Abstract
Recent evidence suggests that presynaptic-acting NMDA receptors (preNMDARs) are important for neocortical synaptic transmission and plasticity. We found that unique properties of the Nr3a subunit enable preNMDARs to enhance spontaneous and evoked glutamate release and that Nr3a is required for spike timing–dependent long-term depression in the juvenile mouse visual cortex. In the mature cortex, Nr2b-containing preNMDARs enhanced neurotransmission in the absence of magnesium, indicating that presynaptic NMDARs may function under depolarizing conditions throughout life. Our findings indicate that Nr3a relieves preNMDARs from the dual-activation requirement of ligand-binding and depolarization; the developmental removal of Nr3a limits preNMDAR functionality by restoring this associative property.
Introduction
Postnatal modifications in the properties of synaptic plasticity allow the environment to sculpt neocortical networks for optimal processing of sensory information1,2. To ensure greater synaptic stability after maturation, some forms of synaptic plasticity are restricted to early life. This is exemplified by the developmental reduction in the expression of long-term depression (LTD) and in the increased threshold for sensory cortices to compensate for deprivation of a sensory input1,3–5. Although orchestrated shifts in many proteins determine the features of synaptic signaling and plasticity, changes in neurotransmitter receptors may be particularly important, since they shape the initial synaptic response. For example, experience-driven changes in postsynaptic NMDA receptor (NMDAR) subunit composition are known to shift the threshold of neuronal activity required to modify glutamatergic synaptic strength2.
NMDARs are crucial for many types of learning and memory, and their dysfunction contributes to a large variety of neurological disorders, including schizophrenia, epilepsy, and pain6,7. Although most research has assumed that these receptors act postsynaptically, presynaptic-acting NMDARs (preNMDARs) may provide a powerful complement to their postsynaptic-acting counterparts8,9. PreNMDARs in the neocortex acutely enhance neurotransmitter release4,10–13, but under certain circumstances their activation can lead to LTD of neurotransmitter release4,5,10,12,14. To date, little is known about the functional mechanisms and developmental regulation of preNMDARs. Interestingly, preNMDARs influence spontaneous release in the absence of strong depolarization. Tonic activation is a unique feature of preNMDARs, as most postsynaptic NMDARs are blocked by magnesium (Mg2+), and therefore require depolarization in conjunction with glutamate binding to become fully active. In the primary visual cortex (V1), preNMDARs are tonically active during early development, but this tonic function is lost by the third postnatal week in mice4. The loss of tonic preNMDAR function coincides with a reduction in the ability to induce spike timing-dependent LTD (tLTD) at layer (L) 2/3 neocortical synapses4,5, a form of plasticity known to rely on preNMDARs4,10,12,14. What underlies the developmental loss in the ability of preNMDARs to tonically enhance glutamate release and to promote tLTD is unknown.
To determine how preNMDARs contribute to neurotransmitter release and synaptic plasticity, we investigated the molecular composition of preNMDARs, and how this influences the conditions under which these receptors function. We found that the developmental downregulation of Nr3a subunits, which impart Mg2+-insensitivity to NMDARs, correlates with the loss of tonic preNMDAR activity. We also found that Nr3a-containing preNMDARs are required both for the ability of preNMDARs to enhance glutamate release and for the induction of tLTD in the juvenile visual cortex. These observations support a previously unappreciated role for Nr3a-containing NMDARs in regulating presynaptic neurotransmitter release and plasticity during a formative period of neocortical development.
Results
Nr3a downregulation coincides with the loss of preNMDARs
We first examined whether shifts in the subunit composition of synaptic NMDARs could explain the observed loss of tonic preNMDAR function late in development. NMDARs are tetramers composed of two obligatory NR1 subunits and two other subunits, either Nr2a-d or Nr3a-d (GluN1-GluN3B)7. Since the loss of the obligatory NMDAR subunit NR1 from the presynaptic terminal could explain the loss of tonic preNMDAR function, which occurs between postnatal day 14 (P14) and P26 in mice4, we first investigated changes in presynaptic NR1 expression through development, using immunogold labeling of NR1. Similar to our previous findings using immunoperoxidase labeling4, we observed a decrease in the expression of NR1 located less than 20 nm from the presynaptic membrane between P14 and P26, but no change in postsynaptic NR1 (Fig. 1a–f). However, despite the developmental downregulation of presynaptic NR1, about 20% of NR1-labeled synapses still contained presynaptic labeling at P26 (Fig. 1e). This finding suggested that the developmental loss in tonic preNMDAR function may be influenced by other NMDAR subunits.
Figure 1.
Both the presynaptic localization of NR1 and the biochemical expression of Mg2+-insensitive NMDAR subunits decrease during early visual cortex development. Electron micrographs demonstrating immunogold localization of NR1 at both presynaptic (pre) and postsynaptic (post) sites in L2/3 of mouse primary visual cortex (V1) at P14 (a,b) and at P26 (c,d). Scale bars indicate 200 nm. (e) While the percentage of synapses containing NR1 immunogold label within the postsynaptic density (PSD) did not change with development (P14: 31 ± 3.6%, n = 3 mice; P26: 35 ± 7.1%, n = 3 mice; >70 synapses/animal were analyzed; P < 0.68), the percentage of synapses positive for NR1 within the presynaptic active zone decreased significantly (P14: 33 ± 2.1% n = 3; P26: 21 ± 2.6%, n = 3, P < 0.03). Despite the reduction, presynaptic NR1 labeling persisted at P26. (f) Open circles represent the ratio of pre- to postsynaptic NR1 labeling per animal; closed circles represent the average of three animals at P14 and P26. (g–k) Quantification and representative immunoblots for NMDAR subunits in synaptosomal fractions from the developing visual cortex. Levels of Nr2d and Nr3a, which confer magnesium insensitivity to NMDARs, decrease during development. Protein levels were normalized to β-actin and presented as percent of maximum expression. Sample sizes were 2–5 for each data point. Each visual cortex sample was pooled from 2–4 mice. For larger blot areas see Supplementary Figure 1. Error bars represent s.e.m. * P < 0.05.
We hypothesized that the NMDAR subunits involved in the developmental regulation of preNMDARs would have properties distinct from their postsynaptic counterparts. Surprisingly, and despite the characteristic voltage-dependence of most NMDARs, neocortical preNMDARs are tonically active in the absence of depolarization4,10–13,15. Because inclusion of the Nr2c/d or Nr3a/b subunits dramatically decreases the receptor’s Mg2+ sensitivity7,16, we wondered whether this could explain the tonic activity of preNMDARs. To determine which subunits have a developmental profile matching that of functional preNMDARs, we quantified protein expression of candidate NMDAR subunits during V1 development (Fig. 1g–k and Supplementary Fig. 1). Similar to the obligatory NR1 subunit (Fig. 1g), protein levels of Nr2a and Nr2b subunits increase with age in V1 (Fig. 1h–i). Temporal and regional expression of Nr2c17 and Nr3b18 suggest that they do not contribute to preNMDAR functions in the visual cortex. Nr2d expression levels are extremely low in V1 compared to the brainstem (Supplementary Fig. 1), and there was no main effect of age on Nr2d expression (ANOVA, P = 0.15), despite a trend suggesting that Nr2d expression levels peak at P16 and become lower in adulthood (Fig. 1j). In contrast, the expression of Nr3a is high early in development and declines dramatically after the third postnatal week (ANOVA group effect, P < 0.001, Fig. 1k), consistent with previous observations16,19,20. Thus, the developmental decreases in Nr3a expression parallels the loss of preNMDAR function observed by the third postnatal week4. These findings raised the possibility that Nr3a- or perhaps Nr2d-containing NMDARs might underlie the tonic activity of preNMDARs.
Nr2b is required for tonic preNMDAR activity
Next, we used genetic and pharmacological approaches to determine which subunits are required for preNMDAR function. To detect the tonic activity of preNMDARs, we examined the effect of the NMDAR antagonist d-AP5 (50 µM) on the frequency and amplitude of miniature excitatory postsynaptic currents (mEPSCs) while postsynaptic NMDARs were blocked by both hyperpolarization and the inclusion of the NMDAR antagonist MK-801 in the recording pipette4,11–14. In L2/3 pyramidal neurons from V1 of P13–18 (juvenile) mice, d-AP5 decreased the frequency but not the amplitude of AMPA receptor (AMPAR)-mediated mEPSCs in wildtype mice (Fig. 2a–d and Supplementary Fig. 2), indicating that preNMDARs are tonically active at this age. We first examined the involvement of NR2 subunits in tonic preNMDAR function. Compared to NR3 subunits, NR2 subunits have a high affinity for glutamate21 and therefore are presumably involved in the response of preNMDARs to NMDA or AP5 application11,22. In Nr2a−/− mice, d-AP5 decreased mEPSC frequency but not amplitude (Fig. 2c–d and Supplementary Fig. 2), demonstrating that Nr2a does not significantly contribute to tonic preNMDAR activity in juvenile V1.
Figure 2.
Glutamate-sensitive preNMDARs containing Nr2b, but not Nr2a or Nr2d, enhance spontaneous neurotransmitter release onto juvenile L2/3 pyramidal neurons. (a) Sample traces showing AMPAR-mediated mEPSCs. Recordings were made during blockade of postsynaptic NMDARs in a L2/3 pyramidal neuron in V1 of a P14 wildtype (WT) mouse before and after bath-applied d-AP5. (b) Cumulative probability histograms from the cell in a show a decrease in mEPSC frequency, but not amplitude, with d-AP5 application. (c) Neither d-AP5 nor the Nr2b-selective antagonist Ro 25-6981 significantly altered mEPSC amplitude in V1 mEPSCs recordings (P > 0.08, D-AP5 in Nr2a wild-type littermates; P > 0.1, D-AP5 in Nr2d wild-type littermates; P > 0.1, Ro 25-6981 in wild-type mice). (d) L2/3 pyramidal cells exhibited a significant reduction in mEPSC frequency in response to d-AP5 application in both Nr2a−/− mice and their wildtype controls (Nr2a−/−, n = 7, P < 0.04; WT, n = 7, P < 0.03). Similarly, d-AP5 reduced the frequency, but not amplitude, in both Nr2d−/− mice and their wildtype controls (WT, n = 5, P < 0.05; Nr2d−/−, n = 6, P < 0.007). Ro 25–6981 (0.5 µM) significantly reduced mEPSC frequency in wildtype mice compared to vehicle controls (controls n = 6, Ro 25–6981 n = 7; P < 0.02). Individual data points are normalized to their respective baseline mEPSC frequencies to allow for the comparison of the effect of d-AP5 across genotypes and conditions. Error bars represent s.e.m. * P < 0.05
To investigate the potential involvement of Nr2d in preNMDAR tonic activity, we measured changes in mEPSCs in response to application of the moderately subunit-selective Nr2c/d antagonist UBP141 (3 µM)23. Unexpectedly, UBP141 decreased the amplitude of spontaneous and evoked AMPAR-currents (Supplementary Fig. 3), suggesting that that UBP141 modulates postsynaptic AMPAR-mediated responses. The effect of UBP141 on AMPAR currents may be direct, but might instead be indirect, since UBP141 has previously been shown to inhibit AMPA receptors only moderately at high concentrations (100 µM)23. This effect of UBP141 on AMPAR mEPSC amplitude precluded us from interpreting its effects on mEPSC frequency. As an alternative and more direct approach, we measured the effects of d-AP5 on mEPSC frequency and amplitude in Nr2d−/− mice to assay this subunit’s contribution to preNMDAR function. Similar to the effect in Nr2a−/− mice, d-AP5 reduced mEPSC frequency, but not amplitude, in mice lacking Nr2d, suggesting that this subunit is not required for tonic preNMDAR activity (Fig. 2c–d and Supplementary Fig. 4). Because previous studies have localized Nr2b to presynaptic terminals in the mature cortex24,25, we next investigated the role of Nr2b subunits in tonic preNMDAR activity. The activity-dependent Nr2b-selective antagonist Ro 25–6981 (0.5 µM) mimicked the effects of d-AP5 by decreasing mEPSC frequency without affecting amplitude (Fig. 2c–d and Supplementary Fig. 5). Another Nr2b-selective antagonist, ifenprodil (3 µM), also decreased mEPSC frequency, but not amplitude, in a manner similar to Ro 25–6981 (Supplementary Fig. 6). Consistent with previous observations11,15,26, our findings indicate that tonically-active preNMDARs contain Nr2b.
Nr3a-containing preNMDARs modulate neurotransmitter release
The involvement of Nr2b in preNMDAR activity explains how these receptors can be activated by low concentrations of glutamate22, since Nr2b has a high affinity for glutamate21, but it does not explain the developmental regulation of preNMDARs, nor their tonic activity in the absence of depolarization. We therefore determined the effect of d-AP5 application on mEPSC frequency in mice lacking Nr3a. Remarkably, the effect of d-AP5 on mEPSC frequency observed in wildtype controls was completely abolished in L2/3 pyramidal neurons from Nr3a−/− mice, without affecting mEPSC amplitude (Fig. 3a–b and Supplementary Fig. 7). This finding indicates Nr3a is required for tonic function of preNMDARs in the juvenile visual cortex.
Figure 3.
The reduced Mg2+-sensitivity of Nr3a-containing preNMDARs promotes spontaneous neurotransmitter release in juvenile V1. (a) Normalized and averaged mEPSC amplitudes confirm that d-AP5 did not affect postsynaptic AMPAR-mediated responses in L2/3 pyramidal neurons from juvenile (P13–P18) wildtype and Nr3a−/− mice. d-AP5 application also did not affect mEPSC amplitude in older (P25–P28) double-transgenic mice that overexpress (OE) Nr3a nor in single-transgenic (STg) control mice expressing only one of the two transgenes necessary for Nr3a overexpression (tet-O-GFPNr3a or CaMKII-tTA transgenes). (b) Normalized mEPSC frequency showing effects of d-AP5 in P13–P18 Nr3a−/− mice (WT n = 8, Nr3a−/− n = 10; P < 0.03), P25–28 OE mice (STg n = 7, OE n = 10; P < 0.0009), and their appropriate littermate controls. (c) Neither d-AP5 nor Ro 25–6981 significantly altered mEPSC amplitude recorded in low Mg2+ solutions in either young (P13–18) Nr3a−/− mice or in older (P25–28) wildtype mice (D-AP5 in Nr3a, P >; 0.13; D-AP5 in P25–28 wild-type mice, P > 0.08; Ro 25-6981 in P25–28 wildtype mice, P > 0.96). (d) In low Mg2+ solutions, d-AP5 reduced mEPSC frequency in both young (P13–18) Nr3a−/− mice (n = 12, P < 0.05) and in older (P25–28) wildtype mice, compared to vehicle controls (n = 9, P < 0.05). Similar to d-AP5, 0.5 µM Ro 25–6981 reduced the mEPSC frequency in older mice in low Mg2+ conditions as compared to vehicle controls (controls, n = 6, Ro 25–6981, n = 7; P < 0.03). (e–h) Electron micrographs demonstrating immunoperoxidase labeling of Nr3a over presynaptic (e–f), postsynaptic (g), and putative dendritic (dend) (h) profiles in the primary visual cortex at P16. Scale bars indicate 0.5 µm in (e-g) and 1 µm in (h). Error bars represent s.e.m. * P < 0.05 and *** P < 0.001.
Since developmental changes in Nr3a expression correlate with changes in tonic preNMDAR functionality (Fig. 1), we hypothesized that a developmental switch in subunit composition, from Nr3a-containing to Nr3a-lacking, might block the tonic function of preNMDARs in more mature neocortex. We therefore tested whether maintaining Nr3a expression later in development19,20 – at an age when preNMDARs have little spontaneous activity4 – enhances tonic preNMDAR activity. To extend the time course of Nr3a expression, we crossed mice expressing Nr3a tagged with EGFP (GFPNr3a) under the control of the tetO promoter to mice expressing the tetracycline-controlled transactivator (tTA) under the CaMKIIα promoter20,27. This system allows overexpression of Nr3a specifically in excitatory neurons in double-transgenic, but not single-transgenic, progeny20,27. In double-transgenic mice (tetO–GFP-Nr3a; Camk2a-tTA), we first observed Nr3a overexpression in excitatory V1 neurons starting at ~P20, when the loss of tonic preNMDAR activity begins4 (Supplementary Fig. 8). In recordings from L2/3 pyramidal cells, we found that the decrease in mEPSC frequency with d-AP5 application was much larger in older (P26–P30) double-transgenic mice overexpressing Nr3a, compared to their single transgenic (STg, tTA or GFPNr3a only) controls (Fig. 1b and Supplementary Fig. 8). Moreover, the overexpression of Nr3a increased the baseline mEPSC frequency prior to d-AP5 application in Nr3a overexpressing mice as compared to STg mice, though this effect did not reach statistical significance (P < 0.07, Supplementary Fig. 8). Thus, genetically increasing Nr3a expression is sufficient to enhance tonic preNMDAR activity in the mature visual cortex.
The developmental removal of Nr3a might limit preNMDAR functions by causing the receptor to gain Mg2+ sensitivity. To test whether Mg2+ normally blocks tonic preNMDAR activity in L2/3 pyramidal neurons from juvenile (P13–18) Nr3a−/− mice, or in older (P25–P28) wildtype mice, we recorded the effect of d-AP5 on mEPSC frequency in ACSF containing only trace amounts of Mg2+. These low Mg2+ conditions revealed functional preNMDARs that were previously masked, as d-AP5 reduced mEPSC frequency in both juvenile Nr3a−/− mice and older wildtype mice (Fig. 3d and Supplementary Figs. 9–10), without altering mEPSC amplitude (Fig. 3c). We found that tonically-active preNMDARs in older wildtype mice contained Nr2b subunits, as Ro 25–6981 mimicked d-AP5 in reducing the mEPSC frequency in the absence of Mg2+ (Fig. 3d). These findings demonstrate that, despite the developmental loss of Nr3a-containing NMDARs in wildtype mice, Mg2+-sensitive Nr2b-containing preNMDARs can continue to influence neurotransmission under certain circumstances. Therefore, preNMDARs in the mature cortex may require simultaneous depolarization-induced removal of Mg2+ and glutamate binding to modulate glutamate release.
A parsimonious explanation for how preNMDARs modulate glutamate release is that Nr3a-containing NMDARs are localized near the presynaptic release machinery. To determine whether Nr3a is expressed at excitatory presynaptic terminals, we performed electron microscopy for Nr3a in juvenile (P16) V1. Postembedding methods provide optimal quantitative data, but are rather insensitive and potentially noisy; to optimize signal detection, we therefore performed pre-embedding immunoperoxidase electron microscopy. While the majority of Nr3a labeling was postsynaptic (Fig. 3g–h), we also observed labeling of Nr3a near presynaptic active zones, where Nr3a would be well-positioned to affect neurotransmitter release early in V1 development (Fig. 3e–f). This signal was specific for Nr3a, because accumulations of reaction product were not detected in comparable material from Nr3a−/− mice. Coupled with previous findings suggesting that Nr3a labeling is absent from presynaptic terminals in the adult rodent brain19,28, our findings suggest that Nr3a-containing preNMDARs are selectively expressed only early in the juvenile visual cortex, where they promote tonic preNMDAR functionality.
Nr3a-containing preNMDARs modulate evoked release
In addition to their role in tonic transmitter release, preNMDARs enhance evoked neurotransmitter release early in development10,11. Synaptic responses evoked by L4 stimulation and recorded in L2/3 undergo a developmental shift, from paired-pulse depression in the juvenile visual cortex to paired-pulse facilitation, at a time when Nr3a is downregulated and preNMDARs are no longer tonically active (>P28)29. To test for a role of Nr3a in evoked transmitter release, we analyzed the paired-pulse ratio (PPR) in V1 L2/3 synapses before and after d-AP5 application in Nr3a−/− mice and their wildtype controls (P13–18). Postsynaptic NMDARs were blocked by loading the NMDAR antagonist MK-801 in the postsynaptic recording pipette, and paired-pulse stimuli were delivered to L4 while recording EPSPs in L2/3 pyramidal neurons. In wildtype mice, d-AP5 application increased the PPR from depressing to facilitating responses and decreased the amplitude of the first EPSP (Fig. 4a–c), suggesting that preNMDARs enhance evoked transmitter release. In contrast, Nr3a−/− mice had facilitating baseline responses, and d-AP5 had no effect on either the PPR or EPSP amplitude (Fig. 4a–c). Thus, Nr3a-containing NMDARs enhance evoked neurotransmitter release early in V1 development.
Figure 4.
Nr3a-containing preNMDARs enhance evoked neurotransmitter release at L2/3 visual cortical synapses. (a) Representative traces of EPSPs evoked by a 30-Hz pair of stimuli in L4 before and after d-AP5 application from wildtype and Nr3a−/− mice. (b) Paired-pulse ratio (PPR) of EPSPs increased after d-AP5 application in the wildtype but not Nr3a−/− mice (WT n = 14, Nr3a−/− n = 12; P < 0.05). (c) The change in PPR was primarily due to a decrease in the amplitude of the first EPSP in the pair in wildtype mice (P < 0.001). Error bars represent s.e.m. * P < 0.05 and *** P < 0.001.
tLTD requires Nr2b- and Nr3a-containing preNMDARs
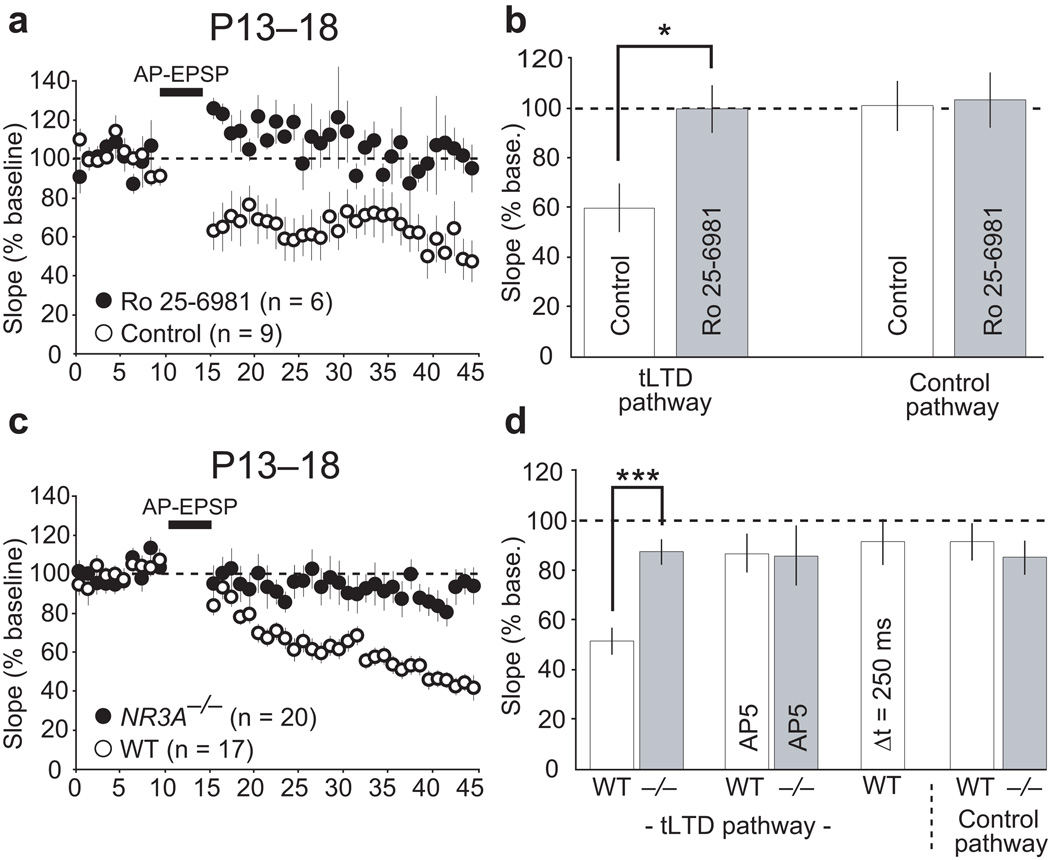
Spike timing-dependent LTD (tLTD), a plausible mechanism for cortical map refinement30, is associated with presynaptic changes in neurotransmitter release that rely on the activation of preNMDARs during early5,10,12,14 —but not later— cortical development4,5. We thus tested whether Nr2b- and Nr3a-containing NMDARs participate in tLTD induction, as they do in tonic preNMDAR functions. To induce tLTD at L2/3 V1 synapses, we monitored L2/3 EPSPs evoked in L4 before and after inducing tLTD with a L4 post-before-presynaptic induction protocol (tLTD pathway). In some experiments, we simultaneously monitored EPSPs evoked in L2/3 which did not receive the tLTD induction protocol (control pathway). In all experiments, postsynaptic NMDARs were blocked by including MK-801 in the recording pipette. In wildtype mice, post-before-pre pairing induced robust tLTD (Fig. 5a). The reduction in EPSP slope was specific to synapses undergoing EPSP–AP pairings, as there was no change in EPSP slope in control pathways which did not undergo EPSP–AP pairings (Fig. 5b). To test for a role of Nr2b-containing receptors in tLTD induction, we examined tLTD induction when the Nr2b-selective antagonist Ro 25–6981 (0.5 µM) was included in the extracellular recording solution. Similar to previous findings in V110, blockade of Nr2b-containing receptors abolished tLTD in wildtype mice without significantly affecting EPSPs evoked in the control L2/3 pathway (Fig. 5a–b). In contrast, Nr2d was not required for tLTD, since Nr2d−/− mice lacking this subunit demonstrated significant synaptic depression following EPSP–AP pairings (Supplemental Fig. 11). Therefore, tLTD induced in V1 L2/3 pyramidal neurons by L4 activation requires Nr2b-containing, but not Nr2d-containing, preNMDARs.
Figure 5.
Nr2b- and Nr3a-containing preNMDARs promote spike timing-dependent long-term depression (tLTD) in juvenile V1. (a) tLTD was induced in L2/3 V1 synapses in response to action potentials (APs) paired with L4-generated EPSPs in wildtype mice (n = 9), but not when the Nr2b-selective antagonist Ro 25–6981 (0.5 µM) was included in the bath (n = 6; P < 0.02). (b) Quantification of the last 10 minutes of the averages shown in a. Quantified data are also shown from a control pathway not receiving AP–EPSP pairings, performed in a subset of experiments, to demonstrate the synapse specificity of tLTD (control, n = 9; Ro 25–6981, n = 6). (c) Robust tLTD was induced in L2/3 synapses of juvenile V1 in wildtype (n = 20), but not Nr3a−/− mice (n = 17), with AP-EPSP pairing. (d) Quantification of the last 10 minutes in c demonstrating Nr3a−/− mice have significantly reduced tLTD magnitude, compared to wildtype mice (P < 0.001). Control AP-EPSP pairing experiments in which either d-AP5 was present throughout the recording session (WT, n = 5; Nr3a−/−, n = 5) or the time between AP–EPSP pairing was increased to 250 ms (n = 7). These control experiments demonstrate tLTD observed in wildtype mice was dependent on NMDARs and the temporal precision of AP–EPSP pairings. Moreover, control pathways not receiving the AP–EPSP pairing also failed to exhibit significant depression (WT, n = 8; Nr3a−/−, n = 9). Error bars represent s.e.m. * P < 0.05 and *** P < 0.001.
Because the developmental switch in the properties of tLTD correlates with the down-regulation of Nr3a and the loss of tonic preNMDAR functions (Fig. 1 and refs 4,19), we tested whether Nr3a is required to induce tLTD at the L4–2/3 synapse in juvenile V1. As expected, wildtype littermate mice demonstrated robust tLTD (Fig. 5c–d) with a magnitude similar to that observed in previous studies where postsynaptic NMDARs were blocked4,12. This tLTD required preNMDAR activation, since including d-AP5 in the bath abolished tLTD in wildtype mice (Fig. 5d). Additionally, tLTD in wildtype mice was timing-dependent, because synaptic depression was not observed when the timing interval between paired action potentials and EPSPs was increased to 250 ms (Fig. 5d), consistent with previous results30,31. To determine if tLTD induction requires Nr3a-containing NMDARs, we examined the effects of EPSP–AP pairings in Nr3a−/− mice and their wildtype controls. In contrast to wildtype mice, no significant tLTD was induced in Nr3a−/− mice (Fig. 5c–d). Therefore, Nr3a is required for the induction of preNMDAR-dependent tLTD in L2/3 pyramidal cells of the visual cortex.
Discussion
Our findings demonstrate a critical and previously unrecognized role for Nr3a-containing NMDARs in enhancing neurotransmitter release and mediating temporally restricted forms of synaptic plasticity in the juvenile visual cortex. We found that Nr3a- and Nr2b-containing NMDARs promote glutamate release at L2/3 synapses in the developing visual cortex. The finding that the loss and gain of Nr3a function have opposing effects on preNMDAR-mediated tonic neurotransmitter release suggests that this NMDAR subunit is critical in modulating release at L2/3 visual cortical pyramidal neurons. Interestingly, both Nr3a- and Nr2b-containing preNMDARs are also required for a timing-dependent form of LTD, which is expressed as a reduction of glutamate release from presynaptic neurons4,10,12,14. Nr3a-containing preNMDARs may therefore promote two functions in the developing visual cortex: to help maintain a necessary high probability of glutamate release acutely, and to weaken synaptic communication in response to uncoordinated synaptic activity30,32. Our results demonstrate that Nr3a-containing preNMDARs are active in the visual cortex just following eye opening in mice (~P13), at a time that corresponds with the development of early receptive field properties in vivo33. Therefore, Nr3a-containing preNMDARs may modulate the formation of early receptive fields by promoting timing-dependent synaptic weakening in response to early visual information.
Subunit composition of preNMDARs through development
What is the heteromeric subunit composition of preNMDARs? While the Nr2b-selective antagonists used in this study would be predicted to block heterotrimeric receptors less effectively than receptors containing NR1–Nr2b alone34 (but see ref. 35), we speculate that during early development these receptors are triheteromeric NMDARs containing NR1, Nr2b, and Nr3a. Triheteromeric Nr3a-containing NMDARs have been suggested to exist in vivo, as Nr3a coimmunoprecipates with both Nr2b and NR1 in the rodent brain36,37. In support of the presence of triheteromeric preNMDARs, we have demonstrated that Nr3a is required for tonic preNMDAR activity in the presence of Mg2+, and that the activity-dependent Nr2b antagonists Ro 25-6981and ifenprodil block preNMDAR activity as effectively as the non-selective NMDAR antagonist d-AP5. Moreover, NR1–Nr3a diheteromeric preNMDARs lacking NR2 are unable to bind glutamate38 and function as excitatory glycine receptors, which have been found on myelin39 but not on neurons36,37,40. Further evidence against a role for NR1–Nr3a diheteromeric preNMDARs is provided by the observation that preNMDARs are activated by NMDA and blocked by AP54,11, which both act on the glutamate binding site22. Therefore, our results raise the possibility that triheteromeric NR1–Nr2b–Nr3a receptors function at excitatory synapses in the juvenile visual cortex, where they promote glutamate release and tLTD.
In contrast to preNMDAR roles in the juvenile neocortex, the activity of Nr3a-lacking preNMDARs in older neocortex is tightly regulated by voltage-sensitive blockade by magnesium. Nr2b-containing, Nr3a-lacking preNMDARs in the mature cortex are not tonically active, but may become active in strongly depolarizing conditions, such as during high frequency bursts, which presumably remove the magnesium block. Thus, Nr2b-containing preNMDARs expressed in the mature neocortex may promote the facilitation of repetitive stimuli, which predominate in the mature visual cortex29.
While this study is the first to test the role of Nr3a in preNMDARs, previous studies have implicated other NR2 subunits in preNMDAR functions. Our finding that Nr2b is required for tonic preNMDAR activity and tLTD is in agreement with the majority of functional and anatomical studies of preNMDARs10,11,15,24–26,41,42 (but see ref. 43).
However, a recent study in mouse somatosensory cortex also demonstrated a potential role for Nr2c/d-containing receptors in tLTD between L4 and L2/3 synapses5, which differs from the results reported here. This apparent discrepancy may be due to regional (somatosensory versus visual cortices) and/or pathway-specific differences in the expression and function of preNMDARs. Segregation of preNMDAR subunits is supported by several findings that suggest that preNMDARs might influence neurotransmitter release differently based on the anatomical region examined9. For example, Nr2b-containing preNMDARs expressed at hippocampal boutons42 might lack Nr3a since preNMDARs at CA1 synapses are not tonically active43, are Mg2+ sensitive42, and because overexpression of Nr3a does not alter the paired pulse ratio20. A similar segregation of preNMDAR subunits based on anatomical region may exist between somatosensory and visual cortices. Additionally, in the somatosensory cortex, the NMDAR-subunit dependence of preNMDARs in the L4–L2/3 pathway is different from that in the L2/3–L2/3 pathway5,11. With these possibilities in mind, we attempted to corroborate the suggestion that preNMDARs contained Nr2c/d8 using UBP141, as previously reported5. We found that commonly employed concentrations of UBP141 reduced AMPAR responses in the visual cortex, suggesting that UBP141 lacks the specificity needed to assess the role of Nr2c or Nr2d in the tonic activity of preNMDARs (Supplementary Fig. 5). However, by using Nr2d−/− mice, we found that Nr2d is not required for tLTD or tonic preNMDAR function in the visual cortex. Nevertheless, we cannot exclude the possibility that Nr2c or Nr2d contribute to the modulation of glutamate release by preNMDARs in certain pathways, regions, or developmental stages.
Subcellular localization of preNMDARs
Nr3a-containing NMDARs can influence neurotransmitter release and presynaptically-expressed forms of synaptic plasticity, but remains unclear where these receptors are localized. Our finding that the NMDAR subunits NR1 and Nr3a can be found at presynaptic terminals raises the possibility that NMDARs may promote glutamate release and tLTD by direct effects at synaptic boutons. However, an important caveat of our studies, and others, is that the precise location of functionally relevant preNMDARs has not been definitively determined. Studies attempting to identify functional preNMDARs in axons have produced variable results. While studies attempting to localize preNMDARs to cerebellar stellate cells and L5 cortical neurons axons have failed to observe NMDAR-mediated axonal calcium influx or depolarization44,45, a study of cortical neurons observed both phenomena in presynaptic boutons46. Furthermore, activation of NMDARs at hippocampal Schaffer collateral boutons selectively enhanced large Ca2+ transients, which themselves underlied increases in the probability of neurotransmitter release42. What might account for the apparent discrepancy between studies? One possibility is that there may be anatomical region differences in the localization of preNMDARs. Alternatively, preNMDAR activation may only contribute to a subpopulation of Ca2+ transients observed at axons. This is supported by the finding that preNMDARs at Schaffer collaterals contribute to only a portion of large action potential-generated calcium influxes, and only at axon boutons, not at axon collaterals42. Another possibility is that the localization of Nr3a-containing NMDARs may be difficult to detect by traditional electrophysiological or calcium imaging methods. Nr3a-containing NMDARs have lower conductances40, rapidly desensitize in the presence of d-serine or glycine38,39, and are 5–10 times less permeable to calcium than NR2-containing NMDARs16. Due to the abundance of functional studies implicating preNMDARs in modulating neurotransmitter release8,9, future studies that both localize active preNMDARs and simultaneously measure their effects on neurotransmitter release are warranted.
In summary, our data provide evidence for a unique role of Nr3a-containing preNMDARs in visual cortical development. Our findings also provide an explanation for how preNMDARs may be tonically active during early development but not later in life. Finally, we suggest that the developmental downregulation of Nr3a may limit the normal ability to induce tLTD at L2/3 neocortical synapses. These findings add a new dimension to our understanding of how NMDAR-subunit composition influences cortical developmental processes that occur in response to visual activity.
Supplementary Material
Acknowledgements
We thank Kris Phend and Susan Burette for processing electron microscopic materials and Portia McCoy and Thorfinn Riday for preparing fixed tissue for this process. We also thank Jayalakshmi Miriyala, Janet Berrios, and Kimberly Williams for mouse colony maintenance and genotyping. We thank D.J. Brasier, Tom Kash, and Deric Corlew for early critical readings of the manuscript and Randy Chitwood for technical assistance. This work was supported by NARSAD, Marie Curie International Program, UTE project CIMA, and Spanish Ministry of Education and Science grants SAF2006-10025, CSD2008-00005 (to I.P.O), an NRSA predoctoral fellowship F31 GM080162 (to R.J.C.), a UNC dissertation completion fellowship (to M.A.H.), a NICHD training grant T32 HD40127 and NARSAD Fellowships (to A.C.R.), NIH grant RO1 NS39444 (to R.J.W.), NARSAD, NEI R01 EY018323, and National Science Foundation grant 0822969 (to B.D.P.), and P01 HD29587, R01 EY05477, and R01 EY09024 (to S.A.L.).
Footnotes
Author Contributions
R.S.L., R.J.C., R.J.W, and B.D.P. designed the study. R.S.L. performed whole cell recordings of mEPSCs, evoked release, and tLTD in all genotypes and conditions and wrote the manuscript. R.J.C. performed the majority of mEPSC recordings in low Mg2+, analyzed immunogold NR1 labeling, contributed to mEPSC recordings in Nr3a−/− and OE mice, and helped prepare figures and the manuscript. M.A.H. performed all biochemical fractionation and quantitative immunoblotting. A.C.R. performed recordings of evoked responses in the presence of UBP141 and contributed to mEPSC recordings in OE mice. M.M. provided Nr2d−/− mice. M.W. designed and provided the anti-Nr3a antibody used for immunoperoxidase labeling. S.A.L. and N.N. provided Nr3a−/− mice, edited the manuscript, and provided experimental suggestions. I.P-O. provided Nr3a OE mice and performed immunohistochemistry on these mice. R.J.W. helped prepare the manuscript and performed immunogold and immunoperoxidase labeling for NR1 and Nr3a. B.D.P. supervised the entire study and edited the manuscript.
References
- 1.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 2.Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 3.Dudek SM, Bear MF. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci. 1993;13:2910–2918. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corlew R, Wang Y, Ghermazien H, Erisir A, Philpot BD. Developmental switch in the contribution of presynaptic and postsynaptic NMDA receptors to long-term depression. J Neurosci. 2007;27:9835–9845. doi: 10.1523/JNEUROSCI.5494-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee A, et al. Double dissociation of spike timing-dependent potentiation and depression by subunit-preferring NMDA receptor antagonists in mouse barrel cortex. Cereb Cortex. 2009;19:2959–2969. doi: 10.1093/cercor/bhp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemp JA, McKernan RM. NMDA receptor pathways as drug targets. Nat Neurosci. 2002;5(Suppl):1039–1042. doi: 10.1038/nn936. [DOI] [PubMed] [Google Scholar]
- 7.Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez-Moreno A, Banerjee A, Paulsen O. Presynaptic NMDA receptors and spike timing-dependent long-term depression at cortical synapses. Frontiers in Synaptic Neuroscience. 2010;2:12. doi: 10.3389/fnsyn.2010.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corlew R, Brasier DJ, Feldman DE, Philpot BD. Presynaptic NMDA receptors: newly appreciated roles in cortical synaptic function and plasticity. Neuroscientist. 2008;14:609–625. doi: 10.1177/1073858408322675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sjöström PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 11.Brasier DJ, Feldman DE. Synapse-specific expression of functional presynaptic NMDA receptors in rat somatosensory cortex. J Neurosci. 2008;28:2199–2211. doi: 10.1523/JNEUROSCI.3915-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J Neurosci. 2006;26:4166–4177. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berretta N, Jones RS. Tonic facilitation of glutamate release by presynaptic N-methyl-D-aspartate autoreceptors in the entorhinal cortex. Neuroscience. 1996;75:339–344. doi: 10.1016/0306-4522(96)00301-6. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez-Moreno A, Paulsen O. Spike timing-dependent long-term depression requires presynaptic NMDA receptors. Nat Neurosci. 2008;11:744–745. doi: 10.1038/nn.2125. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Woodhall GL, Jones RS. Tonic facilitation of glutamate release by presynaptic Nr2b-containing NMDA receptors is increased in the entorhinal cortex of chronically epileptic rats. J Neurosci. 2006;26:406–410. doi: 10.1523/JNEUROSCI.4413-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henson MA, Roberts AC, Perez-Otano I, Philpot BD. Influence of the Nr3a subunit on NMDA receptor functions. Prog Neurobiol. 2010;91:23–37. doi: 10.1016/j.pneurobio.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karavanova I, Vasudevan K, Cheng J, Buonanno A. Novel regional and developmental NMDA receptor expression patterns uncovered in Nr2c subunit-beta-galactosidase knock-in mice. Mol Cell Neurosci. 2007;34:468–480. doi: 10.1016/j.mcn.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishi M, Hinds H, Lu HP, Kawata M, Hayashi Y. Motoneuron-specific expression of Nr3b, a novel NMDA-type glutamate receptor subunit that works in a dominant-negative manner. J Neurosci. 2001;21:RC185. doi: 10.1523/JNEUROSCI.21-23-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong HK, et al. Temporal and regional expression of NMDA receptor subunit Nr3a in the mammalian brain. J Comp Neurol. 2002;450:303–317. doi: 10.1002/cne.10314. [DOI] [PubMed] [Google Scholar]
- 20.Roberts AC, et al. Downregulation of Nr3a-containing NMDARs is required for synapse maturation and memory consolidation. Neuron. 2009;63:342–356. doi: 10.1016/j.neuron.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laurie DJ, Seeburg PH. Ligand affinities at recombinant N-methyl-Daspartate receptors depend on subunit composition. Eur J Pharmacol. 1994;268:335–345. doi: 10.1016/0922-4106(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 22.Laube B, Hirai H, Sturgess M, Betz H, Kuhse J. Molecular determinants of agonist discrimination by NMDA receptor subunits: analysis of the glutamate binding site on the Nr2b subunit. Neuron. 1997;18:493–503. doi: 10.1016/s0896-6273(00)81249-0. [DOI] [PubMed] [Google Scholar]
- 23.Costa BM, et al. N-methyl-D-aspartate (NMDA) receptor NR2 subunit selectivity of a series of novel piperazine-2,3-dicarboxylate derivatives: preferential blockade of extrasynaptic NMDA receptors in the rat hippocampal CA3-CA1 synapse. J Pharmacol Exp Ther. 2009;331:618–626. doi: 10.1124/jpet.109.156752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charton JP, Herkert M, Becker CM, Schroder H. Cellular and subcellular localization of the 2B-subunit of the NMDA receptor in the adult rat telencephalon. Brain Res. 1999;816:609–617. doi: 10.1016/s0006-8993(98)01243-8. [DOI] [PubMed] [Google Scholar]
- 25.DeBiasi S, Minelli A, Melone M, Conti F. Presynaptic NMDA receptors in the neocortex are both auto- and heteroreceptors. Neuroreport. 1996;7:2773–2776. doi: 10.1097/00001756-199611040-00073. [DOI] [PubMed] [Google Scholar]
- 26.Li YH, Wang J, Zhang G. Presynaptic Nr2b-containing NMDA autoreceptors mediate glutamatergic synaptic transmission in the rat visual cortex. Curr Neurovasc Res. 2009;6:104–109. doi: 10.2174/156720209788185632. [DOI] [PubMed] [Google Scholar]
- 27.Mayford M, et al. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- 28.Pérez-Otaño I, et al. Endocytosis and synaptic removal of Nr3a-containing NMDA receptors by PACSIN1/syndapin1. Nat Neurosci. 2006;9:611–621. doi: 10.1038/nn1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheetham CE, Fox K. Presynaptic development at L4 to l2/3 excitatory synapses follows different time courses in visual and somatosensory cortex. J Neurosci. 2010;30:12566–12571. doi: 10.1523/JNEUROSCI.2544-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsen RS, Rao D, Manis PB, Philpot BD. STDP in the developing sensory neocortex. Frontiers in Synaptic Neuroscience. 2010;2:12. doi: 10.3389/fnsyn.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Froemke RC, Poo MM, Dan Y. Spike-timing-dependent synaptic plasticity depends on dendritic location. Nature. 2005;434:221–225. doi: 10.1038/nature03366. [DOI] [PubMed] [Google Scholar]
- 32.McKinney RA, Capogna M, Durr R, Gahwiler BH, Thompson SM. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat Neurosci. 1999;2:44–49. doi: 10.1038/4548. [DOI] [PubMed] [Google Scholar]
- 33.Smith SL, Trachtenberg JT. Experience-dependent binocular competition in the visual cortex begins at eye opening. Nat Neurosci. 2007;10:370–375. doi: 10.1038/nn1844. [DOI] [PubMed] [Google Scholar]
- 34.Hatton CJ, Paoletti P. Modulation of triheteromeric NMDA receptors by N-terminal domain ligands. Neuron. 2005;46:261–274. doi: 10.1016/j.neuron.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Smothers CT, Woodward JJ. Effect of the NR3 subunit on ethanol inhibition of recombinant NMDA receptors. Brain Res. 2003;987:117–121. doi: 10.1016/s0006-8993(03)03315-8. [DOI] [PubMed] [Google Scholar]
- 36.Pérez-Otaño I, et al. Assembly with the NR1 subunit is required for surface expression of Nr3a-containing NMDA receptors. J Neurosci. 2001;21:1228–1237. doi: 10.1523/JNEUROSCI.21-04-01228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das S, et al. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit Nr3a. Nature. 1998;393:377–381. doi: 10.1038/30748. [DOI] [PubMed] [Google Scholar]
- 38.Yao Y, Mayer ML. Characterization of a soluble ligand binding domain of the NMDA receptor regulatory subunit Nr3a. J Neurosci. 2006;26:4559–4566. doi: 10.1523/JNEUROSCI.0560-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piña-Créspo JC, et al. Excitatory glycine responses of CNS myelin mediated by NR1/NR3 "NMDA" receptor subunits. J Neurosci. 2010;30:11501–11505. doi: 10.1523/JNEUROSCI.1593-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chatterton JE, et al. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature. 2002;415:793–798. doi: 10.1038/nature715. [DOI] [PubMed] [Google Scholar]
- 41.Herkert M, Rottger S, Becker CM. The NMDA receptor subunit Nr2b of neonatal rat brain: complex formation and enrichment in axonal growth cones. Eur J Neurosci. 1998;10:1553–1562. doi: 10.1046/j.1460-9568.1998.00164.x. [DOI] [PubMed] [Google Scholar]
- 42.McGuinness L, et al. Presynaptic NMDARs in the Hippocampus Facilitate Transmitter Release at Theta Frequency. Neuron. 2010;68:1109–1127. doi: 10.1016/j.neuron.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 43.Mameli M, Carta M, Partridge LD, Valenzuela CF. Neurosteroid-induced plasticity of immature synapses via retrograde modulation of presynaptic NMDA receptors. J Neurosci. 2005;25:2285–2294. doi: 10.1523/JNEUROSCI.3877-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christie JM, Jahr CE. Dendritic NMDA receptors activate axonal calcium channels. Neuron. 2008;60:298–307. doi: 10.1016/j.neuron.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christie JM, Jahr CE. Selective expression of ligand-gated ion channels in L5 pyramidal cell axons. J Neurosci. 2009;29:11441–11450. doi: 10.1523/JNEUROSCI.2387-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin H, et al. Axonal {alpha}7 nicotinic ACh receptors modulate presynaptic NMDA receptor expression and structural plasticity of glutamatergic presynaptic boutons. Proc Natl Acad Sci U S A. 2010;107:16661–16666. doi: 10.1073/pnas.1007397107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.