Summary
Here we report the discovery of tetracyclic benzothiazepines (BTZ) as highly potent and selective antimalarials along with the identification of the Plasmodium falciparum cytochrome bc1 complex as the primary functional target of this novel compound class. Investigation of the structure activity relationship within this previously unexplored chemical scaffold has yielded inhibitors with low nanomolar activity. A combined approach employing genetically modified parasites, biochemical profiling, and resistance selection validated inhibition of cytochrome bc1 activity, an essential component of the parasite respiratory chain and target of the widely used antimalarial drug atovaquone, as the mode of action of this novel compound class. Resistance to atovaquone is eroding the efficacy of this widely used antimalarial drug. Intriguingly, BTZ-based inhibitors retain activity against atovaquone resistant parasites, suggesting this chemical class may provide an alternative to atovaquone in combination therapy.
Introduction
With an estimated 300-500 million cases and a death toll of 0.8-1.2 million reported in 2008, malaria remains the most deadly parasitic disease, and it along with HIV/AIDS and tuberculosis, comprise the world's most significant infectious diseases. (Aregawi et al., 2008) Of the four Plasmodium species that infect humans, Plasmodium falciparum accounts for most malaria fatalities, which occur primarily in young children and pregnant women in sub-Saharan Africa. Parasite resistance to most of the available falciparum malaria chemotherapies has compromised efforts to reduce disease mortality and morbidity. Since most of the currently used drugs are variations on the molecular templates of their predecessors, new chemotypes exploiting highly sensitive and established targets in a resistance-orthogonal manner represent a powerful approach for the development of antimalarial drugs with activity against otherwise resistant malaria parasites. (Biagini et al., 2008; Winter et al., 2008; Kelly et al., 2009) In an effort to diversify the current stock of antimalarial chemotherapeutic agents, we have instituted a high-throughput screening campaign of small-molecule libraries for novel, drug-like compounds with whole-cell antimalarial activity, limited susceptibility to established mechanisms of drug resistance, and minimal toxicity to mammalian cells, as a pathway to medicinal chemistry optimization and preclinical development of lead compounds. (Baniecki et al., 2007) We recently identified over 100 small molecules with sub-micromolar activity against both drug sensitive and resistant P. falciparum strains from an approximately 79,000 compound screening collection. (Baniecki et al., 2007; Martyn et al., 2010a; Martyn et al., 2010b; Urgaonkar et al., 2010; Barker et al., 2011) The majority of these compounds are structurally unrelated to established antimalarials and may have novel cellular targets or distinct binding modes, and thus may evade existing resistance mechanisms. As with any forward chemical genetics screen, the identification of the molecular target of hit compounds poses one of the greatest challenges. (Stockwell, 2000) In this study, a pathway-specific whole organism screen was used as secondary assay to identify those compounds from our hit set with antimalarial activity that exert their primary activity by inhibiting the parasite mitochondrial electron transport chain (ETC), a previously validated antimicrobial target. (Fry and Pudney, 1992; Kawai et al., 1993; Srivastava et al., 1997)
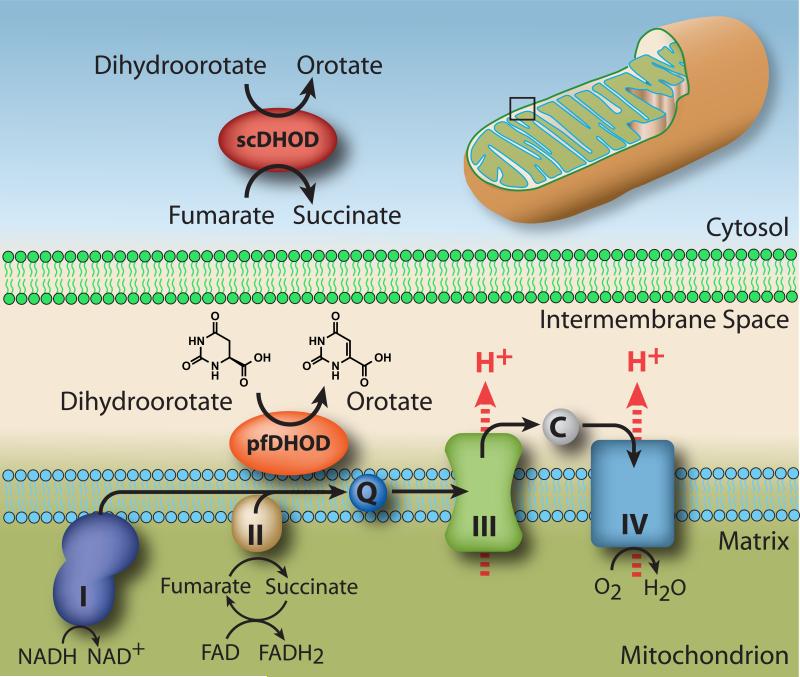
Painter et al. reported that the mitochondrial ETC in the erythrocytic stage of the malaria parasite functions solely to regenerate ubiquinone as the cofactor for the mitochondrial membrane-associated type II dihydroorotate dehydrogenase (DHOD) in the de novo pyrimidine biosynthetic pathway. (Painter et al., 2007) The erythrocytic parasite proliferates in human red blood cells, and in that environment relies completely on de novo pyrimidine biosynthesis. The resulting ubiquinone dependency and, in turn, need for complexes I – III of the ETC, can be circumvented in recombinant parasites by the expression of a type I DHOD from Saccharomyces cerevisiae (scDHOD), a cytosolic form of the enzyme, which uses fumarate or NAD as an electron acceptor in lieu of ubiquinone. (Painter et al., 2007) Compounds that target complexes I – III of the ETC or pfDHOD as their primary mode of action exhibit reduced activity against the transgenic scDHOD-expressing parasite strain compared to the wild-type P. falciparum strain (Fig. 1).
Figure 1.
Electron Transport Chain of P. falciparum. Enzymes of the ETC are associated with the inner mitochondrial membrane. Alternative Complex I (pfNDH2) is a non-proton pumping enzyme, therefore the primary generators of the mitochondrial proton-motive force are Complexes III and IV. Other components contributing to the respiratory chain include Complex II (succinate dehydrogenase), and pfDHOD. Exogenous expression of scDHOD bypasses the electron transport chain through complex III. In addition, glycerol-3-phosphate dehydrogenase and malate:quinone oxidoreductase have been identified as mitochondrial dehydrogenases in P. falciparum that generate reduced CoQ. However, contribution of these additional dehydrogenases to overall flux seem to be small compared to the displayed components and are therefore omitted for clarity. (48)
Here we describe a novel antimalarial drug template identified by the high-throughput screening campaign, its chemical optimization and basic structure-activity relationship (SAR) exploration, and the identification of its ETC molecular target by a combination of a pathway-specific transgenic whole-cell screens, traditional in vitro enzyme assays, and finally by the generation and genetic mapping of compound-specific parasite resistance. In addition to the specific molecules reported, this approach highlights the importance of genetic mapping in developing drugs for neglected diseases.
Results
Tetracyclic benzothiazepines are a novel class of potent inhibitors of the P. falciparum electron transport chain
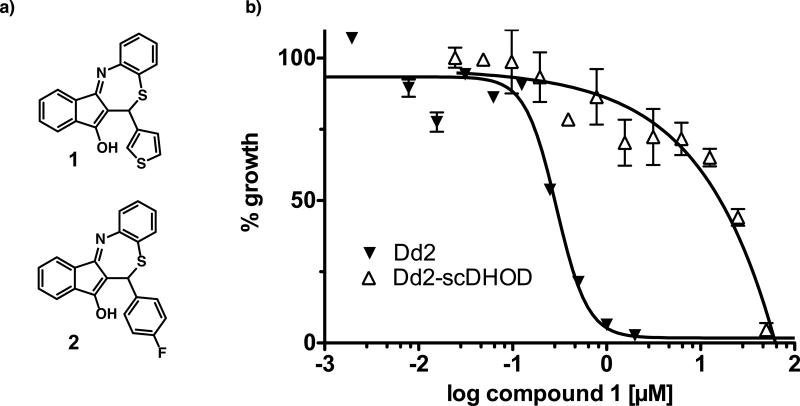
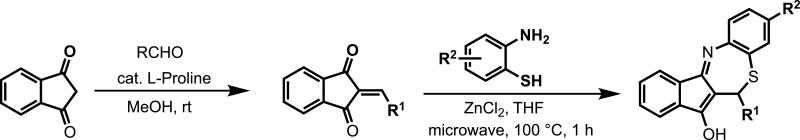
To identify novel antimalarials that act through inhibition of the P. falciparum ETC we profiled compounds identified in the primary screen for differential growth inhibition of a wild-type strain and a scDHOD-expressing transgenic strain with an identical genetic background. Out of all compounds tested, two molecules (1 and 2, Fig. 2a) with a common tetracyclic benzothiazepine (BTZ) molecular scaffold were identified as having significantly reduced activity against the transgenic scDHOD-expressing Dd2 strain with respect to the parental Dd2 strain suggesting that the BTZs target enzymes in the mitochondrial ETC (Fig. 2b). The tetracyclic BTZ scaffold is an unexplored compound class with no reports on its biological activity. To explore the SAR and to identify analogs with increased potency, a focused library of BTZ derivatives was synthesized using a newly developed two-step procedure based on a proline catalyzed Knoevenagel condensation followed by a Lewis acid promoted Michael addition/intramolecular imine formation sequence (Figure 3). (Krysin MY, 2003; Ramachary et al., 2004) This effort identified compound 3 as a potent inhibitor of parasite growth with chloroquine-like potency against wild-type 3D7 strain (IC50 = 16 nM), and more importantly, activity against the multidrug-resistant Dd2 strain (IC50 = 16 nM). Compound 3 is approximately 25-fold less active compared to the clinically optimized activity of atovaquone (IC50 = 0.7 nM). However, a clear SAR trend emerged from the synthesized analogs. We found that the R2 site was well tolerated by small hydrophobic substituents like methoxy and methyl. The importance of non-polar aliphatic groups at R1 for enhanced antimalarial activity was also noted while polar substituents decreased the potency.
Figure 2.
Wild type Dd2 (black triangles) and Dd2-scDHOD (open triangles) parasite strains were grown in the presence of varying drug concentration for 48 hours. Growth was assessed by {3H}-hypoxanthine incorporation. a) Structures of BTZ compounds 1 and 2 identified in the small molecule screen. b) Dose-effect curves for Dd2 and Dd2-scDHOD parasite strains. Growth in the absence of drug was set at 100%. Data represent mean values +/- SD. of triplicate experiments at each condition.
Figure 3.
General synthesis scheme for BTZ analogs.
We next profiled a selected set of eleven BTZ analogs (1-11) against the scDHOD-expressing transgenic strain to assess differential activity compared to the parental Dd2 strain. (Baniecki et al., 2007) The rescue phenotype in the presence of scDHOD expression was associated with compounds 1-9, whereas no significant change in viability was noted for 10 and 11 (Table 1).
Table 1.
Inhibition of in vitro enzyme activity and in vivo parasite proliferation by BTZs. Reported EC50 values for Dd2 and Dd2-scDHOD parasite strains are based on dose-effect curves. IC50 calculations for enzyme activity were determined by best-fit Michaelis-Menten kinetic curves. Cytochrome bc1 enzyme activity was determined using purified mitochondrial fractions.
| EC50 growth (μM) |
IC50 enzyme activity (μM) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | R2 | R1 | 3D7 | Dd2 | Dd2-scDHOD | pfNDH2 | pfDHOD | pfCYT bc1 | hsCYT bc1 |
| 1 | H | 3-thiophenyl | 0.044 | 0.157 | >5 | >20 | >20 | 0.004±0.0014 | >5 |
| 2 | H | 4-fluorophenyl | 0.033 | 0.013 | >5 | n.a | n.a | 0.014±0.0039 | >5 |
| 3 | OMe | cyclopropyl | 0.016 | 0.016 | >5 | >20 | >20 | 0.003±0.0007 | 0.833±0.201 |
| 4 | H | cyclopropyl | 0.048 | 0.02 | >5 | >20 | >20 | 0.007±0.0022 | 1.090±0.093 |
| 5 | H | cyclohexyl | 0.034 | 0.022 | >5 | >20 | >20 | 0.005±0.0016 | >5 |
| 6 | Me | 3-thiophenyl | 0.031 | 0.039 | >5 | >20 | >20 | 0.011±0.0033 | >5 |
| 7 | H | 4-methoxyphenyl | 2 | 1.12 | >5 | >20 | >20 | 0.020±0.0025 | >5 |
| 8 | H | 4-cyanophenyl | 0.79 | 0.27 | >5 | >20 | >20 | 0.049±0.0068 | >5 |
| 9 | H | 4-(3-dimethylamino)propoxy)phenyl | 1.47 | 0.57 | >5 | >20 | >20 | 1.082±0.084 | >5 |
| 10 | H | 2-methoxyphenyl | >5 | >5 | >5 | >20 | >20 | 1.335±0.366 | >5 |
| 11 | H | 3,4-dihydroxyphenyl | >5 | >5 | >5 | >20 | >20 | >5 | >5 |
| atovaquone | 0.001 | 0.001 | >5 | >20 | >20 | 0.0002±0.00003 | 0.350±0.012 | ||
Cytochrome bc1 is the primary target of BTZs
The principal mode of action for the compounds with differential activity was expected to fall within the bypassed region of the ETC comprising complexes I, II, and III, and pfDHOD (Figure 1). To further narrow down the list of possible targets of BTZs 1-11, we profiled these compounds in uncoupled biochemical assays. In vitro assays for three of the four P. falciparum ETC components (complex I, complex III and pfDHOD) had previously been established in our labs. (Patel et al., 2008; Dong et al., 2009) Complex I is an alternative NADH dehydrogenase (pfNDH2) that catalyzes the transfer of electrons from NADH to ubiquinone (CoQ).(Kerscher, 2000) pfDHOD catalyzes the oxidation of L-dihydroorotate (L-DHO) to orotate using a flavin mononucleotide (FMN) cofactor that is re-oxidized by CoQ. (Jones, 1980) None of the evaluated BTZs were found to inhibit either recombinant pfNDH2 or pfDHOD activity at concentrations up to 20 μM (Table 1). Remarkably, however, the BTZ analogs were found to be potent inhibitors of complex III (cytochrome bc1) in a manner that correlated well with the EC50 values determined for parasite growth (Table 1). In contrast, human cytochrome bc1 was relatively insensitive to BTZ treatment, indicating that some members of the BTZ family possess high species selectivity. It should be noted that all compounds with nanomolar activity were 100- to >1250-fold more active against pfCYT bc1 compared to hsCYTbc1, a selectivity window that is generally considered appropriate for drug development, and some were even comparable to the selectivity of atovaquone (1750-fold).
Cytochrome bc1 is a homodimeric, multisubunit mitochondrial inner membrane enzyme complex that transfers electrons from ubiquinol to cytochrome c. It is comprised of a cytochrome b subunit (CYT b), a Rieske iron-sulfur protein, and a cytochrome c1 subunit. (Trumpower, 1990) Inhibition of electron transfer through the cytochrome bc1 complex is linked to proton translocation via the proton motive Q cycle, in which ubiquinol is oxidized at one site (Qo or Center P), and then re-reduced at another site (Qi or Center N). (Hunte et al., 2003) Known inhibitors of the cytochrome bc1 complex have been proposed to act mainly as ubiquinol/ubiquinone antagonists by interfering with the ubiquinol oxidation or ubiquinone reduction steps of the proton-motive Q cycle. (Trumpower and Gennis, 1994) A number of mutants resistant to inhibitors of the cytochrome bc1 complex carry mutations in the ubiquinol or ubiquinone binding sites of the CYT b gene. (Brasseur et al., 1996)
BTZs bind to the quinol-binding site in a mode distinct from atovaquone
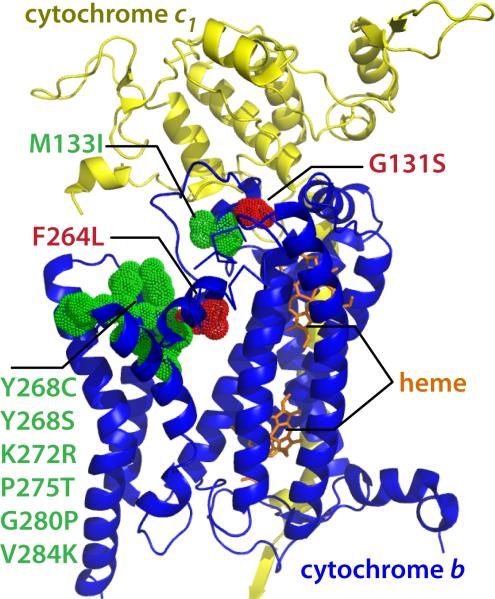
Cytochrome bc1 is the validated target of atovaquone (ATV), a potent inhibitor of both proliferating erythrocytic stage and sexual stage parasites, used in synergistic combination with proguanil, and marketed under the trade name Malarone®.(Fry and Pudney, 1992; Fleck et al., 1996; Srivastava et al., 1997; Chulay, 1998; Korsinczky et al., 2000) ATV resistance is associated with single nucleotide polymorphisms in the Qo and Qi sites in the CYT b gene. (Srivastava et al., 1999; Kessl et al., 2007) In order to validate cytochrome bc1 as a BTZ target and to assess the molecular basis for biological activity of BTZ, we generated resistant P. falciparum parasites using 1 as the selecting agent at 10x IC50 concentration. Starting with 2×109 parasites (Dd2), we were able to isolate a parasite clone (Dd2-BTZ1) after 90 days of continuous culturing that was approximately 5-fold less sensitive to 1. Continuous selection with adjusted drug concentrations for additional 60 days resulted in the generation of a second strain with 80-fold resistance (Dd2-BTZ2). Importantly, the BTZ resistant clones were also resistant to other BTZs but retained sensitivity to ATV. For comparison, previous efforts by Rathod et al. to select for ATV resistant parasite strains, starting at 105-106 parasites (W2 and 3D7), yielded resistant isolates after a two-month selection period. (Rathod et al., 1997) However, the authors also demonstrated that resistance selection depends on both parasite strain and selection agent, making direct comparisons difficult. (Rathod et al., 1997) The CYT b gene was PCR amplified and sequenced from the BTZ-resistant mutant lines. A single point mutation, G391A, resulting in amino acid change, G131S, was identified in Dd2-BTZ1. The G131S mutation lies within the quinol binding site Qo (i.e. site of ubiquinol oxidation) binding regions of CYT b (Fig. 4). Interestingly, Dd2-BTZ2 contained an additional point mutation to the one described above. The second point mutation, T791A, translating to a F264L amino acid change, is located distal to the first mutation in the predicted ubiquinol-binding region (Fig. 4). These results are in accord with observations that ATV-resistance associated point mutations in the same region also accumulate with extended drug pressure.
Figure 4.
Mapping of BTZ resistance mutations on Saccharomyces cerevisiae cytochrome bc1 crystal structure. BTZ resistance mutations (red) and ATV resistance mutations (green) are mapped on the crystal structure coordinates of S. cerevisiae cytochrome bc1 (PDB code: 1EZV). cytochrome c1 (yellow), cytochrome b (blue) and heme (orange). For homology alignment see figure S4.
BTZs are highly synergistic with proguanil and exhibit minimal propensity to cross-resistance with atovaquone
To determine potential cross-resistance between the BTZs and ATV we profiled two established ATV-resistant P. falciparum strains, both of Southeast Asian origin: the FCR3 strain harboring CYT b mutations, M133I and G280D, and TM90C6B, bearing the mutation, Y268S, the most prevalent polymorphism conferring clinical failure of ATV. (Korsinczky et al., 2000) Relative to the ATV-sensitive Dd2 line, FCR3 and TM90C6B showed one and four-fold resistance to 1, respectively. In contrast, ATV was >1000-fold less effective in the two mutant lines. In addition, 2 demonstrated similar efficacy. These results indicate that the antimalarial activity of the BTZs is only modestly compromised in P. falciparum mutants conferring high-level ATV resistance, suggesting that the BTZs may inhibit cytochrome bc1 activity in a different manner than ATV. To further characterize the role of BTZ and cytochrome bc1, wild-type parasites were co-treated with both 1 and proguanil (PG), a drug that does not independently affect electron transport but has been shown to synergistically enhance the ability of ATV to collapse the membrane potential. (Canfield et al., 1995; Srivastava et al., 1999) Indeed, in two culture-adapted lines, Dd2 and D10, 1 demonstrated a synergistic interaction with PG, providing further evidence that the BTZs act on cytochrome bc1 by a mode of action comparable to atovaquone (Table 2, Fig. S1). As previously reported, combination treatment with PG was unexpectedly able to restore sensitivity of the scDHOD-expressing strain to ATV. We tested the BTZ compounds in combination with PG and likewise observed restored sensitivity in the transgenic strain. (Painter et al., 2007) Interestingly, the scDHOD-transgenic strain synergized more potently with PG and was hypersensitive to BTZ compared to the wild type strain even at low concentrations (>0.5 μM) of PG.
Table 2.
Inhibition of in vivo parasite proliferation by 1 and 2 in combination with proguanil. EC50 values for Dd2 and Dd2-scDHOD parasite strains are based on dose-effect curves. See also Figures S1, S2 and S3.
| Compound | Proguanil [nM] | Dd2 [nM] | Dd2-scDHOD [nM] | fold resistance |
|---|---|---|---|---|
| 1 | 0.05 | 370 | 6015 | 16.3 |
| 1 | 0.5 | 81 | 78 | 0.96 |
| 1 | 5 | 29 | 7.9 | 0.27 |
| 2 | 0.05 | 388 | 2978 | 7.7 |
| 2 | 0.5 | 108 | 51 | 0.47 |
| 2 | 5 | 39 | 3.8 | 0.1 |
BTZ liver stage activity and in vivo profiling
Atovaquone is only one of the few known antimalarials that are active against both blood and liver stage parasites (IC50 = 0.3 nM). To confirm comparable characteristics of the BTZs, we profiled two representative analogs, 1 and 2, in a Plasmodium berghei liver stage assay in mammalian Hep2G cells. While Hep2G cells were not sensitive to either compound, both compounds inhibited liver stage P. berghei at IC50= 151± 42 nM and 391±28 nM, respectively.
To assess the in vivo activity of the BTZs, we profiled compounds 1 and 2 in a murine model system. We first established a tolerated dose of these BTZs. Compounds were administered by i.p. injection at two concentrations (50 mg/kg and 100 mg/kg) to uninfected mice b.i.d. Treatment with BTZ 1 resulted in some lethargy for 1 hr following dosing while BTZ 2 was tolerated well without any clinical signs of toxicity. LC/MS/MS analysis of the plasma samples established moderate exposure at approximately 3-5x blood stage IC50 concentrations (see figure S2). We performed a follow-up study exploring the in vivo activity of 1 and 2 against P. berghei according to an acute malaria model adapted from Peters’ “4-day suppressive test”. (Barker et al., 2011) While the compounds were well tolerated at the highest concentrations, indicating an absence of acute toxicity, we did not observe a significant decrease in peripheral blood parasitemia (see figure S3). We speculate that these results are a reflection of the short half-life of the tested compounds leading to insufficient exposure (see supporting information), and possibly decreased activity against blood stage P. berghei compared to P. falciparum. Future medicinal chemistry efforts will be required to identify analogs with improved in vivo DMPK parameters.
Discussion
The synergistic combination of ATV and proguanil (PG) represents one of the most useful antimalarial medications (Malarone®). Unfortunately, P. falciparum quickly develops resistance to ATV compared to other antimalarial drugs. When administered as a single treatment therapy during clinical trials, ATV resistance is observed within 28 days, spurring the search for alternative cytochrome bc1 inhibitors that also synergize with PG. (Looareesuwan et al., 1996) Even when used in combination with PG, which potentiates the activity of ATV, spontaneous resistance, mostly attributed to a mutation of Y268 in CYT b is observed clinically, which has spurred the discovery of alternate inhibitors of Plasmodium cytochrome bc1 complex. (Schwöbel et al., 2003) Once ATV resistance has been established, the potency of the combination with PG is significantly reduced and can lead to treatment failure. (Fivelman et al., 2002) Acridinediones have been characterized as selectively potent inhibitors of parasite cytochrome bc1. (Biagini et al., 2008) Quinolone derivatives, and a series of pyridones related to the anticoccidial drug, clopidol, have also been implicated as cytochrome bc1 inhibitors. (Winter et al., 2008) While these molecules show promise, the ability of the malaria parasite to generate resistance necessitates the continued development of novel electron transport chain inhibitors, ideally with distinct inhibitory modes that are less likely to cause high level resistance as a result of a single point mutation.
Here we have used a combination of forward chemical genetics, pathway-based screens, and Plasmodium genetics to identify tetracyclic BTZs as a novel class of highly potent anti-malarial compounds that are active against both blood and liver stage, and provided evidence that these compounds likely act via inhibition of P. falciparum cytochrome bc1 activity. In addition, we demonstrate that BTZs exhibit synergy with PG comparable to that of ATV. The underlying basis for this synergy is not yet fully understood. It has been speculated that the primary mode of action of ATV is the depolarization of the mitochondrial membrane. (Biagini et al., 2006) Interestingly, we observed that low-dose combination treatment with PG renders scDHOD expressing transgenic parasites hypersensitive to BTZs. Similar findings have been recently reported for ATV. (Painter et al., 2007) This observation suggests that disruption of pyrimidine biosynthesis through indirect inhibition of DHOD, at least in combination with PG, is not the sole basis for the antimalarial activity of BTZs (and ATV). BTZs thus provide an orthogonal set of tool compounds that may enable a better understanding of the enigmatic nature of the synergistic relationship of PG and cytochrome bc1 inhibitors.
Based on drug selection analysis, we defined two mutations in CYT b, G131S and F264L, both within the predicted ubiquinol binding site, that convey resistance to BTZ treatment. The mitochondrial DNA in malaria parasites is remarkably well conserved, and little sequence variation is observed within the parasite species even from geographically isolated clones. (McIntosh et al., 1998) Therefore, rapid sequence changes, not unlike those observed in ATV-resistant parasites, indicates a selective advantage for these mutations in the presence of BTZ. Interestingly, minimal cross-resistance for the BTZs with an ATV-resistant strain indicates that while these molecules likely target the same enzyme, the binding sites are spatially distinct. While ATV is a well-studied inhibitor of cytochrome bc1, the identification of additional inhibitors of this enzyme that do not exhibit cross-resistance with ATV-resistant parasite strains suggests that the mitochondrial ETC can still be profitably exploited as a drug target.
Based upon homologous experiments in yeast, it has been suggested that residue alterations in the CYT b protein may lead to a loss of respiratory fitness in P. falciparum.(Wenz et al., 2007; Fisher and Meunier, 2008) Novel inhibitors with differential binding capacities for cytochrome bc1 used in combination with ATV might therefore restrict the emergence of drug resistance. (Srivastava et al., 1999) In addition, evaluation of novel cytochrome bc1 inhibitors such as the BTZs against diseases for which ATV is used as a prophylactic or acute treatment, such as toxoplasmosis and Pneumocystis pneumonia (PCP), may lead to additional chemotherapeutic applications. (Petersen, 2007; Kazanjian et al., 2001; Rosenberg et al., 2001; Kessl et al., 2007) Although medicinal chemistry optimization for improved exposure will be required to achieve efficiency, the BTZs appear to be well tolerated in vivo, suggesting a promising future lead for the development of cytochrome bc1 targeted antimalarials.
Experimental Procedures
Secondary screening approach
108 small molecules previously identified by Baniecki et al. as highly active against multidrug-resistant parasites were screened against a Dd2 transgenic parasite line expressing chromosomally integrated yeast DHOD (scDHOD). (Baniecki et al., 2007) A transgenic P. falciparum strain expressing scDHOD was generated by amplification of full-length scDHOD from S. cerevisiae genomic DNA and subsequent cloning into the pLN-ENR-GFP plasmid as a AvrII/XhoI insert. This pLN-scDHOD plasmid was cotransfected into the Dd2-attB parasite strain with the Bxb1 integrase expression vector pINT as described by Nkrumah et al., and maintained in vitro by method of Trager et al.. (Trager and Jensen, 1976; Nkrumah et al., 2006) Compounds were screened at two fixed concentrations (2.5 and 5 mM), and inhibition of P. falciparum growth was assessed by the relative reduction of {3H}-hypoxanthine uptake. (Geary et al., 1983; Chulay et al., 1983) IC50 values were determined for compounds showing differential growth between Dd2 and Dd2-scDHOD lines of P. falciparum relative to drug-free control cultures. The susceptibility of parasite strains was also determined for ATV (USP Rockville, Maryland), and chloroquine (Sigma-Aldrich). Synergy experiments were performed in similar fashion. Briefly, BTZ compounds were serially diluted in 5-fold dilutions in quadruplicate in 96 well assay plates in low hypoxanthine RPMI. Synchronous ring stage cultures were plated at 0.75% parasitemia in 2% HC in the 96 well plates in the presence of the indicated concentrations of proguanil. Parasites were grown for 48 hours and re-invasion was monitored in control wells without drug. Following addition of 0.75uCi {3H}-hypoxanthine per well parasites were grown for additional 24 hours and were harvested. {3H}-hypoxanthine incorporation was measured by scintillation counting. IC50 values against 3D7 and Dd2 were determined as reported previously.(Geary et al., 1983)
Activity assays
For pfNDH2 kinetics experiments, the enzymatic activity of purified His-tagged pfNDH2 was measured by fluorescence using an assay adapted from Putt et al. (Putt and Hergenrother, 2004; Dong et al., 2009) 30μL of sample solution in assay buffer (50 mM Tris(8.0), 150 mM KCL, 0.1% Triton) was added in triplicate to wells of a 96-well black plate. Following incubation at 25 °C for 1 hour, 12 μL of aqueous 2 M KOH solution and 12 μL of a 20% acetophenone (in EtOH) solution were added to each well. The plate was incubated at 4 °C for 10 minutes, followed by addition of 46 μL formic acid, resulting in a final concentration of 240 mM KOH, 2.4% acetophenone and 46% formic acid. Plates were then incubated in an oven at 100 °C for 15 minutes. The plate was allowed to cool and fluorescence was measured on a FlexStation III machine (Molecular Devices) at 360/444 ex/em (Supplemental Scheme 1). Thirty microliters of 1 mM to 100 mM NAD+ solution in assay buffer was added in triplicate to wells in order to determine calibration curves for all kinetics experiments.
For DHOD kinetics experiments, the chromogen reduction assay adapted for 384-well format as described by Patel et al was utilized (Supplemental Scheme 2). (Patel et al., 2008) The assay was performed in a final volume of 60 μL of 100 mM HEPES (pH 8.0), 150 mM NaCl, 5% glycerol, 0.05% Triton X-100, 500 mM L-DHO, 115 mM Q0, 50 mM 2,6-dichlorophenol-indophenol (DCIP). Compounds were plated in serial dilution, and the reaction was initiated by addition of pfDHOD (2 mg/mL final concentration.) The reaction proceeded at 25 °C for 10 minutes after which, A600 was measured using an automatic plate reader.
For cytochrome bc1 experiments, mitochondria were isolated as follows: Parasitized erythrocytes were harvested by centrifugation and lysed with 0.05% (w/v) saponin in RPMI. Then parasites were washed three times with H-medium (0.07 M sucrose, 0.21 M mannitol, 1 mM EGTA, 5 mM MgCl2, 5 mM KH2PO4, and 4 mM HEPES, pH 7.4) and resuspended in the same medium in the presence of 1 mM PMSF and protease inhibitor cocktail (Roche Complete). Parasites were disrupted by N2 cavitation (4639 Cell disruption Bomb, Parr, USA) at 1600 psi for 25 min at 4 °C. Unbroken cells and cell debris were removed by centrifugation at 1200 g for 10 min at 4 °C. The mitochondrial fraction was pelleted at 10,000 g for 20 min at 4 °C. The mitochondrial pellet was gently resuspended in 0.8 M sucrose, 1 mM EDTA, 10 mM Tris-HCl pH 7.4 and 0.1% BSA prior to separation on a 1-2 M sucrose gradient. The sample was centrifuged on a gradient at 80000 g for 2 hours at 4 °C. The mitochondria were then recovered and washed with 1 mM EDTA, 10 mM Tris-HCL pH 7.4 to remove sucrose, and resuspended in H-medium with protease inhibitor cocktail and 1 mM PMSF. Samples were stored at –80 °C until use. In the case of human mitochondria, HEK293 human cell line was used as starting material. Cells were pelleted at 400 g 10 min and disrupted by N2 cavitation. Mitochondria then were isolated using the method described above. Cytochrome c reductase activity was assayed by a modification of the method of Fry and Pudney. (Fry and Pudney, 1992) Mitochondria (40 ug/ml) were diluted in reaction buffer (250 mM sucrose, 50 mM KH2PO4, 0.2 mM EDTA, 1 mM NaN3 and 2.5 mM KCN) containing 50 uM cytochrome c. Reactions were started by addition of 25 uM decylubiquinol and monitored by reduction of cytochrome c at 550 nm. To assure the linearity of the enzymatic reaction only data from the first 60 seconds were collected. Decylubiquinol substrate was prepared by reducing decylubiquinone (Sigma-Aldrich Inc., St. Louis, MO, USA) in ethanol with sodium borohydride. Decylubiquinol was aliquoted and stored in acidified ethanol at -80 °C. Dose-response titration curves for P. falciparum cytochrome bc1 assays are provided as supporting information (SI figure 5).
Strain handling
The TM90C6B (MRA205) P. falciparum parasite strain, isolated from a Thai patient who experienced a recrudescence of parasitemia after treatment with ATV (1,000 mg once a day for 3 days) and pyrimethamine, was tested for susceptibility to the BTZ compounds (Malaria Research and Reference Reagent Resource Center). (Fisher and Meunier, 2008) Stepwise in vitro selection of BTZ-resistant parasite lines was carried out as follows. P. falciparum Dd2 parasites were initially cultured in one flask and then split into four 25 mL cultures at 5% parasitemia and 5% hematocrit (approximately 2×109 parasites). Cultures were expanded to 200 mL flasks and grown in medium containing the 1 analog at a concentration 10-fold above the IC50. Three days after the first dosing, each flask was split. One set of four flasks was subjected to continuous drug pressure, while the other set of four flasks was treated at the same concentration intermittently, allowing for the reestablishment of 2-5% parasitemia between drug treatments. Cultures were dosed until no viable parasites were microscopically observed. All flasks were maintained as independent selections. After 90 days two flasks from the original continuous and intermittent splits, BTZ-1 and BTZ-1b, showed evidence of drug resistance, which was confirmed by growth-inhibition assays. Following DNA sequencing of the pfCYTb ORF, the BTZ-1 and -5 strains were subjected to increasing intermittent drug pressure. After 60 days, BTZ-2 and 2b lines were established, showing persistent growth >150 nM 1.
Liver stage assay
The assay was conducted using a luciferase-expressing sporozoite strain of P. berghei ANKA according to a previously published procedure. (Ploemen et al., 2009) Briefly, 4,000 sporozoites harvested from mosquito salivary glands were used to infect 12,000 HepG2 cells (ATCC) in the presence and absence of compounds. The final concentration of DMSO was 0.3%. Parasite growth was determined 45 hours post-infection with ONE-Glo (Promega) according to the manufacturer's instructions. Addition of BTZ post sporozoite infection resulted in similar activity. Data analysis was carried out using GraphPad Prism and all statistic results are given as mean ± SD. The experiments were repeated 2-3 times to ensure reproducibility.
DNA analysis
Parasite DNA was extracted from Dd2 parent and BTZ-5 strains usi ng standard procedures. (Cheng et al., 1997) The CYT b gene was amplified by PCR (5’ ATG AAC TTT TAC TCT ATT AAT TTA G 3’; 5’ TTA TAT GTT TGC TTG GGA GCT GTA A 3’). PCR was performed using 0.2 mM of each deoxynucleoside triphosphate (company), 50 pmol of each primer, 1.25 U Invitrogen Platinum Taq (Invitrogen). The mixture was heated to 94 °C for 2 min and then cycled at 94 °C for 1 min, 55 °C for 1 min, and 62 °C for 3 min over 30 cycles. PCR products were then purified using the PCR Cleanup kit (QIAGEN) and sequenced using primers described by Korsinczky et al., cytb1 (5’ CTC TAT TAA TTT AGT TAA AGC ACA C 3’), cytb2 (5’ ACA GAA TAA TCT CTA GCA CC 3’), cytb3 (5’ AGC AGT AAT TTG GAT ATG TGG AGG 3’) and cytb4 (5’ ATT TTT AAT GCT GTA TCA TAC CCT 3’).(Korsinczky et al., 2000)
Tolerability study
The studies were performed at Genzyme Corporation following standard protocols. Briefly, compounds (formulated in 15% DMSO, 25% polyvinyl pyrrolidone and 5% Tween 80 in water at 5 and 10mg/mL, respectively) were administered i.p. BID to groups of 3 mice (CD-1, female, 5 weeks at 50 mg/kg and 100 mg/kg, respectively. Clinical observations were recorded at fifteen-minute intervals for 1 hour after administration of test articles, then hourly for up to 4 hours daily for signs of acute systemic toxicity. EDTA plasma samples collected from groups 2 and 3 at the following time points were submitted for exposure analysis at 1hr, 1hr(2nd dose), 18hrs.
Liquid chromatography tandem mass spectrometry (LC-MS)
Measurements of plasma exposures of BTZs were carried out using a 4000 QTRAP triple quadrupole mass spectrometer (AB SCIEX; Foster City, CA) that was coupled to an Agilent 1100 HPLC pump (Agilent Technologies; Santa Clara, CA) and an HTS PAL autosampler (Leap Technologies; Carrboro, NC). Plasma samples were extracted (20 μL) using 80 μL of 75/25 acetonitrile/methanol solution containing 0.2 ng/μL valine-d8 internal standard and 10 μL of each supernatant were analyzed. Chromatographic separations were achieved using a 100 × 3 mm Atlantis T3 column (Waters; Milford, MA) that was initially eluted isocratically at 300 uL/min with 95% mobile phase A (0.1% formic acid in water) and 5% mobile phase B (0.1% formic acid in acetonitrile) for 3 minutes, followed by a 5 minute linear gradient to 100% mobile phase B. Multiple reaction monitoring (MRM) transitions were monitored in the positive ion mode using a 100 ms dwell time and collision energy setting of 30. Electrospray ionization source settings were: curtain gas 20, source potential 5 kV, source temperature 400 °C, gas1 30, gas2 35, and declustering potential 40. Calibration curves were generated by analysis of serially diluted reference solutions. MultiQuant software (Version 1.1; AB SCIEX; Foster City, CA) was used for peak integration and data were manually reviewed for quality of integration.
P. berghei in vivo assay
The acute P. berghei rodent model is adapted from the “4-day suppressive test” . (Peters, 1987) The acute portion of the protocol is identical to that recommended by MMV-ESAC. (Fidock et al., 2004) Animals were maintained and housed according to NIH guidelines and were allowed to acclimatize for 1 week prior to study. This protocol has been approved by the IACUC of the University of Puerto Rico. On Day 0, groups of 5 mice are infected by tail vein injection with 0.2 mL heparinized blood diluted to contain 2 × 107 parasites per dose. Compound stocks were prepared in 15% DMSO, 25% polyvinyl pyrrolidone and 5% Tween 80 in water. Animals were dosed approximately 100 μL/dose (adjusted for animal weight), QD on Day 0; b.i.d. i.p., Day 1 - Day 3. (Day 0 = first day of study); animals in the Control-group received vehicle alone.. On Day 4 (5th day of assay) blood was collected by tail-nick; thin smear microscope slides were prepared (3 slides/animal) and stained using Diff Quick. Parasitized erythrocytes were counted and compared with the total number of erythrocytes per microscopic field to determine the per cent parasitemia. A minimum of 450 erythrocytes was counted. In some cases with very low parasitemia data were originally captured as “at least xx parasitemia.” These were later converted to per cent parasitemia by dividing by 450 (the minimum number erythrocytes counted) and multiplying by 100. Animals were euthanized at the end of the study.
General Procedure for the Synthesis of Benzothiazepines
To a glass vial equipped with a magnetic stirring bar was added 1 mmol of 1,3-indandione, 1 mmol of aldehyde, 0.2 mmol of the catalyst L-Proline, and then MeOH (2 mL). The reaction mixture was stirred at room temperature until the LC/MS analysis showed the completion of the reaction (typically 30 min - 1 h). Water was added to the reaction mixture and the solid obtained was filtered off, washed thoroughly with water followed by hexanes, and finally dried under high vacuum. This intermediate, 2-arylidene-1,3-indandione, was used in the next step without any further purification.
2-arylidene-1,3-indandione (1.0 equiv), 2-aminothiophenol (2.5 equiv), and anhydrous ZnCl2 (2.0 equiv) in anhydrous THF (12 mL) were mixed in a Smith™ process 20 mL vial and sealed. The reaction mixture was heated in a microwave reactor at 100 °C for 1 h. The mixture was diluted with CH2Cl2, washed initially with 1M aq. HCl and then with satd. aq. NaHCO3, and dried over Na2SO4. The solvent was removed in vacuo and the crude product was purified using an ISCO column (0-5% MeOH/CH2Cl2) followed by recrystallization from either acetone/hexanes or CH2Cl2/hexanes providing benzothiazepines as red or orange solids. Detailed experimental details are provided as Supplemental Information.
Supplementary Material
Highlights.
- Tetracyclic Benzothiazepines identified as novel class of potent antimalarials
- The molecular target of benzothiazepines has been identified through pathway-based screen
- Benzothiazepines inhibit selectively plasmodium over human cytochrome bc1
- Benzothiazepines are active against atovaquone resistant parasites.
Acknowledgements
We thank Roger Wiegand, Ted Sybertz and the members of the Broad Institute-Genzyme-MMV Malaria Drug Development Initiative for thoughtful discussions; Stuart Schreiber and the Broad Chemical Biology Program for access to key instrumentation and reagents; Chris Johnson, Galina Beletsky and Stephen Jonston for analytical support. This work was supported by grants from Medicines for Malaria Venture (MMV), The Broad Institute (SPARC), the NIH NICHD K12-HD000850 (J.D.D.), the NSF Graduate Research Fellowship Program (V.P.), the Harvard Malaria Initiative (D.F.W.), and partial infrastructure supported by NIH-G12RR03051 (A.E.S.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aregawi M, Cibulskis R, Otten M. World Malaria Report, 2008. World Malaria Report. 2008 [Google Scholar]
- Baniecki ML, Wirth DF, Clardy J. High-Throughput Plasmodium falciparum Growth Assay for Malaria Drug Discovery. Antimicrob. Agents Chemother. 2007;51:716–723. doi: 10.1128/AAC.01144-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker RHJ, Urgaonkar S, Mazitschek R, Celatka C, Skerlj R, Cortese JF, Tyndall E, Liu H, Cromwell M, Sidhu AB, Guerrero-Bravo JE, Crespo-Llado KN, Serrano AE, Lin JW, Janse CJ, Khan SM, Duraisingh M, Coleman BI, Angulo-Barturen I, Jimenez-Diaz MB, Magan N, Gomez V, Ferrer S, Martinez MS, Wittlin S, Papastogiannidis P, O'Shea T, Klinger JD, Bree M, Lee E, Levine M, Wiegand RC, Munoz B, Wirth DF, Clardy J, Bathurst I, Sybertz E. Aminoindoles, a Novel Scaffold with Potent Activity against Plasmodium falciparum. Antimicrob. Agents Chemother. 2011;55:2612–2622. doi: 10.1128/AAC.01714-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagini GA, Fisher N, Berry N, Stocks PA, Meunier B, Williams DP, Bonar-Law R, Bray PG, Owen A, O'Neill PM, Ward SA. Acridinediones: Selective and Potent Inhibitors of the Malaria Parasite Mitochondrial bc1 Complex. Mol. Pharmacol. 2008;73:1347–1355. doi: 10.1124/mol.108.045120. [DOI] [PubMed] [Google Scholar]
- Biagini GA, Viriyavejakul P, O'Neill PM, Bray PG, Ward SA. Functional characterization and target validation of alternative complex I of Plasmodium falciparum mitochondria. Antimicrob. Agents Chemother. 2006;50:1841–1851. doi: 10.1128/AAC.50.5.1841-1851.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasseur G, Saribaş AS, Daldal F. A compilation of mutations located in the cytochrome b subunit of the bacterial and mitochondrial bc1 complex. Biochim. Biophys. Acta. 1996;1275:61–69. doi: 10.1016/0005-2728(96)00051-5. [DOI] [PubMed] [Google Scholar]
- Canfield CJ, Pudney M, Gutteridge WE. Interactions of atovaquone with other antimalarial drugs against Plasmodium falciparum in vitro. Exp. Parasitol. 1995;80:373–381. doi: 10.1006/expr.1995.1049. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Lawrence G, Reed C, Stowers A, Ranford-Cartwright L, Creasey A, Carter R, Saul A. Measurement of Plasmodium falciparum growth rates in vivo: a test of malaria vaccines. Am J Trop Med Hyg. 1997;57:495–500. doi: 10.4269/ajtmh.1997.57.495. [DOI] [PubMed] [Google Scholar]
- Chulay JD. Challenges in the development of antimalarial drugs with causal prophylactic activity. Trans. R. Soc. Trop. Med. Hyg. 1998;92:577–579. doi: 10.1016/s0035-9203(98)90772-6. [DOI] [PubMed] [Google Scholar]
- Chulay JD, Haynes JD, Diggs CL. Plasmodium falciparum: assessment of in vitro growth by [3H]hypoxanthine incorporation. Exp. Parasitol. 1983;55:138–146. doi: 10.1016/0014-4894(83)90007-3. [DOI] [PubMed] [Google Scholar]
- Dong CK, Patel V, Yang JC, Dvorin JD, Duraisingh MT, Clardy J, Wirth DF. Type II NADH dehydrogenase of the respiratory chain of Plasmodium falciparum and its inhibitors. Bioorg. Med. Chem. Lett. 2009;19:972–975. doi: 10.1016/j.bmcl.2008.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S. Antimalarial drug discovery: efficacy models for compound screening. Nat. Rev. Drug Discovery. 2004;3:509–520. doi: 10.1038/nrd1416. [DOI] [PubMed] [Google Scholar]
- Fisher N, Meunier B. Molecular basis of resistance to cytochrome bc1inhibitors. FEMS Yeast Research. 2008;8:183–192. doi: 10.1111/j.1567-1364.2007.00328.x. [DOI] [PubMed] [Google Scholar]
- Fivelman QL, Butcher GA, Adagu IS, Warhurst DC, Pasvol G. Malarone treatment failure and in vitro confirmation of resistance of Plasmodium falciparum isolate from Lagos, Nigeria. Malaria Journal. 2002;1:1. doi: 10.1186/1475-2875-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck SL, Pudney M, Sinden RE. The effect of atovaquone (566C80) on the maturation and viability of Plasmodium falciparum gametocytes in vitro. Trans. R. Soc. Trop. Med. Hyg. 1996;90:309–312. doi: 10.1016/s0035-9203(96)90266-7. [DOI] [PubMed] [Google Scholar]
- Fry M, Pudney M. Site of action of the antimalarial hydroxynaphthoquinone, 2-[trans-4-(4'-chlorophenyl) cyclohexyl]-3-hydroxy-1,4-naphthoquinone (566C80). Biochem. Pharmacol. 1992;43:1545–1553. doi: 10.1016/0006-2952(92)90213-3. [DOI] [PubMed] [Google Scholar]
- Geary TG, Divo AA, Jensen JB. An in vitro assay system for the identification of potential antimalarial drugs. J. Parasitol. 1983;69:577–583. [PubMed] [Google Scholar]
- Hunte C, Palsdottir H, Trumpower BL. Protonmotive pathways and mechanisms in the cytochrome bc1 complex. FEBS Lett. 2003;545:39–46. doi: 10.1016/s0014-5793(03)00391-0. [DOI] [PubMed] [Google Scholar]
- Jones ME. Pyrimidine nucleotide biosynthesis in animals: genes, enzymes, and regulation of UMP biosynthesis. Annu. Rev. Biochem. 1980;49:253–279. doi: 10.1146/annurev.bi.49.070180.001345. [DOI] [PubMed] [Google Scholar]
- Kawai S, Kano S, Suzuki M. Morphologic effects of artemether on Plasmodium falciparum in Aotus trivirgatus. Am J Trop Med Hyg. 1993;49:812–818. doi: 10.4269/ajtmh.1993.49.812. [DOI] [PubMed] [Google Scholar]
- Kazanjian P, Armstrong W, Hossler PA, Lee CH, Huang L, Beard CB, Carter J, Crane L, Duchin J, Burman W, Richardson J, Meshnick SR. Pneumocystis carinii cytochrome b mutations are associated with atovaquone exposure in patients with AIDS. The Journal of infectious diseases. 2001;183:819–822. doi: 10.1086/318835. [DOI] [PubMed] [Google Scholar]
- Kelly JX, Smilkstein MJ, Brun R, Wittlin S, Cooper RA, Lane KD, Janowsky A, Johnson RA, Dodean RA, Winter R, Hinrichs DJ, Riscoe MK. Discovery of dual function acridones as a new antimalarial chemotype. Nature. 2009;459:270–273. doi: 10.1038/nature07937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerscher SJ. Diversity and origin of alternative NADH:ubiquinone oxidoreductases. Biochim. Biophys. Acta. 2000;1459:274–283. doi: 10.1016/s0005-2728(00)00162-6. [DOI] [PubMed] [Google Scholar]
- Kessl JJ, Meshnick SR, Trumpower BL. Modeling the molecular basis of atovaquone resistance in parasites and pathogenic fungi. Trends Parasitol. 2007;23:494–501. doi: 10.1016/j.pt.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Korsinczky M, Chen N, Kotecka B, Saul A, Rieckmann K, Cheng Q. Mutations in Plasmodium falciparum cytochrome b that are associated with atovaquone resistance are located at a putative drug-binding site. Antimicrob. Agents Chemother. 2000;44:2100–2108. doi: 10.1128/aac.44.8.2100-2108.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysin MY PVV, Shikhaliev KS, Gozhina OV, Trefilova IN. New fused thiazepines. (Translated from written in Russian.). Izvestiya Vysshikh Uchebnykh Zavedenii, Khimiya i Khimicheskaya Tekhnologiya. 2003;46:12–14. [Google Scholar]
- Looareesuwan S, Viravan C, Webster HK, Kyle DE, Hutchinson DB, Canfield CJ. Clinical Studies of Atovaquone, Alone or in Combination with other Antimalarial Drugs, for Treatment of Acute Uncomplicated Malaria in Thailand. Am J Trop Med Hyg. 1996;54:62–66. doi: 10.4269/ajtmh.1996.54.62. [DOI] [PubMed] [Google Scholar]
- Martyn DC, Cortese JF, Tyndall E, Dick J, Mazitschek R, Munoz B, Clardy J. Antiplasmodial activity of piperazine sulfonamides. Bioorg. Med. Chem. Lett. 2010a;20:218–221. doi: 10.1016/j.bmcl.2009.10.130. [DOI] [PubMed] [Google Scholar]
- Martyn DC, Nijjar A, Celatka CA, Mazitschek R, Cortese JF, Tyndall E, Liu H, Fitzgerald MM, O'Shea TJ, Danthi S, Clardy J. Synthesis and antiplasmodial activity of novel 2,4-diaminopyrimidines. Bioorg. Med. Chem. Lett. 2010b;20:228–231. doi: 10.1016/j.bmcl.2009.10.133. [DOI] [PubMed] [Google Scholar]
- McIntosh MT, Srivastava R, Vaidya AB. Divergent evolutionary constraints on mitochondrial and nuclear genomes of malaria parasites. Mol. Biochem. Parasitol. 1998;95:69–80. doi: 10.1016/s0166-6851(98)00093-0. [DOI] [PubMed] [Google Scholar]
- Nkrumah LJ, Muhle RA, Moura PA, Ghosh P, Hatfull GF, Jacobs WR, Fidock DA. Efficient site-specific integration in Plasmodium falciparum chromosomes mediated by mycobacteriophage Bxb1 integrase. Nature Methods. 2006;3:615–621. doi: 10.1038/nmeth904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter HJ, Morrisey JM, Mather MW, Vaidya AB. Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum. Nature. 2007;446:88–91. doi: 10.1038/nature05572. [DOI] [PubMed] [Google Scholar]
- Patel V, Booker M, Kramer M, Ross L, Celatka CA, Kennedy LM, Dvorin JD, Duraisingh MT, Sliz P, Wirth DF, Clardy J. Identification and characterization of small molecule inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase. The Journal of biological chemistry. 2008;283:35078–35085. doi: 10.1074/jbc.M804990200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters W. Chemotherapy and Drug Resistance in Malaria. Academic Press; 1987. [Google Scholar]
- Petersen E. Prevention and treatment of congenital toxoplasmosis. Expert Rev. Anti-Infect. Ther. 2007;5:285–293. doi: 10.1586/14787210.5.2.285. [DOI] [PubMed] [Google Scholar]
- Ploemen IHJ, Prudêncio M, Douradinha BG, Ramesar J, Fonager J, van Gemert G-J, Luty AJF, Hermsen CC, Sauerwein RW, Baptista FG, Mota MM, Waters AP, Que I, Lowik CWGM, Khan SM, Janse CJ, Franke-Fayard BMD. Visualisation and quantitative analysis of the rodent malaria liver stage by real time imaging. PLoS ONE. 2009;4:e7881. doi: 10.1371/journal.pone.0007881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putt KS, Hergenrother PJ. An enzymatic assay for poly(ADP-ribose) polymerase-1 (PARP-1) via the chemical quantitation of NAD(+): application to the high-throughput screening of small molecules as potential inhibitors. Anal. Biochem. 2004;326:78–86. doi: 10.1016/j.ab.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Ramachary DB, Anebouselvy K, Chowdari NS, Barbas CF. Direct organocatalytic asymmetric heterodomino reactions: the Knoevenagel/Diels-Alder/epimerization sequence for the highly diastereoselective synthesis of symmetrical and nonsymmetrical synthons of benzoannelated centropolyquinanes. J. Org. Chem. 2004;69:5838–5849. doi: 10.1021/jo049581r. [DOI] [PubMed] [Google Scholar]
- Rathod PK, McErlean T, Lee PC. Variations in frequencies of drug resistance in Plasmodium falciparum. Proc. Natl. Acad. Sci. U S A. 1997;94:9389–9393. doi: 10.1073/pnas.94.17.9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DM, McCarthy W, Slavinsky J, Chan CK, Montaner J, Braun J, Dohn MN, Caldwell PT. Atovaquone suspension for treatment of Pneumocystis carinii pneumonia in HIV-infected patients. AIDS (London, England) 2001;15:211–214. doi: 10.1097/00002030-200101260-00010. [DOI] [PubMed] [Google Scholar]
- Schwöbel B, Alifrangis M, Salanti A, Jelinek T. Different mutation patterns of atovaquone resistance to Plasmodium falciparum in vitro and in vivo: rapid detection of codon 268 polymorphisms in the cytochrome b as potential in vivo resistance marker. Malaria Journal. 2003;2:5. doi: 10.1186/1475-2875-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava IK, Morrisey JM, Darrouzet E, Daldal F, Vaidya AB. Resistance mutations reveal the atovaquone-binding domain of cytochrome b in malaria parasites. Mol. Microbiol. 1999;33:704–711. doi: 10.1046/j.1365-2958.1999.01515.x. [DOI] [PubMed] [Google Scholar]
- Srivastava IK, Rottenberg H, Vaidya AB. Atovaquone, a broad spectrum antiparasitic drug, collapses mitochondrial membrane potential in a malarial parasite. The Journal of biological chemistry. 1997;272:3961–3966. doi: 10.1074/jbc.272.7.3961. [DOI] [PubMed] [Google Scholar]
- Stockwell BR. Chemical genetics: ligand-based discovery of gene function. Nat. Rev. Genet. 2000;1:116–125. doi: 10.1038/35038557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Trumpower BL. The protonmotive Q cycle. Energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc1 complex. The Journal of biological chemistry. 1990;265:11409–11412. [PubMed] [Google Scholar]
- Trumpower BL, Gennis RB. Energy transduction by cytochrome complexes in mitochondrial and bacterial respiration: the enzymology of coupling electron transfer reactions to transmembrane proton translocation. Annu. Rev. Biochem. 1994;63:675–716. doi: 10.1146/annurev.bi.63.070194.003331. [DOI] [PubMed] [Google Scholar]
- Urgaonkar S, Cortese JF, Barker RH, Cromwell M, Serrano AE, Wirth DF, Clardy J, Mazitschek R. A concise silylamine approach to 2-amino-3-hydroxy-indoles with potent in vivo antimalaria activity. Org. Lett. 2010;12:3998–4001. doi: 10.1021/ol101566h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenz T, Covian R, Hellwig P, Macmillan F, Meunier B, Trumpower BL, Hunte C. Mutational analysis of cytochrome b at the ubiquinol oxidation site of yeast complex III. The Journal of biological chemistry. 2007;282:3977–3988. doi: 10.1074/jbc.M606482200. [DOI] [PubMed] [Google Scholar]
- Winter R, Kelly JX, Smilkstein MJ, Dodean RA, Hinrichs DJ, Riscoe MK. Antimalarial quinolones: Synthesis, potency, and mechanistic studies. Exp. Parasitol. 2008;118:487–497. doi: 10.1016/j.exppara.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.