Abstract
Peroxisome proliferator-activated receptor delta (PPARδ) is ubiquitously expressed in the vasculature, including cerebral circulation. The role of PPARδ in metabolism of tetrahydrobiopterin (BH4) has not been studied in the cerebral microvasculature. In the present study, the effects of PPARδ agonist GW501516 on uncoupling of endothelial nitric oxide synthase (eNOS) were determined in cerebral microvessels of BH4-deficient hph-1 mice. Wild-type (B6CBA) and hph-1 mice were orally gavaged with a selective PPARδ activator, GW501516 (2 mg/kg/day) for 14 days, and thereafter, cerebral microvessels were isolated and studied. Treatment of hph-1 mice with GW501516 significantly reduced oxidation of BH4 and increased the ratio of BH4 to 7,8-BH2 (P<0.05, n=6–9). Attenuation of L-NAME-inhibitable superoxide anion levels by GW501516 demonstrated that activation of PPARδ might prevent uncoupling of endothelial nitric oxide synthase (eNOS, P<0.05, n=6–9). Western blotting studies demonstrated that GW501516 selectively increased the endothelial expressions of CuZn superoxide dismutase (P<0.05, n=6–9) and catalase (P<0.05, n=6–8). PPARδ activation increased the total nitrite and nitrate (NO2 + NO3) content in cerebral microvessels (P<0.05, n=6). Obtained results suggest that in vivo activation of PPARδ prevents eNOS uncoupling, restores bioavailability of NO and may help preserve endothelial function in the BH4-deficient cerebral circulation.
Keywords: eNOS uncoupling, cerebral microvessels, GTP cyclohydrolase I, nitric oxide, antioxidant
1. Introduction
Peroxisome proliferator-activated receptor delta (PPARδ) is a member of the ligand-dependent family of PPAR nuclear receptors, and is ubiquitously expressed in the vasculature. Loss of PPARδ in smooth muscle cells of cerebral vasculature resulted in larger infarcts, blood brain barrier breakdown, inflammatory responses and neuronal loss in mice brain exposed to focal cerebral ischemia (Yin et al., 2011). Prior studies have indicated that long-term treatment with PPARδ agonists suppress the expression of pro-inflammatory genes (Barish et al., 2008; Fan et al., 2008; Takata et al., 2008) and exerts anti-atherosclerotic effects. PPARδ activation in cultured endothelial cells also increased expression of antioxidant enzymes – catalase, CuZn superoxide dismutase (CuZn SOD), Mn superoxide dismutase (Mn SOD), and thereby inhibited apoptosis (Inoue et al., 2001; Liou et al., 2006; Piqueras et al., 2007; Jiang et al., 2009).
In a recent study from our laboratory (He et al., 2011), we reported that selective PPARδ agonist GW501516 increased production of tetrahydrobiopterin (BH4), an essential co-factor of endothelial nitric oxide synthase (eNOS) in endothelial progenitor cells. Loss of BH4 and subsequent uncoupling of eNOS causes production of superoxide anions (Vasquez-Vivar et al., 1998; Katusic, 2001; d’Uscio et al., 2011). Relevant to the present study, the in vivo effects of PPARδ activation on cerebrovascular pathologies associated with BH4 deficiency have not been systematically investigated. In this regard, using the GTP cyclohydrolase I (rate-limiting enzyme in BH4 biosynthesis)-deficient hph-1 mice (Canevari et al., 1999; Cosentino et al., 2001; Hyland et al., 2003; Lam and Heales, 2007; d’Uscio et al., 2011), we demonstrated that loss of BH4 resulted in uncoupling of eNOS and elevated concentration of eNOS-derived superoxide anion in the cerebral microvessels (d’Uscio et al., 2011; Santhanam et al., 2012). In the present study, we tested the hypothesis that selective PPARδ agonist GW501516 exerts protective effects in cerebral microvessels of BH4-deficient mice by preventing eNOS uncoupling.
2. Results
2.1. Effect of GW501516 on physiological parameters
We and others have shown that hph-1 mice were normotensive (Nandi et al., 2005; d’Uscio et al., 2011). Treatment of hph-1 mice with GW501516 did not alter systolic blood pressure, body weight, plasma cholesterol, blood glucose and blood cell counts (Table 1).
Table 1.
Characteristics of wild-type and hph-1 mice treated without or with GW501516.
| Parameters | Wild-type | Wild-type + GW501516 | hph-1 | hph-1 + GW501516 |
|---|---|---|---|---|
| SBP (mmHg) | 118±1 | 117±1 | 120±1 | 116±1 |
| Body weight (g) | 31±1.0 | 30±1 | 31±1 | 30±1 |
| Glucose (mg/dL) | 161±8 | 160±10 | 183±12 | 182±11 |
| Cholesterol (mmol/L) | 2.0±0.1 | 2.2±0.1 | 2.3±0.2 | 2.2±0.1 |
| HDL (mmol/L) | 1.6±0.1 | 1.8±0.1 | 1.8±0.2 | 1.7±0.1 |
| White blood cells (103/mm3) | 6.8±0.4 | 6.8±0.6 | 9.9±0.8 | 9.7±1.1 |
| Red blood cells (106/mm3) | 9.9±0.1 | 9.8±0.1 | 10.2±0.2 | 10.1±0.2 |
| Platelets (103/mm3) | 682±38 | 712±31 | 823±24 | 792±62 |
SBP indicates systolic blood pressure. Data are means ± SEM (n=6–9). P= not significant (ANOVA with Bonferroni’s).
2.2. Effect of PPARδ agonist on BH4
Consistent with our previous studies on hph-1 mice (d’Uscio et al., 2011; Santhanam et al., 2012), we observed that oxidation of BH4 and the ratio of BH4 to 7,8-BH2 were significantly reduced in the cerebral microvessels of hph-1 mice used in the present study. Treatment of wild-type mice with GW501516 did not alter BH4 or 7,8-BH2 levels (Figure 1). In hph-1 mice treated with GW501516, BH4 levels in cerebral microvessels (Figure 1A) were significantly increased (P<0.05), while the levels of 7,8-BH2 were significantly reduced (Figure 1B, P<0.05). The ratio of BH4 to 7,8-BH2 was significantly elevated in cerebral microvessels of hph-1 mice treated with GW501516 (Figure 1C, P<0.05).
Figure 1.
GW501516 increases bioavailability of BH4 in hph-1 mice. (A) Levels of BH4 were decreased in cerebral microvessels (* P<0.05, n=9) of hph-1 mice. Treatment with GW501516 tended to decrease BH4 levels in cerebral microvessels of wild-type mice, but GW501516 significantly increased BH4 only in cerebral microvessels of hph-1 mice (# P<0.05, n=9). (B) Levels of 7,8-BH2 were significantly increased in hph-1 mice, as compared to wild-type mice (* P<0.05, n=9). Treatment with PPARδ agonist GW501516 inhibited this increase in 7,8-BH2 levels (# P<0.05, n=9). (C) The ratio of BH4 to 7,8-BH2 were significantly decreased in cerebral microvessels of hph-1 mice (* P<0.05, n=9) and treatment with GW501516 significantly increased the BH4 to 7,8-BH2 ratio (# P<0.05, n=9). (D) The reduced enzymatic activity of GTP cyclohydrolase I in cerebral microvessels of hph-1 mice (* P<0.05, n=6) were reversed on treatment with GW501516 (# P<0.05, n=6).
Enzymatic activity of GTP cyclohydrolase I was also significantly increased by GW501516 in the cerebral microvessels of hph-1 mice (Figure 1D, P<0.05).
2.3. Effect of GW501516 on superoxide anions
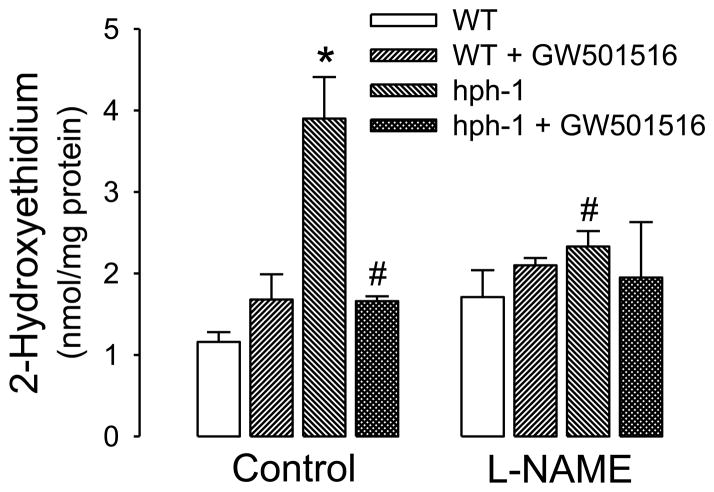
Consistent with findings from other laboratories (Cosentino et al., 2001; Khoo et al., 2005) as well as with our prior studies on hph-1 mice (d’Uscio et al., 2011; Santhanam et al., 2012), we confirmed that loss of BH4 caused increase in formation of superoxide anion, as indicated by increased formation of 2-hydroxyethidium, the product generated by chemical reaction of superoxide with dihydroethidium. Treatment with L-NAME, a NOS inhibitor, reduced superoxide anion levels selectively in hph-1 mice, indicative of increased superoxide anion production by eNOS activity (Figure 2). Treatment of hph-1 mice with PPARδ agonist GW501516 also significantly attenuated the increased superoxide anion levels in the cerebral microvessels (Figure 2, P<0.05). Importantly, in cerebral microvessels of hph-1 mice treated with GW501516, L-NAME did not further reduce this attenuated superoxide anion production (Figure 2).
Figure 2.
GW501516 reduced superoxide anion generation in hph-1 mice. Superoxide anion levels were significantly increased in cerebral microvessels of vehicle-treated hph-1 mice (* P<0.05, n = 8–9, as compared to wild-type mice). Treatment of hph-1 mice with GW501516 significantly attenuated this increased production of superoxide anions (# P<0.05, n = 8–9, as compared to vehicle-treated hph-1 mice). The increased superoxide anion production in cerebral microvessels of hph-1 mice was attenuated selectively by NOS inhibitor L-NAME (30 μmol/L; 30 min; # P<0.05; n=8–9, as compared to vehicle-treated hph-1 mice).
2.4. Effect of PPARδ activation on expression of antioxidant proteins
Expression of antioxidant proteins, namely CuZn SOD, Mn SOD and catalase were not different between wild-type and hph-1 mice (data not shown). GW501516 did not affect these antioxidant enzymes in wild-type mice (data not shown). Treatment of hph-1 mice with GW501516 selectively increased the expression of CuZn SOD in cerebral microvessels (Figure 3A), while expression of Mn SOD remained unchanged (Figure 3B). Protein expression of EC SOD was undetectable in the cerebral microvessels of hph-1 mice (data not shown).
Figure 3.
GW501516 increased protein expressions of CuZn SOD and catalase in hph-1 mice. Representative Western blot and densitometric analysis demonstrating selectively increased expression of CuZn SOD (*P <0.05, n = 6–9) in cerebral microvessels (A), while Mn SOD (B) expression remained unchanged. Expression of catalase was significantly increased by GW501516 in cerebral microvessels (C) of hph-1 mice.
Expression of catalase, a H2O2 scavenging enzyme, was also significantly increased by GW501516 in cerebral microvessels of hph-1 mice (Figure 3C, P<0.05).
2.5. Effect of GW501516 on bioavailability of NO
GW501516 did not affect expression of eNOS in the cerebral microvessels of hph-1 mice (Figure 4A). However, levels of nitrite and nitrate (NOx = NO2 +NO3) were significantly increased in the cerebral microvessels of hph-1 mice treated with GW501516 (Figure 4B).
Figure 4.
GW501516 increased total nitrite content in hph-1 mice. (A) Representative Western blot and densitometric analysis demonstrating that expression of eNOS remained unaffected in cerebral microvessels of hph-1 mice treated with GW501516 (P=0.83, n=4). (B) Levels of total nitrate and nitrite (NOx = NO2 + NO3) was significantly increased in the cerebral microvessels of hph-1 mice treated with GW501516 (*P<0.05, n = 6).
3. Discussion
In the present study, we report several novel findings. First, treatment with PPARδ agonist GW501516 increased the bioavailability of BH4 in the cerebral microvessels of hph-1 mice. Second, activation of PPARδ decreased oxidative stress as demonstrated by the reduced production of superoxide anions. Third, GW501516 increased the expression of antioxidant enzymes, CuZn SOD and catalase. Finally, PPARδ agonist GW501516 significantly increased the levels of NO, as evidenced by increased content of nitrite and nitrate in cerebral microvessels.
To the best of our knowledge, this is the first in vivo study to demonstrate the cerebrovascular protection conferred by a PPARδ agonist in mouse model of eNOS uncoupling. In the present study, we used GW501516 to selectively activate PPARδ in the hph-1 deficient mice. Indeed, GW501516 at the dose (of 2 mg/kg per day for 14 days) used in the present study has previously been demonstrated to selectively activate PPARδ (Oliver et al., 2001; Brunelli et al., 2007; Barish et al., 2008; He et al., 2011). Recently, we reported that PPARδ activation stimulated biosynthesis of BH4, an essential co-factor for eNOS activation in endothelial progenitor cells (He et al., 2011). In the present study, we expanded understanding of the benefits of PPARδ activation to include protection of cerebral microvessels under conditions of oxidative stress induced by eNOS uncoupling. Lack of effects on plasma cholesterol and blood glucose by GW501516 ruled out the contribution of metabolic effects of PPARδ activation to the vascular protective effects observed in the present study.
Activation of PPARδ in hph-1 deficient mice increased the ratio of BH4 to 7,8-BH2, by two mechanisms: (a) increasing the biosynthesis of BH4, mediated by activation of GTP cyclohydrolase I, and (b) reducing the levels of 7,8-BH2, the oxidized derivative of BH4 that competitively binds and inactivates eNOS (Vasquez-Vivar et al., 2002; d’Uscio et al., 2003). The protective effects of GW501516 on attenuation of 7,8-BH2 levels are explained by up-regulation of antioxidant enzymes and subsequent protection of BH4 against oxidation by peroxynitrite, generated by chemical reaction between superoxide anion and nitric oxide (Milstien and Katusic, 1999). Indeed in a previous study, we demonstrated that peroxynitrite is a potent oxidant of BH4 (Milstien and Katusic, 1999).
Further characterization of the biochemical mechanism responsible for attenuated superoxide anion production by GW501516 identified selective up-regulation of CuZn SOD in the cerebral microvasculature hph-1 mice. CuZn SOD gene is the predominant and primary antioxidant defense enzyme of the vasculature. Promoter of CuZn SOD gene has a peroxisome-proliferator activated receptor response element (PPRE), and our results are consistent with published reports on other PPAR ligands that demonstrate binding to the PPREs and activation of CuZn SOD transcription (Yoo et al., 1999; Fan et al., 2008). In addition, PPARδ agonist seems to prevent the accumulation of H2O2 by increasing the expression of catalase.
Treatment with GW501516 increased availability of NO, as reflected in elevation of NO2/NO3. This effect is most likely caused by prevention of eNOS uncoupling and protection of NO from inactivation by superoxide anion. Since NO is a key vascular protective molecule, our findings support the concept that, besides reported anti-atherosclerotic and neuroprotective effects, PPARδ agonists may prevent endothelial dysfunction induced by uncoupling of eNOS. The effects of PPARδ are mediated by enhanced activity of GTP cyclohydrolase I, increased expression of CuZn SOD and subsequent inactivation of superoxide anion. In aggregate, presented findings suggest that PPARδ is a potential therapeutic target for prevention of endothelial dysfunction in the cerebral microvasculature.
4. Experimental procedures
4.1. Mice
Breeder pairs of homozygous hph-1 mutant mice, on B6CBA background, were provided by Dr. Alex Chen (Michigan State University, East Lansing, MI). Age-matched wild-type (B6CBA) mice were procured from The Jackson Laboratory (Bar Harbor, ME). Prior studies established that wild-type (B6CBA) mice are appropriate control animals (Nandi et al., 2005; d’Uscio et al., 2011). All experiments were performed on 3–4 months old (20 – 25 g) male mice according to the guidelines of the Institutional Animal Care and Use Committee of Mayo Clinic. Mice were orally gavaged with a selective PPARδ activator GW501516 (2 mg/kg/day) for 14 days. Prior studies established that this dosing regimen selectively activated PPARδ (Oliver et al., 2001; Barish et al., 2008). Control mice were treated with the appropriate vehicle (0.5% carboxy methyl cellulose in 2% dimethylsulfoxide). Following treatment with GW501516, mice were killed by injection of an overdose of pentobarbital (250 mg/kg body weight, i.p.).
4.2. Physiological parameters in mice
Systolic blood pressure was measured in non-anesthetized mice by tail-cuff method, as described earlier (d’Uscio et al., 2011). For blood cell counts, mice were anesthetized in a bell jar containing isoflurane (1%) and 150 μL blood was drawn from the orbital venous sinus and transferred immediately to EDTA containing tubes (Microtainer®; Becton Dickinson; Franklin Lakes, NJ). For measurement of glucose and cholesterol levels, blood was obtained as previously reported (d’Uscio et al., 2011).
4.3. Isolation of cerebral microvessels
Brain were removed and placed in cold (4° C) modified Krebs-Ringer bicarbonate solution (in mmol/l: NaCl 118.6; KCl 4.7; CaCl2 2.5; MgSO4 1.2; KH2PO4 1.2; NaHCO3 25.1; glucose 10.1; EDTA 0.026). Mice brains were exposed under a surgical microscope, anterior and posterior cerebral, middle cerebral and basilar arteries were isolated and discarded. Cerebral microvessels were subsequently isolated from the brains by Dextran centrifugation, as described earlier (Austin et al., 2010; Santhanam et al., 2012). The isolated microvessels contained a heterogenous mixture of small arteries, arterioles, small veins, venules and capillaries.
4.4. Measurement of biopterin levels and GTP cyclohydrolase I activity
Cerebral microvessels were homogenized in extraction buffer containing 50 mmol/l Tris (pH 7.4), 1 mmol/L dithiothreitol, and 1 mmol/L EDTA at 4°C and were centrifuged at 10,000 g (8 min at 4°C). Tetrahydrobiopterin (BH4), 7,8-dihydrobiopterin (7,8-BH2) and enzymatic activity of GTPCH I were measured by reverse phase HPLC, as reported previously (d’Uscio et al., 2003; d’Uscio et al., 2011; Santhanam et al., 2012).
4.5. Detection of intracellular superoxide anion
Intracellular superoxide anion levels in cerebral microvessels were quantified using a HPLC-based fluorescence detection of the oxidation of dihydroethidium. Briefly, microvessels were incubated in Krebs-HEPES buffer containing 50 μmol/L of dihydroethidium (Molecular Probes) at 37°C for 15 minutes. In some experiments, microvessels were incubated with L-NAME (30 μmol/L) for 30 minutes prior to addition of dihydroethidium (d’Uscio et al., 2010; d’Uscio et al., 2011; Santhanam et al., 2012). The samples were washed to remove the free dihydroethidium and incubated in Krebs-HEPES buffer for 1 additional hour at 37°C. Microvessels were homogenized in cold methanol and centrifuged at 12,000 rpm. The supernatant was analyzed by fluorescence detection by HPLC (Beckman Coulter) in 37% acetonitrile in 0.1% trifluoroacetic acid aqueous solution. Data were quantified using 2-hydroxyethidium standard from the reaction between dihydroethidium and Fremy’s salt and normalized against tissue levels (d’Uscio et al., 2010; d’Uscio et al., 2011; Santhanam et al., 2012).
4.6. Western blot analysis
Cerebral microvessels were homogenized in lysis buffer containing [50 mmol/L NaCl, 50 mmol/L NaF, 50 mmol/L sodium pyrophosphate, 5 mmol/L EDTA, 5 mmol/L EGTA, 0.1 mmol/L Na3VO4, 1% Triton X-100, 10 mmol/L HEPES, pH 7.4, and protease inhibitor cocktail (Sigma)]. The experimental methods for protein expression studies are published elsewhere (Santhanam et al., 2007; Santhanam et al., 2012). Monoclonal antibodies against eNOS (1:500) [BD Transduction], catalase (1:1000), β-actin (1:5000) [Sigma] and polyclonal antibodies (1:1000 dilution) against CuZn superoxide dismutase (CuZn SOD), manganese superoxide dismutase (Mn SOD), extracellular SOD (EC SOD) [Stressgen] were used, and bands were visualized by enhanced chemiluminescence (Super Signal West Pico Chemilumiscent Substrate, Thermo Fisher Scientific, Rockford, IL).
4.7. Measurement of nitric oxide
Nitric oxide in the cerebral microvessels were measured as total nitrite and nitrate (NOx = NO3 + NO2) using a commercially available fluorometric nitrite/nitrate assay kit according to manufacturer’s instructions (Cayman Chemical Co.) (Austin et al., 2010; Santhanam et al., 2012).
4.8. Statistical analysis
Data are represented as mean ± SEM, n represents the number of mice used in each group. Un-paired Student’s ‘t’ test was used to determine statistical difference between two groups, and multiple comparisons were performed by two-way ANOVA. A value of P<0.05 was considered statistically significant.
Highlights.
We studied the effects of GW501516 in cerebral microvessels of hph-1 mice.
Selective PPARδ agonist GW501516 significantly increased bioavailability of BH4.
GW501516 reversed eNOS uncoupling and increased nitrite/nitrate content.
GW501516 may preserve endothelial function in BH4-deficient cerebral circulation.
Acknowledgments
This study was funded in part by American Heart Association Scientist Development Grants (08-35436N to AVS; 07-30133N to LVD; 09SDG2190046 to TH), National Institutes of Health Grants (HL 53524, HL 91867 to ZSK), and the Mayo Foundation. The authors would like to thank Suzanne M. Greiner for assistance with blood cell counts.
Footnotes
Conflict of Interest / Disclosure(s):
The authors disclose no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Austin SA, Santhanam AV, Katusic ZS. Endothelial nitric oxide modulates expression and processing of amyloid precursor protein. Circ Res. 2010;107:1498–502. doi: 10.1161/CIRCRESAHA.110.233080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish GD, Atkins AR, Downes M, Olson P, Chong LW, Nelson M, Zou Y, Hwang H, Kang H, Curtiss L, Evans RM, Lee CH. PPARdelta regulates multiple proinflammatory pathways to suppress atherosclerosis. Proc Natl Acad Sci U S A. 2008;105:4271–6. doi: 10.1073/pnas.0711875105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli L, Cieslik KA, Alcorn JL, Vatta M, Baldini A. Peroxisome proliferator-activated receptor-delta upregulates 14-3-3 epsilon in human endothelial cells via CCAAT/enhancer binding protein-beta. Circ Res. 2007;100:e59–71. doi: 10.1161/01.RES.0000260805.99076.22. [DOI] [PubMed] [Google Scholar]
- Canevari L, Land JM, Clark JB, Heales SJ. Stimulation of the brain NO/cyclic GMP pathway by peripheral administration of tetrahydrobiopterin in the hph-1 mouse. J Neurochem. 1999;73:2563–8. doi: 10.1046/j.1471-4159.1999.0732563.x. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Barker JE, Brand MP, Heales SJ, Werner ER, Tippins JR, West N, Channon KM, Volpe M, Luscher TF. Reactive oxygen species mediate endothelium-dependent relaxations in tetrahydrobiopterin-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:496–502. doi: 10.1161/01.atv.21.4.496. [DOI] [PubMed] [Google Scholar]
- d’Uscio LV, Milstien S, Richardson D, Smith L, Katusic ZS. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circ Res. 2003;92:88–95. doi: 10.1161/01.res.0000049166.33035.62. [DOI] [PubMed] [Google Scholar]
- d’Uscio LV, Smith LA, Katusic ZS. Erythropoietin increases expression and function of vascular copper- and zinc-containing superoxide dismutase. Hypertension. 2010;55:998–1004. doi: 10.1161/HYPERTENSIONAHA.110.150623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Uscio LV, Smith LA, Katusic ZS. Differential effects of eNOS uncoupling on conduit and small arteries in GTP-cyclohydrolase I-deficient hph-1 mice. Am J Physiol Heart Circ Physiol. 2011;301:H2227–34. doi: 10.1152/ajpheart.00588.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Wang Y, Tang Z, Zhang H, Qin X, Zhu Y, Guan Y, Wang X, Staels B, Chien S, Wang N. Suppression of pro-inflammatory adhesion molecules by PPAR-delta in human vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:315–21. doi: 10.1161/ATVBAHA.107.149815. [DOI] [PubMed] [Google Scholar]
- He T, Smith LA, Lu T, Joyner MJ, Katusic ZS. Activation of peroxisome proliferator-activated receptor-{delta} enhances regenerative capacity of human endothelial progenitor cells by stimulating biosynthesis of tetrahydrobiopterin. Hypertension. 2011;58:287–94. doi: 10.1161/HYPERTENSIONAHA.111.172189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland K, Gunasekara RS, Munk-Martin TL, Arnold LA, Engle T. The hph-1 mouse: a model for dominantly inherited GTP-cyclohydrolase deficiency. Ann Neurol. 2003;54(Suppl 6):S46–8. doi: 10.1002/ana.10695. [DOI] [PubMed] [Google Scholar]
- Inoue M, Itoh H, Tanaka T, Chun TH, Doi K, Fukunaga Y, Sawada N, Yamshita J, Masatsugu K, Saito T, Sakaguchi S, Sone M, Yamahara K, Yurugi T, Nakao K. Oxidized LDL regulates vascular endothelial growth factor expression in human macrophages and endothelial cells through activation of peroxisome proliferator-activated receptor-gamma. Arterioscler Thromb Vasc Biol. 2001;21:560–6. doi: 10.1161/01.atv.21.4.560. [DOI] [PubMed] [Google Scholar]
- Jiang B, Liang P, Zhang B, Song J, Huang X, Xiao X. Role of PPAR-beta in hydrogen peroxide-induced apoptosis in human umbilical vein endothelial cells. Atherosclerosis. 2009;204:353–8. doi: 10.1016/j.atherosclerosis.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Katusic ZS. Vascular endothelial dysfunction: does tetrahydrobiopterin play a role? Am J Physiol Heart Circ Physiol. 2001;281:H981–6. doi: 10.1152/ajpheart.2001.281.3.H981. [DOI] [PubMed] [Google Scholar]
- Khoo JP, Zhao L, Alp NJ, Bendall JK, Nicoli T, Rockett K, Wilkins MR, Channon KM. Pivotal role for endothelial tetrahydrobiopterin in pulmonary hypertension. Circulation. 2005;111:2126–33. doi: 10.1161/01.CIR.0000162470.26840.89. [DOI] [PubMed] [Google Scholar]
- Lam AA, Heales SJ. Nitric oxide accelerates the degradation of tetrahydrobiopterin but not total neopterin in cerebrospinal fluid; potential implications for the assessment of tetrahydrobiopterin metabolism. Ann Clin Biochem. 2007;44:394–6. doi: 10.1258/000456307780945741. [DOI] [PubMed] [Google Scholar]
- Liou JY, Lee S, Ghelani D, Matijevic-Aleksic N, Wu KK. Protection of endothelial survival by peroxisome proliferator-activated receptor-delta mediated 14-3-3 upregulation. Arterioscler Thromb Vasc Biol. 2006;26:1481–7. doi: 10.1161/01.ATV.0000223875.14120.93. [DOI] [PubMed] [Google Scholar]
- Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun. 1999;263:681–4. doi: 10.1006/bbrc.1999.1422. [DOI] [PubMed] [Google Scholar]
- Nandi M, Miller A, Stidwill R, Jacques TS, Lam AA, Haworth S, Heales S, Vallance P. Pulmonary hypertension in a GTP-cyclohydrolase 1-deficient mouse. Circulation. 2005;111:2086–90. doi: 10.1161/01.CIR.0000163268.32638.F4. [DOI] [PubMed] [Google Scholar]
- Oliver WR, Jr, Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, Lewis MC, Winegar DA, Sznaidman ML, Lambert MH, Xu HE, Sternbach DD, Kliewer SA, Hansen BC, Willson TM. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci U S A. 2001;98:5306–11. doi: 10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piqueras L, Reynolds AR, Hodivala-Dilke KM, Alfranca A, Redondo JM, Hatae T, Tanabe T, Warner TD, Bishop-Bailey D. Activation of PPARbeta/delta induces endothelial cell proliferation and angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27:63–9. doi: 10.1161/01.ATV.0000250972.83623.61. [DOI] [PubMed] [Google Scholar]
- Santhanam AV, Smith LA, He T, Nath KA, Katusic ZS. Endothelial progenitor cells stimulate cerebrovascular production of prostacyclin by paracrine activation of cyclooxygenase-2. Circ Res. 2007;100:1379–88. doi: 10.1161/01.RES.0000265848.55035.5d. [DOI] [PubMed] [Google Scholar]
- Santhanam AV, d’Uscio LV, Smith LA, Katusic ZS. Uncoupling of eNOS causes superoxide anion production and impairs NO signaling in the cerebral microvessels of hph-1 mice. J Neurochem. 2012 doi: 10.1111/j.1471–4159.2012.07872.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata Y, Liu J, Yin F, Collins AR, Lyon CJ, Lee CH, Atkins AR, Downes M, Barish GD, Evans RM, Hsueh WA, Tangirala RK. PPARdelta-mediated antiinflammatory mechanisms inhibit angiotensin II-accelerated atherosclerosis. Proc Natl Acad Sci U S A. 2008;105:4277–82. doi: 10.1073/pnas.0708647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A. 1998;95:9220–5. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Martasek P, Whitsett J, Joseph J, Kalyanaraman B. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: an EPR spin trapping study. Biochem J. 2002;362:733–9. doi: 10.1042/0264-6021:3620733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin KJ, Deng Z, Hamblin M, Zhang J, Chen YE. Vascular PPARdelta protects against stroke-induced brain injury. Arterioscler Thromb Vasc Biol. 2011;31:574–81. doi: 10.1161/ATVBAHA.110.221267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo HY, Chang MS, Rho HM. Induction of the rat Cu/Zn superoxide dismutase gene through the peroxisome proliferator-responsive element by arachidonic acid. Gene. 1999;234:87–91. doi: 10.1016/s0378-1119(99)00176-6. [DOI] [PubMed] [Google Scholar]