Abstract
The orexin/hypocretin system has the potential to significantly modulate affect, based on both the neuroanatomical projection patterns of these neurons and on the sites of orexin receptor expression. However, there is little data supporting the role of specific orexin receptors in the modulation of depression-like behavior. Here we report behavioral profiling of mice after genetic or pharmacologic inhibition of hcrtr1 and 2 receptor signaling. Hcrtr1 null mice displayed a significant reduction in behavioral despair in the forced swim test and tail suspension test. Wild-type mice treated with the hcrtr1 antagonist SB-334867 also displayed a similar reduction in behavioral despair. No difference in anxiety-like behavior was noted following hcrtr1 deletion. In contrast, hcrtr2-null mice displayed an increase in behavioral despair with no effect on measures of anxiety. These studies suggest that the balance of orexin action at either the hcrtr1 or the hcrtr2 receptor produces an anti-depressant or pro-depressant like effect, depending on the receptor subtype activated.
Keywords: Orexin, Depression, Anxiety
1. Introduction
Orexin (also known as hypocretin) neurons exist as a small cluster of cells found exclusively in the perifornical area, dorsal medial hypothalamus and lateral hypothalamus [1,2]. Orexin neurons secrete the neuropeptides orexin-A and B, synthesized from the common precursor pre-pro-orexin [2,3]. These neuromodulatory peptides signal through two G-protein coupled receptors, orexin receptor 1 and orexin receptor 2 (Hcrtr1 and Hcrtr2), with orexin-A showing equal affinity for both receptors while orexin-B demonstrates a higher affinity for Hcrtr2 [2]. Orexin signaling has been implicated in processes as diverse as sleep-wake transitioning [4], the control of food intake [5], and autonomic output [6]; acting to match the activity of homeostatic circuits with the appropriate level of vigilance. Interestingly, although orexin neurons innervate most areas of the brain, there is minimal co-expression of the two orexin receptors throughout structures of the CNS [7], suggesting that little redundancy exists in the orexinergic modulation of behavior. For example, while hcrtr1 exhibits high levels of expression in the locus coeruleus, hcrtr2 expression is seen in histaminergic, sleep-state regulatory tuberomammillary neurons in the absence of hcrtr1 expression [7]. Consequently, while Hcrtr1 receptor deletion produces no measurable effect on sleep wake cycling, hcrtr2 deletion produces an increase in waking during the rest phase or light cycle coupled with an inability to maintain wakefulness during the active phase or dark cycle [4]. Hcrtr1 signaling, meanwhile, has been implicated in the re-instatement of cocaine seeking and the development of anhedonia [8]. This divergence in orexin receptor function likely extends to the modulation of mood, particularly depression, as orexin receptors are differentially expressed in many regions traditionally associated with the modulation of depression-like behavior including the cerebral cortex, hippocampus, dorsal raphe, and ventral tegmental area [7]. Although little data is available on the role of orexin in the acute modulation of depression, in a model of drug-induced depression, clomipramine induces an elevation in orexin-A and B levels along with an induction of a depressive-like state [9], suggesting orexin acts to negatively modulate affect. To date, however, the role of specific receptors in the regulation of depression-like behavior has not been investigated. To begin to explore the role of both hcrtr1 and 2 receptors in the regulation of mood, specifically depression, we performed behavioral tests on mice lacking either the hcrtr1 or hcrtr2 receptor. We employed two novel mouse lines that have been engineered with a removable transcription-blocking cassette inserted into either the hcrtr1 or 2 genes, arresting the production of the receptor. Subsequent exposure to Cre recombinase allows for the re-expression of receptor in select CNS nuclei, while maintaining the non-recombinase expressing neurons null. In this report, we present an initial characterization of these receptor null mice, illustrating that hcrtr1 and 2 receptor signaling differentially regulates depression-like behaviors.
2. Materials and methods
2.1. Animal husbandry
All animal procedures were conducted in accordance with UT Southwestern’s Institutional Animal Care and Use Committee guidelines and those of the Association for Assessment and Accreditation of Laboratory Animal Care. Mice were housed individually on a 12 h light/dark cycle with free access to food and water. Adult male mice (8–10 weeks of age) were used for all studies. Hcrtr1 and 2 null mice were backcrossed for 10 generations onto C57BL/6. Mice heterozygous for the hcrtr1 or 2 null allele were bred to generate wild-type and hcrtr1 or 2 null littermates for all behavioral testing conducted within our mouse housing facility.
2.2. Generation of mice
Hcrtr2-null mice were generated by inserting a loxP-flanked transcriptional and translational blocking cassette as described previously [10,11]. These mice have been demonstrated to be functionally null for the hcrtr2 receptor [11]. Similarly, hcrtr1 null mice were produced by the insertion of a transcription and translational blocking cassette between exons 3 and 4 of the hcrtr1 gene. Mice were subsequently shown to be null for the hcrtr1 by in situ hybridization, as the receptor was shown to be absent from previously described sites of elevated receptor expression (Supplemental Fig. 1) and from all other CNS nuclei (not shown).
2.3. In situ hybridization
In situ hybridization was performed as described previously [12], using probes generated from mouse hcrtr1 cDNA. The probe sequence spanned exons 4–8 and covered sequence unique to hcrtr1, with minimal similarity to hcrtr2. Probe validation was performed on wild-type mouse tissue, exhibiting an expression pattern similar to that reported for rat hcrtr1 receptor expression (data not shown). Expression was strongest in the locus coeruleus (Supplemental Fig. 1).
2.4. Behavioral testing
Mice of each genotype were initially subjected to tests of anxiety, first the elevated plus maze followed by the light dark assay the next day. Seven days after these tests, mice were tested using the porsolt forced swim paradigm. An independent group of mice was then employed in the tail suspension test. For the orexin receptor antagonist studies, wild-type mice were either tested in the porsolt forced swim test or tail suspension test. All testing was performed at the same time of day, from 12 p.m. to 3 p.m. The number of animals used in each experiment is specified in figure legends.
2.5. Forced swim test
The Porsolt forced swim test was performed according to protocols employed in prior published studies [13]. Briefly, mice were placed in 4 L Pyrex beakers filled with 3 L of 24 ± 1 °C water for 6 min. Swimming activity was recorded on videotape and scored manually by two trained and blinded observers. After 2 min of acclimatization, the final 4 min of the test were scored. Immobility time was defined as time spent without any motion other than a single limb paddling to maintain flotation. Latency to immobility was scored as the amount of time (after the 2 min acclimatization period) that elapsed before the first period of immobility.
2.6. SB-334867 injections
Eight to ten week old C57Bl6/j wild-type mice were injected I.P. with either the hcrtr1 receptor antagonist SB-334867 (10 mg/kg) (Tocris, Ellisville, MI) dissolved in a 50% (w/v) 2-hydroxypropyl-beta cyclodextrin (Sigma, St. Louis, MI) saline solution or control vehicle solution 30 min before exposure to either the TST or FST. This particular dose and route of administration has been demonstrated to modulate behavior through actions in the CNS [14].
2.7. Tail suspension test
Mice were attached from the tail to a force transducer (Med Associates, St. Albans. VT) suspended from the bench in a plexiglass chamber. Time spent immobile was subsequently scored for 6 min [15].
2.8. Elevated plus maze
Anxiety-like behavior was measured using an elevated plus maze apparatus according to published procedures [16]. The 12 cm × 50 cm maze was elevated 55 cm from the floor in a low-light environment. Time in the open arm was measured during a 5 min period. Scoring was done automatically using a videotracking system (Noldus Ethovision, Noldus Information Technology, Sterling, VA).
2.9. Light dark box
The procedure was performed according to a previously published procedure from our institute [17]. The dark–light two chamber apparatus (Med Associates) was made of black and white Plexiglass, with each chamber measuring 25 cm × 26 cm × 25 cm. The dark chamber was not illuminated, while the light side was illuminated by a fluorescent lamp. Mouse movement within each chamber was measured by photocell beam breaks. Mice were placed in the dark chamber for 2 min. The divider separating the 2 chambers was then removed and the mice were allowed to freely investigate both chambers for 10 min.
2.10. Locomotor activity
Mice were placed in fresh cages and locomotor activity was measured using photo cell beams linked to computer data acquisition software (San Diego Instruments, San Diego, CA) as described previously [13]. Both locomotion in the X and Y planes were summated to generate a measure of total locomotor activity. Counts are presented, as number of beam breaks binned into 15 min intervals.
2.11. Statistical analysis
Data are reported as the mean ± SEM for the specified number of animals. Graph-Pad Prism 5 software (GraphPad Software Inc., San Diego, CA) was used to perform all statistical analyses. Two–tailed Student’s t-test was used in the analysis of all data unless otherwise described in figure legends. Significance was defined as p < 0.05.
3. Results
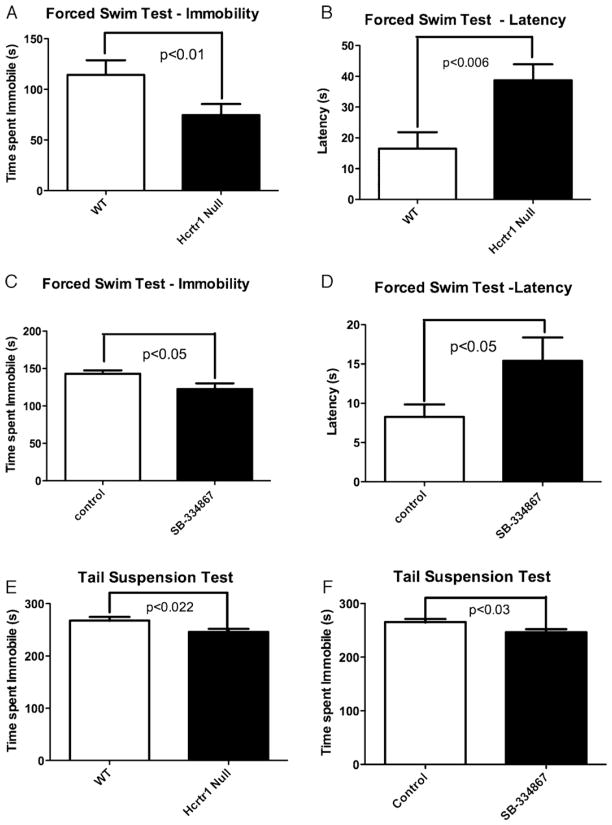
We first examined whether orexin receptor signaling modulated depression-like behavior using the forced swim test (FST), a measure of behavioral despair [18]. Hcrtr1 null mice displayed a significant reduction in time immobile and in latency to first bout of immobility (both measures of despair [19,20]) in the FST, consistent with reduced depression-like behavior (Fig. 1A and B). Because genetic deletion can produce developmental compensation, we next sought to confirm our findings by treating wild-type adult mice with the hcrtr1 antagonist SB-334867. As was observed in the Hcrtr1 knockout, pharmacologic inhibition of hcrtr1 also resulted in a significant reduction in immobility and latency to first bout of immobility in the FST (Fig. 1C and D). We then extended this initial observation using a complimentary test of behavioral despair, the tail suspension test (TST). Consistent with the FST result, inhibition of hcrtr1 signaling by genetic (Fig. 1E) or pharmacologic (Fig. 1F) methods also reduced immobility in the TST.
Fig. 1. Disruption of hcrtr1 reduces behavioral despair.
Eight to ten week old male mice were tested in mouse models of depression, the porsolt forced swim test and tail suspension test. (A) Time spent immobile in the FST. (B) Latency to immobility in the FST. (Data presented as mean ± SEM, Student’s t-test, N = 10–15/group.) (C) Time spent immobile and latency to immobility. (D) After SB-334867 (10 mg/kg) injection. (Data presented as mean ± SEM, Student’s t-test, N = 18/group.) (E) Time spent immobile in the TST. (Data presented as mean ± SEM, Student’s t-test, n = 17–19/group.) (F) Time spent immobile in the TST after injection of vehicle or SB-334867 (10 mg/kg). Data presented as mean ± SEM, Student’s t-test, n = 18/group.
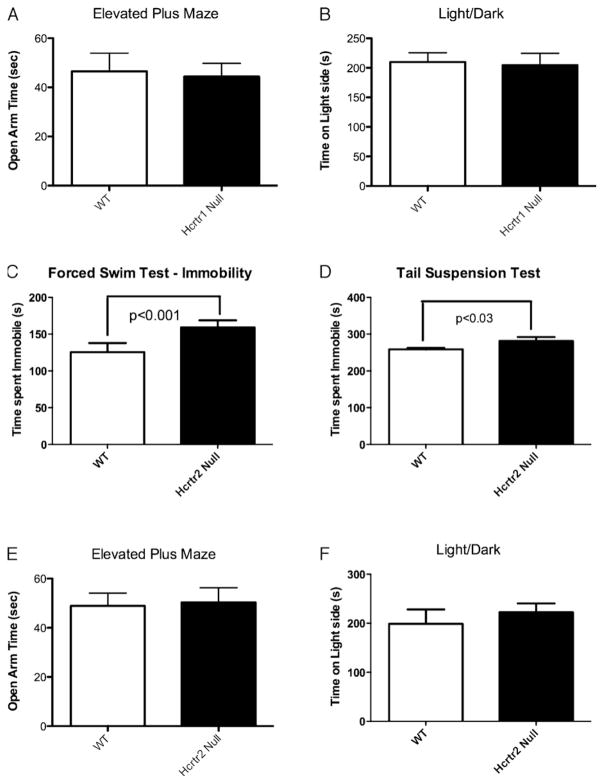
Because depression and anxiety are often co-morbid, we next analyzed hcrtr1 null mice in two measures of anxiety: the elevated plus maze (Fig. 2A) and the light–dark box (Fig. 2B). Deletion of hcrtr1 had no effect in either test indicating that the effect of hcrtr1 inhibition on depression-like behaviors was dissociable from anxiety-like responses.
Fig. 2. Hcrtr1 and 2 deletions have no effect on measures of anxiety like behavior while hcrtr2 deletion enhances depression like behavior.
(A and E) Time spent in the open arm of the EPM. (Data presented as mean ± SEM, Student’s t-test, n = 14–20/group.) (B and F) Time spent on the light side of light/dark box. (Student’s t-test, n = 10–15/group.) (C and D) Time spent immobile in the FST and time spent immobile in the TST. (Data presented as mean ± SEM, Student’s t-test, n = 7–10/group.)
Unlike hcrtr1 null mice, mice lacking orexin ligand display no phenotype in the FST [13]. This finding suggests that hcrtr2 signaling may oppose the actions of orexin receptor 1 on mood regulation such that loss of signaling at both receptors (as is the case in the ligand deletion) would result in the absence of any phenotype. We subsequently tested this hypothesis by examining depression-like behaviors in hcrtr2-null mice. Compared to wild-type littermates, hcrtr2 deletion produced a significant increase in total time spent immobile in the FST (Fig. 2C) and TST (Fig. 2D), although latency to first bout of immobility in the FST was not significantly different from control (not shown). Furthermore, in examining the role of hcrtr2 in anxiety like behaviors, similar to hcrtr1, no effect was observed in the elevated plus maze or light/dark tests (Fig. 2E and F), again demonstrating the specificity of the orexin effect in the modulation of depression like behavior. Unlike hcrtr1, published reports of compounds that produce selective hcrtr2 antagonism in vivo in mice have yet to be described. Thus, we did not pursue a pharmacological study of the effect of Hcrtr2 disruption on behavior.
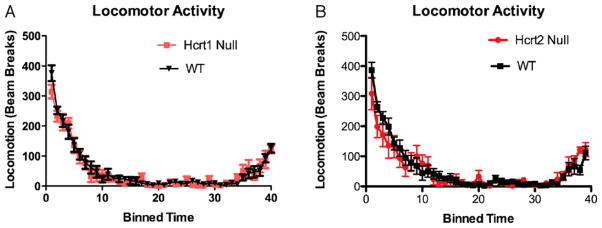
Finally, since differences in locomotor activity may confound the interpretation of behavioral tests, we determined whether deletion of hcrtr1 or hcrtr2 affected locomotor activity in a novel environment. Deletion of either receptor had no effect on locomotion during a 9 h trial suggesting that differences in locomotor activity did not contribute to the observed behavioral phenotypes (Fig. 3A and B). At no point was there any difference observed in locomotor activity between the null hcrtr1 and 2 mice and control animals (Fig. 3A and B), as initial and total exploratory activity was identical between nulls and wildtypes. This is not surprising, as the sleep state transition phenotype observed in the Hcrtr2 null animals is most evident during the dark phase, while all behavioral testing was performed during the light phase [4]. Lastly, the effects of the orexin receptor antagonist SB-334867 on locomotor activity were not analyzed, as this compound has yet to demonstrate any effect on locomotion [21,22].
Fig. 3. Disruption of hcrtr1 and 2 does not affect locomotor activity.
A and B individually housed mice were placed in a novel cage and then tested for locomotor activity. No difference was found between wild-type and (A) Hcrt1r-null (two way ANOVA with repeated measures, p > 0.05, n = 13 wild-type, n = 9 Hcrt1-null) or (B) Hcrtr2-null mice (two way ANOVA with repeated measures, p > 0.05, n = 13 wild-type, n = 7 Hcrtr2-null). Data are presented as mean ± SEM.
4. Discussion
Our results indicate that hcrtr1 and 2 signaling oppose one another in the regulation of depression-like behaviors. More specifically, these data suggest that hcrtr1 and hcrtr2 signaling may act to counterbalance each other in regions of the brain that regulate antidepressant-like responses such as in the hippocampus, ventral tegmental area, or prefrontal cortex [23] We postulate that states that favor activation of orexin neurons projecting to regions that express hcrtr1, such as occurs during highly stressful situations [24], may create a negative affective state that worsens depression-like symptoms. The finding that orexin receptor signaling may promote a negative emotional state is consistent with previous reports demonstrating that orexin-A peptide promotes the development of anhedonia, increasing intracranial self-stimulation thresholds [8], while re-instatement of drug seeking is blocked by the hcrtr1 antagonist SB-334867 [8]. Our data is also in agreement with a significant number of studies demonstrating a role for hcrtr1 in the modulation of reward dependent behavior. The enhancement of cocaine [25] and morphine place preference [26,27] is dependent on hcrtr1 activation, while operant responding for palatable food also requires functional hcrtr1 receptor signaling [28]. We contend that a negative emotional state may be produced by hcrtr1 signaling, acting to enhance both the conditioning to and responding for a particular reward.
Furthermore, our data also illustrate how a negative emotional state along with the development of anhedonia (based on other reports [8]) may be enhanced, through disruption of hcrtr2 signaling, leaving hcrtr1 transmission intact. For example, states that favor activation of hcrtr2 expressing neurons, such as calorie restriction, may promote antidepressant-like responses [13]. An important prediction of this hypothesis is that unique hcrtr1 and hcrtr2 neural circuits exist with potentially distinct patterns of innervation and activation by different stimuli. The existence of unique circuits would allow for the orexin system to integrate various inputs into coordinated behavioral and physiological responses. Interestingly, recent data highlight the differential regulation of orexin receptor expression in the medial prefrontal cortex. In this particular example, the authors describe a shift in orexin receptor expression, with enhanced hcrtr1 expression accompanying a trend in the reduction of hcrtr2 expression [29]. In this case, the change in orexin receptor expression is expected to drive operant responding following exposure to social stress. Our data compliment these findings, as we demonstrate that changes in orexin receptor expression modulate depression like behavior.
Testing this hypothesis, that selective activation of orexin receptor subtypes can produce distinct effects on behavior, can subsequently be examined using the mouse model systems described for the first time in this report. Using cre recombinase delivered either virally or expressed from a transgene, restoration of either hcrtr1 or 2 may be accomplished in select brain nuclei. These receptor-restored mice could then be analyzed for depression and anxiety-like behavior, permitting a mapping of the sites of orexin receptor expression that are responsible for the modulation of affect.
The transcriptionally blocked orexin receptor mice described here are a valuable tool in the investigation of orexinergic modulation of behavior. While developmental compensation, due to embryonic gene deletion, is often a concern when analyzing genetic mutations, there is no evidence, at least in terms of the effect of orexin on depression like behavior, that deletion of orexin receptors has produced such compensation in the reactivatable hcrtr1 and 2 lines. While deficits in sleep state transitioning are clearly evident in the transcriptionally blocked hcrtr2 null [11], data presented in this report demonstrates that the hcrtr1 selective antagonist SB-334867 mimics the effect of the hcrtr1 deletion on depression like behavior.
The studies presented here are the first to describe the effect of hcrtr1 and 2 receptor-specific modulation of depression like behavior. Interestingly, in limited literature describing orexin action in modulation of depression like behavior, chronic hcrtr1 receptor antagonism was shown to enhance the presentation of depression-like behavior [30,31]. Our data is then highly relevant to the discussion of the role of orexin receptor-acting compounds in the treatment of depression; that there may be significant differences in the modulation of affect between the acute and chronic actions of orexin receptor targeted therapeutics. Alternately, the use of the non-selective hcrtr receptor agonist, orexin-A, along with the hcrtr1 selective antagonist at potentially non-selective doses in vivo, may result in the non-specific targeting of both hcrtr1 and 2. This would make it difficult to discern any differences in the modulation of depression like behavior by either hcrtr1 or 2.
Our data suggests that modulation of depression like behavior depends on the balance between hcrtr1 and 2 receptor signaling, thus the use of non-selective hcrtr1 and 2 agonists and antagonists at non-selective doses would not be expected to be able to discern the true roles of both receptors in the modulation of behavior.
Finally, considering that the orexin system has become an attractive target for the development of novel pharmacologic treatments for a variety of neuropsychiatric disorders including narcolepsy, insomnia, and drug addiction, careful monitoring of behavioral responses to these medications will be required to determine the potential for both novel applications as well as potentially unwanted side effects, based on the conclusions drawn from our studies.
Supplementary Material
Acknowledgments
We would like to thank the Behvioral Core at UT Southwestern for their help in conducting the assays described in this report. This work was supported by the following grants: K08 MH084058-1A1, UL1RR024923, RL1 DK081182, RL1 DK081185, K99 DA024719-02, NARSAD Young Investigator Award, and The Disease Oriented Clinical Scholars Program.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bbr.2011.02.044.
References
- 1.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 3.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, et al. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–30. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 5.Clegg DJ, Air EL, Woods SC, Seeley RJ. Eating elicited by orexina, but not melanin-concentrating hormone, is opioid mediated. Endocrinology. 2002;143:2995–3000. doi: 10.1210/endo.143.8.8977. [DOI] [PubMed] [Google Scholar]
- 6.Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA. 1999;96:748–53. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 8.Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, et al. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA. 2005;102:19168–73. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng P, Vurbic D, Wu Z, Hu Y, Strohl KP. Changes in brain orexin levels in a rat model of depression induced by neonatal administration of clomipramine. J Psychopharmacol. 2008;22:784–91. doi: 10.1177/0269881106082899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115:3564–72. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mochizuki EAT, Marcus JN, Clark EL, Yamamoto M, Honer M, Borroni E, et al. Orexin receptor 2 expression in the posterior hypothalamus rescues sleepiness in narcoleptic mice. Proc Natl Acad Sci. 2011;108:4471–6. doi: 10.1073/pnas.1012456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, et al. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518–32. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutter M, Krishnan V, Russo SJ, Jung S, McClung CA, Nestler EJ. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J Neurosci. 2008;28:3071–5. doi: 10.1523/JNEUROSCI.5584-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, Rovinsky SA, et al. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–70. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 16.Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11:752–3. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherjee S, Coque L, Cao JL, Kumar J, Chakravarty S, Asaithamby A, et al. Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biol Psychiatry. 2010;68:503–11. doi: 10.1016/j.biopsych.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porsolt RD, Brossard G, Hautbois C, Roux S. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci. 2001;Chapter 8(Unit 8):10A. doi: 10.1002/0471142301.ns0810as14. [DOI] [PubMed] [Google Scholar]
- 19.Eisch AJ, Bolanos CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, et al. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl) 2005;177:245–55. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen K, Hsu MA, Noone S, Johnson BG, Thompson LK, Hemrick-Luecke SK. The orexin-1 antagonist SB-334867 blocks antipsychotic treatment emergent catalepsy: implications for the treatment of extrapyramidal symptoms. Schizophr Bull. 2007;33:1291–7. doi: 10.1093/schbul/sbm087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 24.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–7. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Hutcheson DM, Quarta D, Halbout B, Rigal A, Valerio E, Heidbreder C. Orexin-1 receptor antagonist SB-334867 reduces the acquisition and expression of cocaine-conditioned reinforcement and the expression of amphetamine-conditioned reward. Behav Pharmacol. 2011 doi: 10.1097/FBP.0b013e328343d761. [DOI] [PubMed] [Google Scholar]
- 26.Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res. 2007;183:43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taslimi Z, Haghparast A, Hassanpour-Ezatti M, Safari MS. Chemical stimulation of the lateral hypothalamus induces conditioned place preference in rats: involvement of OX1 and CB1 receptors in the ventral tegmental area. Behav Brain Res. 2011;217:41–6. doi: 10.1016/j.bbr.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Sharf R, Sarhan M, Brayton CE, Guarnieri DJ, Taylor JR, DiLeone RJ. Orexin signaling via the orexin 1 receptor mediates operant responding for food reinforcement. Biol Psychiatry. 2010;67:753–60. doi: 10.1016/j.biopsych.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis JF, Krause EG, Melhorn SJ, Sakai RR, Benoit SC. Dominant rats are natural risk takers and display increased motivation for food reward. Neuroscience. 2009;162:23–30. doi: 10.1016/j.neuroscience.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 30.Ito N, Yabe T, Gamo Y, Nagai T, Oikawa T, Yamada H, et al. I.c.v. administration of orexin-A induces an antidepressive-like effect through hippocampal cell proliferation. Neuroscience. 2008;157:720–32. doi: 10.1016/j.neuroscience.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 31.Ito N, Yabe T, Nagai T, Oikawa T, Yamada H, Hanawa T. A possible mechanism underlying an antidepressive-like effect of Kososan, a Kampo medicine, via the hypothalamic orexinergic system in the stress-induced depression-like model mice. Biol Pharm Bull. 2009;32:1716–22. doi: 10.1248/bpb.32.1716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.