thickness was decreased (central thickness = 188 μm OD; average thickness = 230 μm OD) with normal retinal nerve fiber layer thickness as measured on the Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, California). Data were consistent with decreased visual function associated with an outer retinal disruption.
Figure 1.
Clinical presentation of the patient. Results are shown for the right eye, although all findings were seen bilaterally. A fundus photograph (A) and an autofluorescence image with asterisks indicating the locations imaged with adaptive optics shown in Figure 2(B) demonstrate pigment irregularities throughout the macula. Visual fields (C) and multifocal electroretinography results (D) were consistent with a decrease in cone function. E and F, High-resolution spectral-domain optical coherence tomographic images using a broadband illumination with the Bioptigen spectral-domain optical coherence tomographic system (Bioptigen, Durham, North Carolina) show pronounced outer retinal disruption.
High-resolution images of the macula were obtained using a Bioptigen spectral-domain (SD) OCT equipped with a 186-nm broadband light source (Bioptigen, Durham, North Carolina). Images of the cone photoreceptor mosaic were obtained using a newly developed high-speed, flood-illuminated, AO ophthalmoscope. Images were analyzed using custom Matlab programs (Math-works, Natick, Massachusetts) and ImageJ software (National Institutes of Health, Bethesda, Maryland).
The high-resolution SD-OCT images from the patient’s right eye revealed a disruption of the inner segment–outer segment layer in most parafoveal locations with preservation of cone remnants in the central fovea (Figure 1E and F). The external limiting membrane was intact, indicating the presence of remaining inner segment structures, although measurement of the outer nuclear layer revealed significant thinning compared with 97 healthy control subjects (Figure 2A).
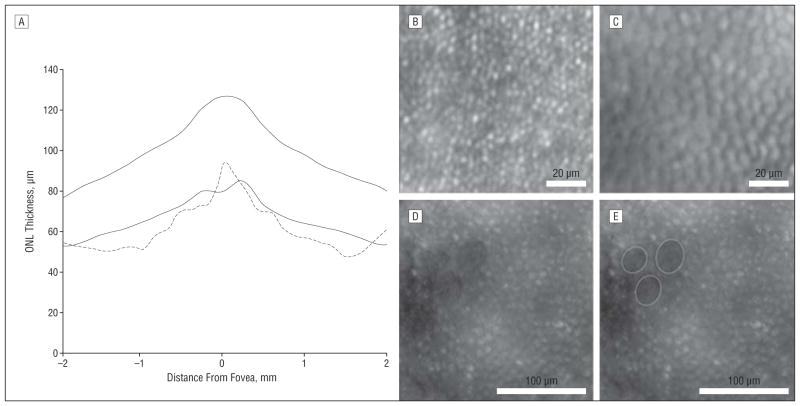
Figure 2.
Evidence for cone photoreceptor degeneration. A, Comparison of the patient’s outer nuclear layer (ONL) thickness (dashed line) with the average ONL thickness in 97 healthy control subjects reveals significant thinning. Solid lines indicate ±2 SDs from the mean. An adaptive optics image of the right eye from a normal retina at a 1° temporal location (B) and a corresponding image from the patient (C) are shown. The density of the “bubble wrap” structures is 14 917 cells/mm2. Normal cone density at this location is about 37 000 cones/mm2, based on histologic findings6 and adaptive optics data4 from other groups. D and E, Images from a 3° nasal location show heterogeneous areas of variably sized reflecting structures interleaved with 3 craterlike lowly reflecting patches (outlined in E).
The AO images revealed significant photoreceptor mosaic heterogeneity (Figure 2B–E). While some structures were consistent with normal cone photoreceptor appearance, these were rare. Of particular interest were patches of hexagonally packed structures with a bubble wrap appearance. The density of these structures was less than 50% of a normal cone array for this retinal location.4 A similar morphological appearance was reported in a patient with X-linked cone-rod dystrophy.4 Finally, craterlike lowly reflecting patches were seen interleaved among variably sized reflecting structures. While these changes in reflectivity may have their origins in the pigment irregularities, precise correlation was not possible owing to the low resolution of the autofluorescence image.
Comment
High-resolution imaging (SD-OCT and AO) was able to reveal cellular damage in this case of bilateral maculopathy. Specifically, the AO images greatly enhanced our understanding of the cause of this patient’s condition and SD-OCT revealed significant thinning of the outer nuclear layer, confirming degeneration of the foveal cones. A challenge ahead in introducing AO as a standard clinical modality is that we need better understanding of how the various stages of cone degeneration manifest on AO imaging. Additional studies like these will help elucidate the etiology of various conditions and will also contribute to the evolving atlas of in vivo photoreceptor pathological changes. Despite there being no definitive treatment for this case, findings like these where AO is used to interpret SD-OCT demonstrate the complementary roles these modalities can play in the clinical setting.
Acknowledgments
Funding/Support: This work was supported by grants EY001931 and EY017607 from the National Institutes of Health, by the Posner Foundation, Fight for Sight, the E. Matilda Ziegler Foundation for the Blind, the R. D. and Linda Peters Foundation, and Hope for Vision, and by an unrestricted grant from Research to Prevent Blindness. Dr Carroll is the recipient of a career development award from Research to Prevent Blindness.
Footnotes
Author Contributions: Dr Carroll had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosure: None reported.
References
- 1.Liang J, Williams DR, Miller D. Supernormal vision and high-resolution retinal imaging through adaptive optics. J Opt Soc Am A Opt Image Sci Vis. 1997;14(11):2884–2892. doi: 10.1364/josaa.14.002884. [DOI] [PubMed] [Google Scholar]
- 2.Carroll J, Neitz M, Hofer H, Neitz J, Williams DR. Functional photoreceptor loss revealed with adaptive optics: an alternate cause for color blindness. Proc Natl Acad Sci U S A. 2004;101(22):8461–8466. doi: 10.1073/pnas.0401440101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi SS, Doble N, Hardy JL, et al. In vivo imaging of the photoreceptor mosaic in retinal dystrophies and correlations with visual function. Invest Ophthalmol Vis Sci. 2006;47(5):2080–2092. doi: 10.1167/iovs.05-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duncan JL, Zhang Y, Gandhi J, et al. High-resolution imaging with adaptive optics in patients with inherited retinal degeneration. Invest Ophthalmol Vis Sci. 2007;48(7):3283–3291. doi: 10.1167/iovs.06-1422. [DOI] [PubMed] [Google Scholar]
- 5.Joeres S, Jones SM, Chen DC, et al. Retinal imaging with adaptive optics scanning laser ophthalmoscopy in unexplained central ring scotoma. Arch Ophthalmol. 2008;126(4):543–547. doi: 10.1001/archophthalmol.2007.33. [DOI] [PubMed] [Google Scholar]
- 6.Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292(4):497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]