Abstract
Rationale
PROTECT DC examines whether stroke navigators can improve cardiovascular risk factors in urban underserved individuals newly hospitalized for stroke or ischemic attack. Within one year of hospital discharge, up to one third of patients no longer adhere to secondary prevention behaviors. Adherence rates are lower in minority-underserved groups, contributing to health disparities. In-hospital programs increase use of stroke prevention therapies but may not be as successful in underserved individuals. In these groups, low literacy, limited health care access, and sparse community resources may reduce adherence. Lay community health workers (‘navigators’) improve adherence in other illnesses through education and assisting in overcoming barriers to achieving desired health behaviors and obtaining needed healthcare services.
Aims and design
PROTECT DC is a Phase II, single-blind, randomized, controlled trial comparing in-hospital education plus stroke navigators to usual care. Atherogenic ischemic stroke and transient ischemic attack survivors are recruited from Washington DC hospitals. Navigators meet with participants during the index hospitalization, perform home visits, and meet by phone. They focus on stroke education, medication compliance, and overcoming practical barriers to adherence. The interventions are driven by the theories of reasoned action and planned behavior.
Study outcomes
The primary dependent measure is a summary score of four objective measures of stroke risk factor control: systolic blood pressure, low-density lipoprotein, hemoglobin Hb A1C, and antiplatelet agent pill counts. Secondary outcomes include stroke knowledge, exercise, dietary modification, smoking cessation.
Conclusion
PROTECT DC will determine whether a Phase III trial of stroke navigation for urban underserved individuals to improve adherence to secondary stroke prevention behaviors is warranted.
Keywords: stroke, secondary prevention, community health education, healthcare disparities, clinical trials, health behavior, patient adherence, patient compliance
Introduction
Stroke remains the third leading cause of death and a leading cause of adult disability in the United States. Health care costs related to stroke total over $50 billion per year in the US alone1. Decades of research in cardiovascular risk reduction have led to guidelines which, if optimally implemented, could prevent secondary stroke in 50–80% of patients2.
Stroke is particularly prominent in urban underserved populations. Sacco and colleagues found a 2.4 fold greater stroke incidence in African Americans and a two-fold increase in Hispanics compared to Caucasians, with higher mortality and lower three-year survival3. At older ages, when stroke mortality is the highest, the stroke mortality rate in non-Hispanic Caucasians approached the stroke mortality rate of African Americans4. Compared with Caucasians, minority groups suffered greater neurological impairment and had poorer outcomes5, 6
Adherence to evidence-based therapies for the prevention of ischemic stroke in patients remains inadequate. Even in the general population, there is marked room for improvement in the implementation of antithrombotics, lipid-lowering therapies, antihypertensives, and smoking cessation counseling in individuals who have experienced a cerebrovascular event7, particularly in African Americans. Superimposed are racial differences in the use of aspirin and smoking cessation for secondary prevention in veterans with coronary artery disease8. African Americans are less likely to receive comprehensive diagnostic evaluation compared to white patients and were less likely to have a neurologist as their attending physician9.
Several barriers to stroke prevention have been identified in minority populations. A telephone study in African Americans found that stroke knowledge was related to stroke risk factors and that stress and inadequate finances were the most frequently reported barriers10. Schneider and colleagues found that individuals with the highest risk and incidence of stroke, including African Americans, were the least knowledgeable about stroke warning signs and risk factors11. Reasons for these disparities likely include cultural, biological, and environmental factors (e.g. access to health care, education and socio-economic status, variations in lifestyle, religious and cultural beliefs, language barriers, and genetic factors). Resolution of these disparities is urgently needed to improve the health status of underserved communities.
Education of patients is one approach to overcoming these issues, and in-hospital education has been emphasized by many organizations. For example, the American Heart Association “Get with the Guidelines” effort12 uses in-hospital presentation of education materials about stroke and management of cardiovascular risk factors13. Other efforts have increased the intensity of the patient’s in-hospital education experience by incorporating a nurse educator into this process. For example, the original PROTECT LA study14 used a nurse to educate a predominantly white high socioeconomic status (SES) stroke population who later self-reported increased adherence to medications and other risk factor modification efforts14, 15.
Other programs have demonstrated success in targeting underserved populations. Rimmer et al16 evaluated a 12 week health promotion intervention for a predominantly black population of stroke. Improvements in total serum cholesterol, cardiovascular fitness, and strength were achieved compared to the control group. A related example of coordinated care for stroke patients is STEPS CARE: A Post Discharge Intervention to Improve Stroke Outcomes. STEPS CARE was an RCT of a geriatrics-based model of post acute care provided by a geriatrician and advanced practice geriatrics nurse. Results of a preliminary study showed improvements at three-months in a health and function global endpoint composed of five domains17.
The overall aim of this project is to perform a phase II randomized clinical trial (RCT) designed to prepare for a phase III assessment of whether PROTECT DC (hospital-based initiation of secondary prevention strategies coupled with stroke navigation) can significantly reduce secondary vascular events (stroke, MI and vascular death) rates in an underserved population at high risk for subsequent stroke or serious cardiovascular events. Health navigators are lay health workers recruited from the community to be served; they are trained and supervised by physicians, nurses, social workers, or health educators18. The goal of navigation is to improve self-management of chronic diseases and to reduce the barriers to health care. Since only 20% of disease self-management skills are disease specific19, we expect that techniques developed in other conditions will be relevant to stroke patients. Navigation is effective in increasing compliance in primary care20, diabetes21, 22, cancer23, cardiovascular disease and HTN24, 25 and asthma26. Our goals are to refine the intervention and gather data necessary to design that phase III trial.
Methods
Design
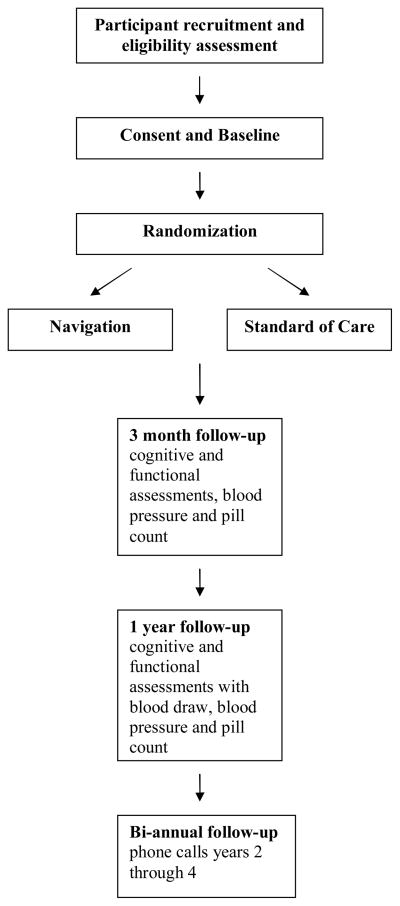
In this phase II trial, a total of 250 participants admitted to four acute care urban hospitals and one rehabilitation hospital for atherogenic stroke are being randomized. In the experimental arm, community based ‘stroke navigators’ facilitate compliance and health care access. The usual and customary care control arm consists of the American Heart Association (AHA) materials tailored for African Americans (‘Power to End Stroke’ www.powertoendstroke.org) distributed during hospitalization.
The primary dependent measure will be the percentage of four objective markers of stroke risk that are normal one year after stroke onset. Effects on secondary behavioral goals will also be evaluated; the 8 PROTECT DC goals are listed in Table 1. Vascular event rates will be measured to optimize study methodology and inform sample size calculation for a subsequent Phase III trial.
Table 1.
Primary PROTECT DC goals
| Primary goals | 1. Compliance with prescribed antithrombotic therapy confirmed by pill count |
| 2. Normal systolic blood pressure | |
| 3. Normal LDL | |
| 4. Normal Hemoglobin A1c | |
| Secondary goals | |
| 5. Compliance with smoking cessation | |
| 6. Compliance with American Heart Association diet | |
| 7. Compliance with exercise regimen | |
| 8. Stroke awareness |
The National Rehabilitation Hospital is the coordinating site and the IRB of record is Georgetown University. PROTECT DC is designated a minimal risk study. The study is funded by the National Institute of Neurological Disorders and Stroke at the National Institutes of Health and a supplemental grant from National Center on Minority Health and Health Disparities.
Patient population
The study sample is intended to be representative of stroke patients in the District of Columbia (DC), a region overrepresented in African Americans and low SES individuals. No racial, ethnic or gender groups are excluded. Participants are recruited from the inpatient stroke services from five hospitals that serve a majority of the stroke patients within DC: Washington Hospital Center, Howard University Hospital, Georgetown University Hospital, Providence Hospital and National Rehabilitation Hospital (NRH). Potential participants are identified from emergency department and hospital admission records; coordinators screen and consent participants.
The sample consists of adults (age >18) hospitalized within 30 days of an ischemic stroke or TIA due to atherogenic cerebrovascular or cardiac disease. Ischemic stroke is defined as rapidly developing clinical signs of focal disturbance of cerebral function lasting more than 24h. In the case of TIA or clinical stroke with no lesion visualized on neuroimaging, a stroke neurologist confirms the diagnosis. Atherogenic stroke is defined as large vessel, small vessel, or cryptogenic etiology with at least one stroke risk factor27. Persons with embolic stroke due to atherogenic cardiac disease are also included. Participants reside within DC or within five miles of the DC border. A caregiver or interested party must be available if the participant is moderately or severely disabled. To minimize loss to follow-up, a sufficient number of collateral contacts (>3 preferred) are required. Subjects must be judged likely to return to community setting at completion of post-acute care.
Individuals are excluded if they have one or more of the following:
non-atherogenic cause of stroke or embolic stroke due to non-atherogenic heart disease
NIHSS > 20
any medical condition that would limit participation in follow up assessments
baseline dementia per informant report (AD 828) or screening assessment (Short Blessed Memory Orientation Concentration Test29).
Randomization
Study participants are randomized to navigation or control in a 1:1 ratio using a baseline adaptive randomization algorithm, stratified by recruitment site. The algorithm uses Pearson’s chi-square statistic to measure treatment imbalance in baseline NIHSS (≤ 6 vs. >6), age (≤ 65 vs. >65) or gender30, 31. A new subject is randomly assigned with probability 0.75 to the group that would achieve the best treatment balance among these three characteristics. The first twelve participants in each site were randomized with a fair coin, and their baseline characteristics used to start the adaptive randomization. The randomization algorithm is embedded in a web based data management application, and treatment assignments are available immediately upon baseline data entry. The inclusion/exclusion criteria were incorporated into the application to ensure all randomized patients are eligible. We chose to stratify randomization based on the admitting hospital because of concerns that differences in hospital practices and procedures could be a source of systematic bias. These biases could stem from differences in patient populations, acute management, post-discharge planning and support, or other unanticipated factors. Stratified randomization by hospital will minimize these effects.
Experimental intervention
Participants randomized to the experimental arm initially meet the stroke navigator as an inpatient; that same navigator becomes the primary navigator for that participant during the one year intervention. At hospital discharge, the navigator ensures that the patient has a participant handbook specific to the assigned treatment group, tailored AHA educational materials, and prescriptions. After discharge, the stroke navigator assesses adherence and actively screens for barriers to medication adherence and access to health care services. The navigators also provide tailored health education regarding each of the primary and secondary study goals (medication compliance, smoking cessation, AHA diet, physical activity/exercise and stroke awareness). Interactions occur via at least two home visits and at least monthly phone calls. Many individuals need more frequent contact to help resolve family and social barriers to adherence that arises. Techniques used include motivational interviewing, application of the principles of health behavior, and practical problem solving regarding issues such as transportation, insurance, and fear of medication side effects. Navigators interact with primary care doctors when necessary, and assist the participant in obtaining medications. Aside from navigation, no additional resources are provided to participants, by the study navigators are supervised by a team of physicians, health educators and social workers on a daily basis.
The PROTECT DC intervention is based on the Theories of Reasoned Action and Planned Behavior (TRA, TPB)32, 33. Thus, navigators focus efforts on increasing the participant’s behavioral intention towards taking medications, not simply towards avoiding stroke. They also focus on increasing the participant’s actual and perceived control over barriers to adherence. The TRA/TPB model provides several targets for improving medication compliance for secondary stroke prevention. For example, education of the individual/family caregivers/health care providers about medications’ beneficial effects can tilt behavioral and normative beliefs more firmly towards compliance. Motivation is likely to be highest immediately after stroke, during the acute hospitalization. Education about solutions to difficulties in taking meds, and assistance in elimination of barriers increases the individual’s perception of control over their situation, again increasing the likelihood of adherence.
Control intervention: usual and customary care
The control intervention retains subjects while minimizing any study-related impact on stroke prevention behaviors. The control intervention is a standardized version of the usual and customary care delivered at each hospital, and participants receive the same tailored AHA materials as the experimental arm, but no navigator input. Control subjects are provided with a participant handbook specific to their study assignment. Control subjects are contacted by phone monthly to confirm contact information and to inquire about hospitalizations. If the participant requests information, study staff mail relevant materials.
Blinding
Blinded raters obtain all follow up data. Raters are supervised by research staff outside the study; participants are instructed not to reveal their treatment assignment. Vascular events, re-hospitalizations, and other clinical event are adjudicated by a physician blinded to treatment assignment.
Baseline data
Trained study coordinators collect data regarding demographics, cognitive status (1), and stroke type and severity. Pre-stroke functional status, health behaviors and health beliefs are also assessed. Selected baseline measurements can be found in Table 2.
Table 2.
Selected study assessments
| Domain Assessed | Baseline | 3 months | 12 months | |
|---|---|---|---|---|
|
| ||||
| History and physical | Medical status | X | ||
|
| ||||
| Vital signs | Medical status | X | X | X |
|
| ||||
| Demographics | Social and economic status | X | X | X |
|
| ||||
| NIH Stroke Scale | Stroke severity | X | X | X |
|
| ||||
| Cognitive assessments Geriatric depression scale Vascular dementia battery |
X | X | X | |
|
| ||||
| Functional assessments Barthel Index Modified Rankin Scale Lawton instrumental ADL Activity card sort WISSE diet Physical activity log Short form-12 |
Disability, participation | X | X | X |
|
| ||||
| Laboratory data (LDL, Hb A1C,) | Risk factor management | X | X | |
Primary outcome measures
The primary dependent measure is defined as the proportion of 4 objective measures of stroke risk (SBP, LDL, Hb A1C, and antiplatelet compliance documented by pill count) which are within normal range34 (see table below for definitions) at the primary time point of 1 year. Compliance with antiplatelet agents is defined as pill counts documenting use of 90% of prescribed medication35. Risk factors that are within normal limits at study enrollment (e.g., normal pre-randomization Hb A1C, LDL, SBP) will be included in this analysis. These are physiological indicators of stroke risk that are the targets of secondary stroke reduction efforts, and are intermediate steps to vascular event rate reduction. Each is associated with medication compliance. A secondary analysis of these measures will examine whether PROTECT DC improved laboratory values by a clinically significant amount. For those participants prescribed more than one antiplatelet agent, compliance with each will be averaged into a single value.
Secondary outcome measures
Four stroke prevention behaviors were defined as secondary goals of navigation. These are displayed in Table 3. The rate of primary vascular events is another secondary measure; these are defined as the documented occurrence of a subsequent stroke, MI, or vascular death. Subjects and families will be contacted annually, and medical records will be obtained where available for review for blinded adjudication.
Table 3.
Medication compliance goals
| Medication goal | Primary analysis: normalization | Secondary analysis: clinically significant improvement |
|---|---|---|
| LDL | LDL < 100 mg/dl (< 70 for very high risk)* | 1 mmol/dl |
| SBP | SBP < 120 mm Hg | SBP 10 mm Hg reduction |
| Hb A1C | < 7% | 1% reduction |
| Antiplatelet therapy | Pill count documentation of 80% of prescribed medications taken | Pill count documentation of 50% of prescribed medications taken |
Very high risk defined as patients who have established cardiovascular disease plus (1) multiple major risk factors (especially diabetes), (2) severe and poorly controlled risk factors (especially continued cigarette smoking), (3) multiple risk factors of the metabolic syndrome (especially high triglycerides [200 mg/dL] with low HDL cholesterol [40 mg/dL]), and (4) patients with acute coronary syndromes.34
A secondary Aim of the study is to assess the contribution of health status, depression, cognition, socio-economic status, race and other factors to the incidence of barriers and the rate of response to the study interventions. Covariates collected to achieve this aim include measures of disability (Barthel Index, Functional Independence Measure, Lawton Instrumental ADL scale), social participation (Activity Card Sort), and HR-QOL (SF-12 and Stroke Impact Scale). These measures are collected at baseline and at the one year assessment by a blinded rater.
Ethics approval
All hospitals participating in this study have received IRB approval; Georgetown University is the primary study IRB. This trial is registered in clinicaltrials.gov (NCT00703274).
Data quality
Data quality control includes data checks that are built into this data system to ensure the integrity of all data entered for each study participant. These checks include range validator for continuous variables, date and time pickers and comparison validators for consistency checks. In addition, the database contains an audit trail of all data entries and edits with the username and timestamp to monitor data entry and updates.
The web-based system also contains reports of forms status (completed, pending, or missing) and a report of key variables to regularly check for data completeness and accuracy of these data elements.
The second component of the quality control procedure is a 100% data check of the baseline data in addition to a yearly 10% CRF verification audits at each hospital to compare data in the study database to data on CRF documents. The audit includes checks that all participants have signed informed consents, checks for lab procedures and records, verification of measurement tool specifications, and checks that the clinical staff has up to date study documentation available. Data errors are reported to the study team for correction and a data entry error rate is established after each audit.
Adverse event monitoring
Adverse events from this protocol are reported to the IRB. Because navigation is a minimal risk intervention, adverse events are monitored by the study investigators and a formal Data Safety and Monitoring Board was not appointed.
Sample size
Sample size was determined based on a two-sided Wilcoxon rank-sum test to compare the proportion of normal secondary prevention stroke risk measures—a non-normally distributed outcome—in navigation and control groups. To achieve this, a sample size using Student’s t-statistic assuming a 0.05 significance level and 80% power was first calculated, and then adjusted based on the lower bound of the asymptotic relative efficiency of the Wilcoxon rank-sum test relative to the t-test (i.e. inflating the sample size by 15.7%)36. Pilot data was obtained from the PROTECT LA study15 in which the mean and standard deviation of the proportion of risk factor compliance were 0.690 and 0.21 in the intervention group, and 0.585 and 0.27 in the control group. The sample size required is 198. However, a 20% drop out rate was assumed; hence the target for enrollment is 248 participants.
Statistical analyses
Descriptive statistics will be computed for all study variables, including average follow-up time, retention rate, protocol deviations and violations, and will be compared by treatment group. The primary analysis will be performed according to the principle of intention to treat. Given that adaptive randomization was used to assign patients to treatment or control, all outcome analyses will be conducted by regression analyses adjusted by the factors used in the adaptive randomization37, 38. A nominal p value of 0.05 or less will be considered as statistically significant in the primary analysis. Linear, logistic or ordered logistic regression will be used as appropriate depending on the distributions of the outcome measure. Assessment of the fit of the models will be made using residual plots for continuous variables and other measures of goodness of fit such as the Hosmer Lemeshow test for categorical response models.
A sequence of post hoc analysis will be conducted depending on the results of the analyses of the primary outcome. If no statistically significant difference is found between the intervention groups, we will perform post-hoc analyses to elucidate whether the negative finding was likely due to true absence of a treatment effect, or to design factors that resulted in an underpowered study. Careful examination of these issues is critical for considering the feasibility and utility of a future phase III trial of the PROTECT DC intervention. Analyses will also be performed by sub groups, such as by race and age, in order to determine if there is any differential efficacy found among any key subgroups of patients. If statistically significant differences are found between treatment and control arms, we will conduct regression analyses further adjusted by variables selected based on a combination of clinical judgment and descriptive statistical findings comparing baseline characteristics between the control and PROTECT DC intervention groups. These adjustments will help describe the likely pathways by which the treatment influenced outcome. Additional analyses will assess the patient’s adherence to intervention. Similar methods will be used to assess the impact of the PROTECT DC intervention on secondary behavioral goals.
Finally, we will conduct analyses to refine the inclusion/exclusion criteria to identify those who cannot respond to the intervention. This will help in planning a subsequent Phase III trial of the PROTECT DC intervention. Specifically, we will seek to identify baseline barriers such as poorer health status, depression, socio-economic status, and other potential barriers that are associated with less favorable response to the PROTECT DC intervention at one-year follow-up. Any barriers found to be associated with a significantly reduced response to intervention will be further examined at the patient level to ensure that all the possible barriers/factors are considered. Participant-specific barriers will be assessed by the stroke navigators using various sources of information including participants, caregivers, and the navigators themselves. These analyses will be restricted to the intervention group because some barrier interview questions are specifically related to the intervention group.
Summary
Improved secondary stroke prevention may reduce the frequency of subsequent stroke or other vascular events. This reduction is of great importance to society for reducing disability and health care expenditures. Improving access to health care for underserved populations will also reduce rates of illness, disability and poor quality of life. PROTECT DC will determine whether navigation in combination with TRA can improve health behavior and adherence to prevent future strokes.
To date, PROTECT DC has recruited 162 participants across five sites in Washington, DC. Findings will be reported in 2012.
Fig. 1.
Study design
Acknowledgments
Supported by NINDS/NCMHD (U54NS057405)
Study organization and funding
Supported by grants from the National Institute for Neurological Disorders and Stroke (U54 057405 and 3U54NS 057405–02S1). The Clinical Trials identifier is NCT00703274.
Footnotes
Conflict of interest: None declared
References
- 1.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Hankey GJ, Warlow CP. Treatment and secondary prevention of stroke: evidence, costs, and effects on individuals and populations*. Lancet. 1999;354:1457–1463. doi: 10.1016/S0140-6736(99)04407-4. [DOI] [PubMed] [Google Scholar]
- 3.Sacco RL, Boden-Albala B, Gan R, et al. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol. 1998;147:259–68. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- 4.Morgenstern LB, Spears WD, Goff DC, Grotta J, Nichaman MZ. African Americans and women have the highest stroke mortality in Texas. Stroke. 1997;28:15–18. doi: 10.1161/01.str.28.1.15. [DOI] [PubMed] [Google Scholar]
- 5.Shen JJ, Washington EL, Aponte-Soto L. Racial disparities in the pathogenesis and outcomes for patients with ischemic stroke. Manged Care Interface. 2004;17:28–34. [PubMed] [Google Scholar]
- 6.Bian J, Oddone EZ, Samsa GP, Lipscomb J, Matchar DB. Racial differences in survival post cerebral infarction among the elderly. Neurology. 2003;60:285–90. doi: 10.1212/01.wnl.0000041492.58594.4d. [DOI] [PubMed] [Google Scholar]
- 7.Holloway RG, Benesch C, Rush SR. Stroke prevention: narrowing the evidence-practice gap. Neurology. 2000;54:1899–1906. doi: 10.1212/wnl.54.10.1899. [DOI] [PubMed] [Google Scholar]
- 8.Ambriz EH, Woodard LD, Kressin NR, Petersen LA. Use of smoking cessation interventions and aspirin for secondary prevention: are there racial disparities? Am J Med Qual. 2004;19:166–71. doi: 10.1177/106286060401900405. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell JB, Ballard DJ, Matchar DB, Whisnant JP, Samsa GP. Racial variation in treatment for transient ischemic attacks: impact of participation by neurologists. Health Serv Res. 2000;34:1413–28. [PMC free article] [PubMed] [Google Scholar]
- 10.Pratt CA, Ha L, Levine SR, Pratt CB. Stroke knowledge and barriers to stroke prevention among African Americans: implications for health communication. J Health Commun. 2003;8:369–81. doi: 10.1080/10810730305725. [DOI] [PubMed] [Google Scholar]
- 11.Schneider AT, Pancioli AM, Khoury JC, et al. Trends in community knowledge of the warning signs and risk factors for stroke. JAMA. 2003;289:343–6. doi: 10.1001/jama.289.3.343. [DOI] [PubMed] [Google Scholar]
- 12.LaBresh KA, Reeves MJ, Frankel MR, Albright D, Schwamm LH. Hospital Treatment of Patients With Ischemic Stroke or Transient Ischemic Attack Using the “Get With The Guidelines” Program. Arch Intern Med. 2008;168:411–17. doi: 10.1001/archinternmed.2007.101. [DOI] [PubMed] [Google Scholar]
- 13.Bushnell CD, Zimmer LO, Pan W, et al. Persistence With Stroke Prevention Medications 3 Months After Hospitalization. Arch Neurol. 2010;67:1456–63. doi: 10.1001/archneurol.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ovbiagele B, Saver JL, Fredieu A, et al. PROTECT: a coordinated stroke treatment program to prevent recurrent thromboembolic events. Neurology. 2004;63:1217–22. doi: 10.1212/01.wnl.0000140493.83607.f1. [DOI] [PubMed] [Google Scholar]
- 15.Ovbiagele B, Saver JL, Fredieu A, et al. In-hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow-up. Stroke. 2004;35:2879–83. doi: 10.1161/01.STR.0000147967.49567.d6. [DOI] [PubMed] [Google Scholar]
- 16.Rimmer JH, Braunschweig C, Silverman K, Riley B, Creviston T, Nicola T. Effects of a short-term health promotion intervention for a predominantly African-American group of stroke survivors. Am J Prev Med. 2000;18:332–8. doi: 10.1016/s0749-3797(00)00129-x. [DOI] [PubMed] [Google Scholar]
- 17.Allen KR, Hazelett S, Jarjoura D, et al. Effectiveness of a postdischarge care management model for stroke and transient ischemic attack: a randomized trial. J Stroke Cerebrovasc Dis. 2002;11:88–98. doi: 10.1053/jscd.2002.127106. [DOI] [PubMed] [Google Scholar]
- 18.Lemak CH, Johnson C, Goodrick EE. Collaboration to improve services for the uninsured: Exploring the concept of health navigators as interorganizational integrators. Health Care Manage Rev. 2004;29:196–206. doi: 10.1097/00004010-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Health RWJFaCftAo. Essential elements of self-management interventions. Vol. 2001. Seattle, Washington: 2001. [Google Scholar]
- 20.Sarfaty M, Turner CH, Damotta BA. Use of a patient assistant to facilitate visits for Latino patients with low health literacy. J Community Health. 2005;30:299–307. doi: 10.1007/s10900-005-3707-2. [DOI] [PubMed] [Google Scholar]
- 21.Fisher EB, Brownson CA, O’Toole ML, Shetty G, Anwuri VV, Glasgow RE. Ecological Approaches to Self-Management: The Case of Diabetes. Am J Public Health. 2005;95:1523–35. doi: 10.2105/AJPH.2005.066084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Two Feathers J, Kieffer EC, Palmisano G, et al. Racial and Ethnic Approaches to Community Health (REACH) Detroit partnership: improving diabetes-related outcomes among African American and Latino adults. Am J Public Health. 2005;95:1552–60. doi: 10.2105/AJPH.2005.066134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dohan D, Schrag D. Using navigators to improve care of underserved patients. Cancer. 2005;104:848–55. doi: 10.1002/cncr.21214. [DOI] [PubMed] [Google Scholar]
- 24.Fedder DO, Chang RJ, Curry S, Nichols G. The effectiveness of a community health worker outreach program on healthcare utilization of west Baltimore City Medicaid patients with diabetes, with or without hypertension. Ethn Dis. 2003;13:22–7. [PubMed] [Google Scholar]
- 25.Allen JK, Scott LB. Alternative models in the delivery of primary and secondary prevention programs. J Cardiovasc Nurs. 2003;18:150–6. doi: 10.1097/00005082-200304000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Krieger JW, Takaro TK, Song L, Weaver M. The Seattle-King County Healthy Homes Project: A Randomized, Controlled Trial of a Community Health Worker Intervention to Decrease Exposure to Indoor Asthma Triggers. Am J Public Health. 2005;95:652–9. doi: 10.2105/AJPH.2004.042994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams H, Jr, Bendixen B, Kappelle L, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 28.Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65:559–64. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 29.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140:734–9. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 30.Frane JW. A method of biased coin candomization, its Implementation and validation. Drug Inf J. 1998;32:423–32. [Google Scholar]
- 31.Smoak CG, Lin J-S. A SAS program to perform adaptive randomization. Twenty-Sixth Annual SAS Users Group International Conference; 2001; Cary, N.C: SAS Institute, Inc; 2001. Paper 242–26. [Google Scholar]
- 32.Godin G, Kok G. The theory of planned behavior: a review of its applications to health-related behaviors. Am J Health Promot. 1996;11:87–98. doi: 10.4278/0890-1171-11.2.87. [DOI] [PubMed] [Google Scholar]
- 33.Milne S, Sheeran P, Orbell S. Prediction and intervention in health-related behavior: A meta-analytic review of protection motivation theory. Journal of Applied Social Psychology. 2000;30:106–143. [Google Scholar]
- 34.Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- 35.Boudes P. Drug compliance in therapeutic trials: a review. Control Clin Trials. 1998;19:257–68. doi: 10.1016/s0197-2456(98)00005-1. [DOI] [PubMed] [Google Scholar]
- 36.Lehmann E. Nonparametics: Statistical Methods Based on Ranks. Prentice Hall; 1998. [Google Scholar]
- 37.Wier CJ, Lees KR. Comparison of stratification and adaptive methods for treatment allocation in an acute stroke trial. Stat Med. 2003;22:705–26. doi: 10.1002/sim.1366. [DOI] [PubMed] [Google Scholar]
- 38.Forsythe AB. Validity and power of tests when groups have balanaced for prognostic factors. Computational Statistics & Data. 1987;5:193–200. [Google Scholar]