Abstract
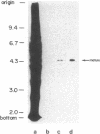
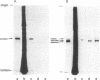
Recently, we showed that completion of the polypeptide chains on the polysomes isolated from germinating rice seed scutellum in a cell-free translation system can direct the synthesis of (i) unprocessed polypeptide containing the signal sequence (precursor 1), (ii) signal sequence-cleaved but nonglycosylated polypeptide (precursor 2), and (iii) the fully processed and glycosylated form of alpha-amylase molecules. The two precursors as well as the mature form of alpha-amylase can thus be produced in almost the same condition. Here, the binding affinity of the enzyme molecule to the substrate analogue beta-cyclodextrin was used as a probe to compare the conformations of the three distinctly different polypeptide chains produced on the polysomes. It was found that the mature secretory form and the nonglycosylated precursor form (precursor 2) specifically bind to beta-cyclodextrin immobilized on an epoxy-activated Sepharose 6B column but the form that has an attached signal sequence (precursor 1) does not. The results provide evidence that the NH2-terminal signal sequence prevents acquisition of beta-cyclodextrin-binding activity, indicating that, in rice seed alpha-amylase, the signal sequence impairs conversion of the unprocessed polypeptide to the enzymically active configuration.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. L., Wold F. Detection of alpha-amylase activity in unprocessed preamylase produced in the cell-free translation of porcine pancreatic RNA. J Biol Chem. 1981 Nov 10;256(21):10743–10746. [PubMed] [Google Scholar]
- Gorecki M., Zeelon E. P. Cell-free synthesis of rat parotid preamylase. J Biol Chem. 1979 Jan 25;254(2):525–529. [PubMed] [Google Scholar]
- Haugen T. H., Heath E. C. De novo biosynthesis of an enzymatically active precursor form of bovine pancreatic RNase. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2689–2693. doi: 10.1073/pnas.76.6.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreil G. Transfer of proteins across membranes. Annu Rev Biochem. 1981;50:317–348. doi: 10.1146/annurev.bi.50.070181.001533. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Przybyla A. E., Rutter W. J. Isolation and in vitro translation of the messenger RNA coding for pancreatic amylase. J Biol Chem. 1977 Aug 10;252(15):5522–5528. [PubMed] [Google Scholar]
- Miyata S., Akazawa T. Enzymic mechanism of starch breakdown in germinating rice seeds : 12. Biosynthesis of alpha-amylase in relation to protein glycosylation. Plant Physiol. 1982 Jul;70(1):147–153. doi: 10.1104/pp.70.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S., Akazawa T. alpha-Amylase biosynthesis: evidence for temporal sequence of NH2-terminal peptide cleavage and protein glycosylation. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6566–6568. doi: 10.1073/pnas.79.21.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S., Okamoto K., Watanabe A., Akazawa T. Enzymic Mechanism of Starch Breakdown in Germinating Rice Seeds: 10. IN VIVO AND IN VITRO SYNTHESIS OF alpha-AMYLASE IN RICE SEED SCUTELLUM. Plant Physiol. 1981 Dec;68(6):1314–1318. doi: 10.1104/pp.68.6.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vretblad P. Immobilization of ligands for biospecific affinity chromatography via their hydroxyl groups. The cyclohexaamylose-beta-amylase system. FEBS Lett. 1974 Oct 1;47(1):86–89. doi: 10.1016/0014-5793(74)80431-x. [DOI] [PubMed] [Google Scholar]