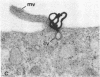
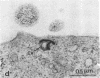
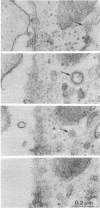
Abstract
When 125I-labeled insulin (125I-insulin) is incubated with 3T3-L1 adipocytes and cells processed for electron microscopic autoradiography, the ligand initially localizes preferentially to microvilli and coated pits. As a function of time and temperature, this initial preferential localization to microvilli is lost, and the ligand is internalized by the cell. Serial sections of apparent coated vesicles near the cell surface indicate that about half of these structures are true vesicles and, therefore, intermediates in this receptor-mediated endocytotic process. With time, 125I-insulin localizes to larger intracellular membrane-bounded structures. When cells are incubated with another ligand, cationic ferritin, that is taken up by adsorptive endocytosis, essentially the same structures are involved as for the endocytosis of 125I-insulin. The data suggest that specificity for receptor-mediated endocytosis is conferred by the specific ligand receptor and possibly by ligand-induced receptor mobility in the plane of the plasma membrane. Other structures such as coated pits, coated vesicles, larger vesicles, and secondary lysosomes are common for different ligands.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackett N. M., Parry D. M. A simplified method of "hypothetical grain" analysis of electron microscope autoradiographs. J Histochem Cytochem. 1977 Mar;25(3):206–214. doi: 10.1177/25.3.839062. [DOI] [PubMed] [Google Scholar]
- CARO L. G., VAN TUBERGEN R. P., KOLB J. A. High-resolution autoradiography. I. Methods. J Cell Biol. 1962 Nov;15:173–188. doi: 10.1083/jcb.15.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Amherdt M., Van Obberghen E., Kahn C. R., Orci L. 125I-insulin binding to cultured human lymphocytes. Initial localization and fate of hormone determined by quantitative electron microscopic autoradiography. J Clin Invest. 1978 Apr;61(4):1057–1070. doi: 10.1172/JCI109005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Anderson R. G., Goldstein J. L., Brown M. S., Cohen S., Orci L. Co-localization of 125I-epidermal growth factor and ferritin-low density lipoprotein in coated pits: a quantitative electron microscopic study in normal and mutant human fibroblasts. J Cell Biol. 1982 Oct;95(1):73–77. doi: 10.1083/jcb.95.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Freychet P., Le Cam A., Orci L. Lysosomal association of internalized 125I-insulin in isolated rat hepatocytes. Direct demonstration by quantitative electron microscopic autoradiography. J Clin Invest. 1979 Jun;63(6):1249–1261. doi: 10.1172/JCI109420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Van Obberghen E., Gorden P., Orci L. Surface redistribution of 125I-insulin in cultured human lymphocytes. J Cell Biol. 1981 Oct;91(1):17–25. doi: 10.1083/jcb.91.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. B., Willingham M. C., Pastan I. alpha 2-macroglobulin adsorbed to colloidal gold: a new probe in the study of receptor-mediated endocytosis. J Cell Biol. 1981 Apr;89(1):29–34. doi: 10.1083/jcb.89.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlmann M., Carpentier J. L., Le Cam A., Thamm P., Saunders D., Brandenburg D., Orci L., Freychet P. Biochemical and morphological evidence that the insulin receptor is internalized with insulin in hepatocytes. J Cell Biol. 1982 Apr;93(1):82–87. doi: 10.1083/jcb.93.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Fan J. Y., Orci L. Receptor mediated endocytosis of polypeptide hormones: mechanism and significance. Metabolism. 1982 Jul;31(7):664–669. doi: 10.1016/0026-0495(82)90196-2. [DOI] [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Freychet P. O., Orci L. Internalization of polypeptide hormones: mechanism, intracellular localization and significance. Diabetologia. 1980 Apr;18(4):263–274. doi: 10.1007/BF00251003. [DOI] [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Moule M. L., Yip C. C., Orci L. Direct demonstration of insulin receptor internalization. A quantitative electron microscopic study of covalently bound 125I-photoreactive insulin incubated with isolated hepatocytes. Diabetes. 1982 Jul;31(7):659–662. doi: 10.2337/diab.31.7.659. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Tobleman M. Q., Hackenbrock C. R. The distribution and mobility of anionic sites on the surfaces of baby hamster kidney cells. J Cell Biol. 1975 Sep;66(3):470–479. doi: 10.1083/jcb.66.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J., Simons K., Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry D. M., Blackett N. M. Electron microscope autoradiography of erythroid cells using radioactive iron. J Cell Biol. 1973 Apr;57(1):16–26. doi: 10.1083/jcb.57.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. Methods for assessing immunologic and biologic properties of iodinated peptide hormones. Methods Enzymol. 1975;37:223–233. doi: 10.1016/s0076-6879(75)37018-3. [DOI] [PubMed] [Google Scholar]
- Salpeter M. M., Fertuck H. C., Salpeter E. E. Resolution in electron microscope autoradiography. III. Iodine-125, the effect of heavy metal staining, and a reassessment of critical parameters. J Cell Biol. 1977 Jan;72(1):161–173. doi: 10.1083/jcb.72.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall D. A., Wilson G., Hubbard A. L. The galactose-specific recognition system of mammalian liver: the route of ligand internalization in rat hepatocytes. Cell. 1980 Aug;21(1):79–93. doi: 10.1016/0092-8674(80)90116-6. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Rutherford A. V., Gallo M. G., Wehland J., Dickson R. B., Schlegel R., Pastan I. H. Receptor-mediated endocytosis in cultured fibroblasts: cryptic coated pits and the formation of receptosomes. J Histochem Cytochem. 1981 Sep;29(9):1003–1013. doi: 10.1177/29.9.6169759. [DOI] [PubMed] [Google Scholar]
- de Petris S. Preferential distribution of surface immunoglobulins on microvilli. Nature. 1978 Mar 2;272(5648):66–68. doi: 10.1038/272066a0. [DOI] [PubMed] [Google Scholar]