Abstract
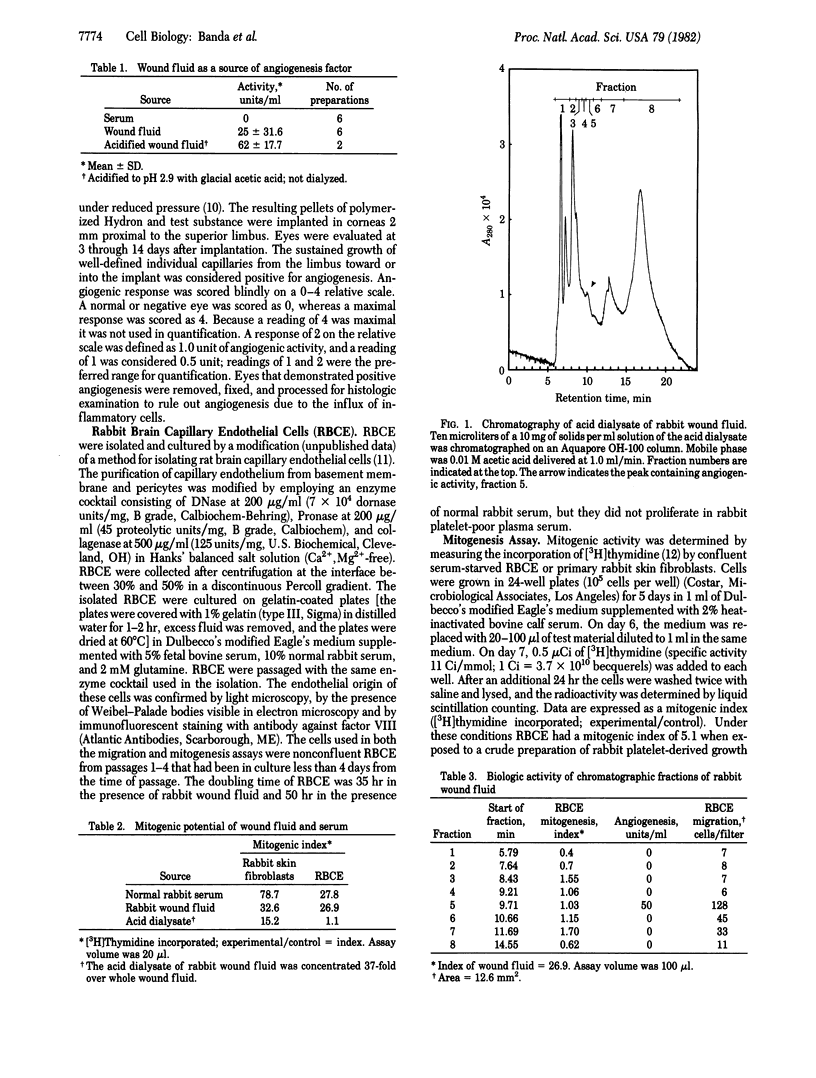
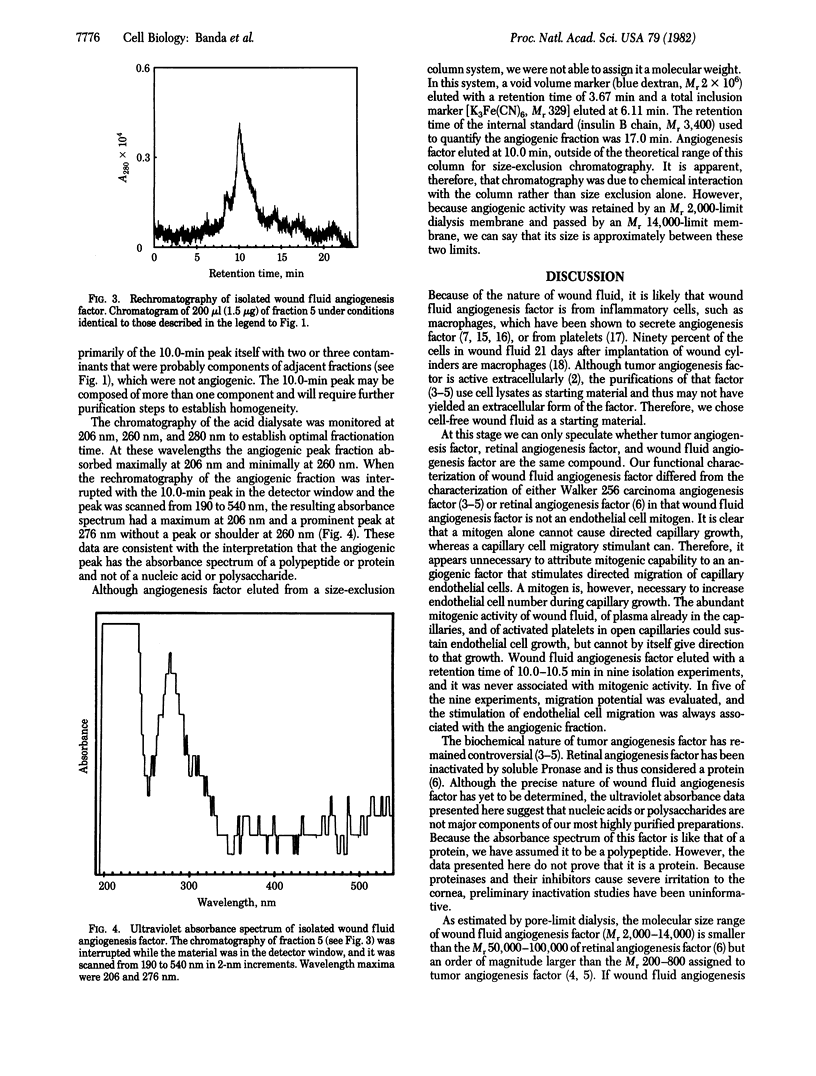
Angiogenesis, or new capillary growth, is essential to normal growth and wound healing. It is also active in several pathologic states, including the growth of malignant tumors. An extracellular, nonneoplastic angiogenesis factor has been isolated from cell-free rabbit wound fluid by pore-limit dialysis and chromatography on a size-exclusion HPLC column. The isolated angiogenesis factor was purified 9,600-fold with a yield of 81% and has a molecular weight between 2,000 and 14,000. Wound fluid angiogenesis factor was completely separated from the mitogenic activity of wound fluid; it did not increase the number of capillary endothelial cells in vitro or stimulate [3H]thymidine uptake by these cells. The isolated angiogenesis factor stimulated endothelial cell migration in vitro, and less than 200 ng of the factor stimulated angiogenesis in vivo in the corneal implant assay.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ausprunk D. H., Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977 Jul;14(1):53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- Bowman P. D., Betz A. L., Ar D., Wolinsky J. S., Penney J. B., Shivers R. R., Goldstein G. W. Primary culture of capillary endothelium from rat brain. In Vitro. 1981 Apr;17(4):353–362. doi: 10.1007/BF02618147. [DOI] [PubMed] [Google Scholar]
- D'Amore P. A., Glaser B. M., Brunson S. K., Fenselau A. H. Angiogenic activity from bovine retina: partial purification and characterization. Proc Natl Acad Sci U S A. 1981 May;78(5):3068–3072. doi: 10.1073/pnas.78.5.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenselau A., Watt S., Mello R. J. Tumor angiogenic factor. Purification from the Walker 256 rat tumor. J Biol Chem. 1981 Sep 25;256(18):9605–9611. [PubMed] [Google Scholar]
- Folkman J., Merler E., Abernathy C., Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med. 1971 Feb 1;133(2):275–288. doi: 10.1084/jem.133.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S., Leapman S. B., Folkman J. Tumor growth and neovascularization: an experimental model using the rabbit cornea. J Natl Cancer Inst. 1974 Feb;52(2):413–427. doi: 10.1093/jnci/52.2.413. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D. Purification of a fibroblast growth factor from bovine pituitary. J Biol Chem. 1975 Apr 10;250(7):2515–2520. [PubMed] [Google Scholar]
- Greenblatt M., Shubi P. Tumor angiogenesis: transfilter diffusion studies in the hamster by the transparent chamber technique. J Natl Cancer Inst. 1968 Jul;41(1):111–124. [PubMed] [Google Scholar]
- Greenburg G. B., Hunt T. K. The proliferative response in vitro of vascular endothelial and smooth muscle cells exposed to wound fluids and macrophages. J Cell Physiol. 1978 Dec;97(3 Pt 1):353–360. doi: 10.1002/jcp.1040970310. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Hunt T. K., Thakral K. K., Goodson W. H., 3rd Role of platelets and fibrin in the healing sequence: an in vivo study of angiogenesis and collagen synthesis. Ann Surg. 1982 Oct;196(4):379–388. doi: 10.1097/00000658-198210000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer R., Folkman J. Polymers for the sustained release of proteins and other macromolecules. Nature. 1976 Oct 28;263(5580):797–800. doi: 10.1038/263797a0. [DOI] [PubMed] [Google Scholar]
- Polverini P. J., Cotran P. S., Gimbrone M. A., Jr, Unanue E. R. Activated macrophages induce vascular proliferation. Nature. 1977 Oct 27;269(5631):804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Snyderman R., Kang A. H. The chemotactic attraction of human fibroblasts to a lymphocyte-derived factor. J Exp Med. 1976 Nov 2;144(5):1188–1203. doi: 10.1084/jem.144.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILLING J. A., FAVATA B. V., RADAKOVICH M. Studies of fibroplasia in wound healing. Surg Gynecol Obstet. 1953 Feb;96(2):143–149. [PubMed] [Google Scholar]
- Seppä H., Grotendorst G., Seppä S., Schiffmann E., Martin G. R. Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol. 1982 Feb;92(2):584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Cohen S., Mitchell W. M. Epidermal growth factor: high and low molecular weight forms. Proc Natl Acad Sci U S A. 1970 Sep;67(1):164–171. doi: 10.1073/pnas.67.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Mitchell W. M., Cohen S. Characterization of the high molecular weight form of epidermal growth factor. J Biol Chem. 1974 May 25;249(10):3198–3203. [PubMed] [Google Scholar]
- Weiss J. B., Brown R. A., Kumar S., Phillips P. An angiogenic factor isolated from tumours: a potent low-molecular-weight compound. Br J Cancer. 1979 Sep;40(3):493–496. doi: 10.1038/bjc.1979.206. [DOI] [PMC free article] [PubMed] [Google Scholar]




