Abstract
Objective
To investigate the incidence of noncardiac vascular disease in a community-based incidence cohort of patients with rheumatoid arthritis (RA) and compare to that in the general population. To investigate trends in incidence of noncardiac vascular disease in patients with RA.
Methods
A population-based inception cohort of patients with RA in Olmsted County, Minnesota with incident RA between 1/1/1980 and 12/31/2007 and a cohort of non-RA subjects from the same population base was assembled and followed until 12/31/2008. Venous thromboembolic events (VTE), cerebrovascular events, and peripheral arterial events were ascertained by medical record review.
Results
The study population included 813 patients with RA (mean age [SD] 55.9 [15.7] years, 68% women), with average length of follow-up of 9.6 years [SD 6.9]. Compared to non-RA subjects of similar age and sex, patients diagnosed with RA between 1995 and 2007 had a higher incidence (%) of VTE compared to non-RA subjects (cumulative incidence [±SE] 6.7 ± 1.7 vs 2.8 ± 1.1, respectively; p=0.005), but similar rates of cerebrovascular and peripheral arterial events. Among patients with RA, the incidence of VTE, cerebrovascular events, and peripheral arterial events was similar in the 1995–2007 time period compared to the 1980–1994 time period.
Conclusion
The incidence of VTE appears to be increased in patients with RA compared to non-RA subjects. The incidence of cerebrovascular events and peripheral vascular disease events was similar in patients with RA compared to non-RA subjects. Among patients with RA, the incidence of noncardiac vascular disease has remained stable in recent decades.
Patients with rheumatoid arthritis (RA) have an increased incidence of cardiovascular disease (CVD) compared to the general population (1, 2). Patients with RA also have increased premature mortality, some of which is due to this increased risk of CVD (3). Like CVD, noncardiac vascular disease may be a manifestation of systemic involvement in RA, and may have important impact on the health of patients with RA.
While there is a considerable body of literature regarding the risk of heart disease as a component of the overall CVD risk burden in patients with RA, there is very little known about the incidence, risk, and outcome of noncardiac vascular disease among these patients. The primary focus of interest in vascular disease in the study of patients with RA has been on coronary artery disease, but it is likely that the entire vasculature is affected. Less well studied is noncardiac vascular disease in RA, including venous thromboembolism (VTE) (4–7), transient ischemic attack (8, 9), stroke (10–13), aortic aneurysm (14), arterial thromboembolism (6), and peripheral artery disease (15–17).
Little is known regarding the potential risk factors for noncardiac vascular disease and what influence the traditional cardiovascular risk factors of previous myocardial infarction, obesity, diabetes, hypertension, and smoking have on development of these events in patients with RA. The contribution of these risk factors has been examined in relation to ischemic stroke (11), but as yet no study has addressed these risk factors in relation to other noncardiac vascular diseases. There are some data regarding the effect of RA therapies on ischemic stroke in patients with RA (11, 18), but there is little information about how medications used to treat RA might influence the incidence of other noncardiac vascular diseases (6).
The purpose of this study was to investigate the incidence of noncardiac vascular disease events in a community-based incidence cohort of patients with RA and to compare this incidence to that in the general population in the same community. Among patients with RA, the incidence of noncardiac vascular disease events was also compared to that found in an earlier decade in a previous study by our group (10). In addition, we examined risk factors that may be associated with development of these events in patients with RA.
Patients and Methods
The population of Olmsted County, Minnesota is well suited for investigation of the epidemiology of RA and noncardiac vascular disease because comprehensive medical records on all residents who have sought medical care are available. A record linkage system allows ready access to the medical records from all health care providers for the local population, including the Mayo Clinic and its affiliated hospitals, the Olmsted Medical Group, the Olmsted Community Hospital, local nursing homes, and the few private practitioners. The potential of this data system for use in population-based studies has been described previously (19, 20). This system ensures virtually complete ascertainment of all clinically recognized cases of RA among the residents of Olmsted County, Minnesota.
Using this data resource, an inception cohort of all cases of RA first diagnosed between January 1, 1980 and December 31, 2007 (n=813) among Olmsted County residents ≥18 years of age was assembled as previously described (21–23). The incidence date was defined as the earliest date at which the patient fulfilled at least 4 of the 7 American College of Rheumatology (ACR; formerly, the American Rheumatism Association) 1987 classification criteria for RA (24). The population of Olmsted County in 2000 was estimated to be 124,277, and the annual incidence of RA during 1995–2007 was estimated to be 40.9 per 100,000 population (23). All cases were followed up longitudinally through their entire medical records until death, migration from Olmsted County, or 12/31/2008. A comparison cohort of Olmsted County residents without RA with similar age, sex and calendar year was identified. The index date for these non-RA subjects was defined as the RA incidence date of the corresponding patient with RA.
A comprehensive code list for diagnoses of noncardiac vascular diseases was electronically cross matched with the medical records of the patients in the RA cohort and the non-RA comparison cohort. The subset of patients in each cohort whose medical records contained one or more of the codes of interest were reviewed (by AKB) to confirm the diagnosis based on the specified criteria. To characterize vascular morbidity in this cohort of patients with RA, the date of first occurrence of unique noncardiac vascular disease was recorded. Noncardiac vascular disease included VTE (deep venous thrombosis (DVT) or pulmonary embolism (PE)), cerebrovascular events (hemorrhagic stroke, nonhemorrhagic stroke, transient ischemic attack, or amaurosis fugax), and peripheral arterial events (abdominal aortic aneurysm, renal artery stenosis, peripheral artery disease, or arterial thromboembolism), as determined by fulfillment of the criteria for each disease entity (Table 1). Among the non-RA comparison cohort, the date of first occurrence of unique noncardiac vascular disease was recorded.
Table 1.
Criteria for inclusion in the study of noncardiac vascular disease in rheumatoid arthritis (RA) and non-RA subjects*
| 1. Hemorrhagic stroke | Clinical diagnosis by neurologist + verified by CT/MRI or autopsy or cerebrospinal fluid analysis |
| 2. Nonhemorrhagic stroke or nonspecified stroke | Clinical diagnosis by neurologist + verified by CT/MRI or autopsy |
| 3. Transient ischemic attack | Clinical diagnosis by neurologist |
| 4. Amaurosis fugax | Clinical diagnosis |
| 5. Aortic aneurysm | Diameter increased >50% compared with normal values; diameter ≥3.0 cm in abdominal aorta (43); verified by ultrasound/CT or at autopsy |
| 6. Renal artery stenosis | Verified by ultrasound/renal scintigraphy/angiography; assumed clinically significant if detected |
| 7. Peripheral artery disease or atherosclerosis obliterans | Clinical diagnosis supported by documented vascular physical examination; ankle/brachial index <0.9 or angiography confirming disease, if performed |
| 8. Arterial thromboembolism | Clinical diagnosis supported by angiography or autopsy |
| 9. Deep vein thrombosis | Verified by phlebography/venography or ultrasound or autopsy |
| 10. Pulmonary embolism | Verified by angiography, CT angiography, scintigraphy, or autopsy |
CT = computed tomography; MRI = magnetic resonance imaging
A clinical diagnosis of a noncardiac vascular disease listed above was accepted if physician or other medical provider documentation was present in the medical record that the patient has the condition of interest. Several conditions (e.g. stroke, peripheral artery disease, arterial thromboembolism, and VTE) were verified with objective data (imaging and related examination) in addition to the clinical diagnosis. For conditions where there was no objective data (e.g. transient ischemic attack and amaurosis fugax), we accepted the clinician diagnosis if the physician documented that this condition was present. A consensus discussion among the investigators with medical record review was held to resolve any unclear situations or discrepancies. Disease prevalence was assessed by recording those cases in which a vascular disease occurred prior to the diagnosis of RA or prior to the index date in the non-RA comparison cohort. Prevalent cases were excluded from the incidence analyses.
Cardiovascular risk factor data have been collected on patients in the RA cohort and the non-RA comparison cohort as previously described (25–27). The cardiovascular risk factor data include: cigarette smoking status (current, former, or never); presence of dyslipidemia, hypertension, diabetes mellitus; personal cardiac history including presence of angina pectoris, coronary artery disease, ischemic heart disease, myocardial infarction, heart failure (defined by Framingham criteria (28)), pulmonary edema, or coronary revascularization procedures (e.g. coronary artery bypass graft, percutaneous angioplasty, insertion of stents, and atherectomy); height and weight measurements at baseline and computed body mass index (BMI); and family history of coronary heart disease. Data were also collected on medication use in the RA cohort including methotrexate, hydroxychloroquine, other disease modifying anti-rheumatic drugs (DMARD; includes gold, sulfasalazine, azathioprine, cyclophosphamide, cyclosporine, D-penicillamine or leflunomide), biologic agents, corticosteroids, non-steroidal anti-inflammatory drugs (NSAID) including cox-2 inhibitors, and aspirin. Atrial fibrillation was defined as the date that atrial fibrillation was first noted on electrocardiogram. The dates of all hospital admissions were collected in both cohorts. In the RA cohort, data indicating RA disease severity including rheumatoid factor (RF) positivity, anti-citrullinated peptide antibody (ACPA), erosive disease, extra-articular manifestations, joint swelling, erythrocyte sedimentation rates, or joint arthroplasty were previously collected.
Statistical Methods
The cumulative incidence of each noncardiac vascular disease event adjusted for the competing risk of death was estimated (29). These methods are similar to Kaplan-Meier method with censoring of patients who are still alive at last follow-up. However, patients who die before experiencing noncardiac vascular disease events are appropriately accounted for to avoid overestimation of the rate of occurrence of noncardiac vascular disease events, which can occur if such subjects are simply censored at death. For each type of noncardiac vascular disease, patients whose first occurrence was prior to the diagnosis of RA, or prior to the index date for subjects in the non-RA comparison cohort, were excluded from the analysis of cumulative incidence. In addition, the cumulative incidence of combinations of related diagnoses of noncardiac vascular disease entities was estimated, including VTE events, cerebrovascular events, and peripheral arterial events. The date of occurrence of each of these combinations was the earliest date of occurrence of the any of the individual diseases in that category. Cumulative incidence rates for the two cohorts (1995–2007 and 1985–1994) were compared using methods by Gray (30).
Cox proportional hazards models were used to compare the rate of development of noncardiac vascular diseases, individually and in combination, between patients with RA and the non-RA comparison cohort. In addition, Cox proportional hazards models were used to assess the trends in noncardiac vascular disease over time and the association of risk factors on the development of noncardiac vascular events among patients with RA. Specifically, the differences in the rates of occurrence of noncardiac vascular disease comparing patients with incident RA in the 1995–2007 time period to patients with incident RA in the 1980–1994 time period were assessed. Age was used as the time scale for these models to provide optimal adjustment for age under the assumption that age is likely the most important time determinate of noncardiac vascular disease.
Subjects entered the model at the age they met criteria for RA and remained in the model until the age of each unique noncardiac vascular disease event. Subjects without events were censored at the age of death or last follow-up. The models were stratified by sex. Risk factors of interest include those described above. Time-dependent covariates were used to model risk factors that developed over time. These time-dependent covariates allowed patients to be modeled as unexposed to the risk factor during the follow-up time prior to development of the risk factor, then change to exposed following development of the risk factor. Only events affecting an adequate number of patients (n ≥ 20) were examined in these risk factor analyses. Risk factor analyses were limited to combined events due to the relative paucity of individual event types and the large number of risk factors to be assessed.
Results
The RA cohort consisted of 813 patients with RA who received their diagnosis between January 1, 1980 and December 31, 2007. All patients were 18 years or older and were residents of Olmsted County, Minnesota at the time of RA diagnosis. There were 556 women and 257 men. The mean age [SD] at diagnosis was 55.9 [15.7] years. The average length of followup was 9.6 [6.9] years, and 537 (66%) had positive rheumatoid factor. The 464 patients diagnosed with RA between January 1, 1995 and December 31, 2007 were compared to 464 subjects without RA of similar age and sex (mean age [SD] 55.6 [15.5] years, 69% women) who were also residents of Olmsted County, Minnesota at index date.
Among medications used for treatment of RA, 469 patients (58%) had received methotrexate, 480 (59%) had received hydroxychloroquine, 258 (32%) had received another DMARD, 137 (17%) had received a biologic, 627 (77%) had received corticosteroids, 390 (48%) had received a cox-2 inhibitor, and 737 (91%) had taken an NSAID for treatment of RA. In the RA cohort, 349 (43%) used low dose aspirin for cardioprotection.
Patients diagnosed with RA between 1995 and 2007 were compared to non-RA subjects. Among patients with RA diagnosed in this time period, there were 37 patients with at least one noncardiac vascular disease event (15 with VTE, 11 with cerebrovascular, 6 with peripheral arterial, 1 with VTE and cerebrovascular, 3 with VTE and peripheral arterial, and 1 with cerebrovascular and peripheral arterial) after RA incidence date. In the same time period, there were 25 non-RA subjects with at least one noncardiac vascular disease event (7 with VTE, 9 with cerebrovascular, 7 with peripheral arterial, and 2 with cerebrovascular and peripheral arterial) after the index date. Notably, there were 52 noncardiac vascular disease events (16 VTE, 16 cerebrovascular, 20 peripheral arterial) among 47 patients with RA prior to the RA incidence date and 39 noncardiac vascular disease events (12 VTE, 17 cerebrovascular, 10 peripheral arterial) among 33 non-RA subjects prior to the index date; patients with each individual noncardiac vascular disease entity were removed from the incidence analysis for that entity.
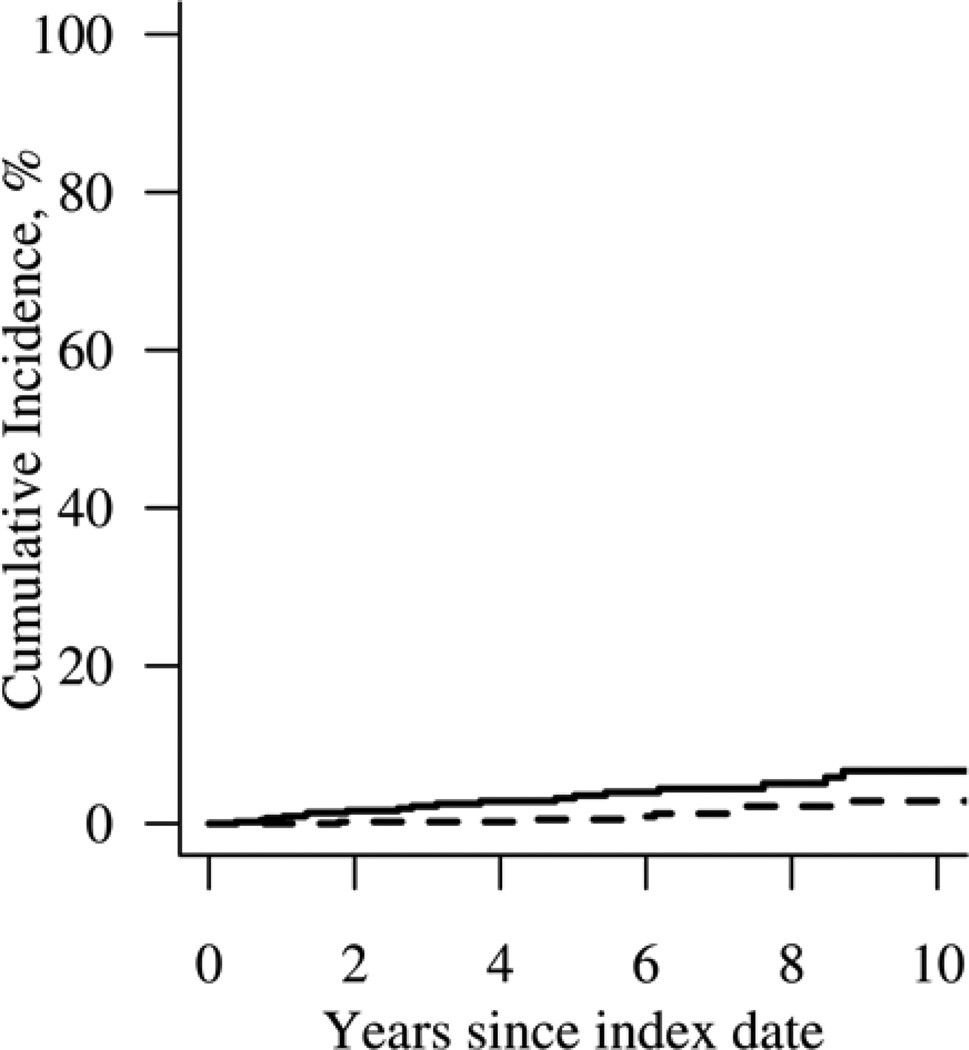
Patients with RA had a higher cumulative incidence (%) at 10 years of VTE compared to non-RA subjects (cumulative incidence [±SE] 6.7 ± 1.7 vs 2.8 ± 1.1, respectively; p=0.005). This increased risk of VTE in patients with RA was noted to occur steadily throughout the follow-up period (Figure 1). When VTE events were analyzed individually, patients with RA had a higher 10-year cumulative incidence of both DVT (cumulative incidence [±SE] 3.1 ± 1.0 vs 1.7 ± 0.9; p=0.034) and PE (cumulative incidence [±SE] 4.8 ± 1.5 vs 1.6 ± 0.7; p=0.050) compared to non-RA subjects. Patients with RA were more than 3 times more likely to experience VTE compared to non-RA subjects (HR 3.6; 95% CI 1.5 – 8.6). The risk of VTE was significantly higher within 90 days following hospital admission in both cohorts (p<0.001). Following adjustment for hospitalization, the difference in risk of VTE between patients with RA and non-RA subjects persisted (HR 4.4; 95% CI 1.6 – 12.0).
Figure 1.
Cumulative incidence of venous thromboembolic events in 464 patients with incident rheumatoid arthritis (solid line) in 1995–2007 compared to 464 non-RA subjects (dashed line; p=0.005).
The 10-year cumulative incidence (%) of cerebrovascular events was similar between patients with RA and non-RA subjects (cumulative incidence [±SE] 4.1 ± 1.2 vs 4.3 ± 1.5, respectively; p=0.47). There was no evidence of an increased risk of cerebrovascular events in patients with RA compared to non-RA subjects (HR: 1.4; 95% CI: 0.6–3.0). Likewise, the 10-year cumulative incidence of peripheral arterial events (cumulative incidence [±SE] 3.3 ± 1.1 vs 3.8 ± 1.4, respectively; p=0.58) was similar between patients with RA and non-RA subjects (HR: 1.5; 95% CI: 0.6 – 3.7). The combined 10-year cumulative incidence [±SE] of cerebrovascular and peripheral arterial events was 6.4 ± 1.5 among patients with RA compared with 7.2 ± 2.0 among non-RA subjects (p=0.39).
Cox proportional hazards models adjusted for calendar year were used to assess potential risk factors for VTE events in patients with RA. Among the patients with RA, 537 (66%) had a positive rheumatoid factor, 105 (13%) had 3 or more erythrocyte sedimentation rates ≥ 60 mm/hour, 433 (53%) had joint erosions or destructive changes, 267 (33%) had rheumatoid nodules, 90 (11%) had severe extra-articular RA (31) and 147 (52% of 267 tests) had a positive ACPA; none of these characteristics were associated with an increased risk of VTE. At RA incidence or during followup, obesity (BMI ≥30 kg/m2) (hazard ratio [HR] 2.2, 95% confidence interval [CI] 1.2 – 3.9; p=0.010) was a time-dependent risk factor for VTE. Among medications used to treat RA, exposure to a DMARD other than methotrexate or hydroxychloroquine (e.g. gold, sulfasalazine, azathioprine, cyclophosphamide, cyclosporine, D-penicillamine or leflunomide) was a time dependent risk factor for VTE (HR 1.9, 95% CI 1.02 – 3.6; p=0.042) as was exposure to a biologic (HR 2.7, 95% CI 1.2 – 6.3; p=0.016). When all medications to treat RA were included in the same model, these associations were attenuated (other DMARDs HR 1.7; 95% CI 0.9 – 3.3; p=0.11 and biologic HR 2.3; 95% CI 0.9 – 5.8; p=0.07). The use of low dose aspirin for cardioprotection was also a time-dependent risk factor for VTE (HR 2.3; 95% CI 1.2 – 4.3; p=0.009). A recent lower extremity arthroplasty (within 90 days post-surgery) was a statistically significant time-dependent risk factor for VTE (HR 11.4; 95% CI 2.5 – 51.8; p=0.002). Heart failure was not significantly associated with VTE (HR 1.5; 95% CI 0.6 – 3.5; p=0.36). Atrial fibrillation was marginally associated with VTE (HR 2.0, 95% CI 0.9 – 4.4; p=0.07).
To assess for change in the incidence of noncardiac vascular disease events over time among patients with RA, those diagnosed with RA between 1995 and 2007 (n=464) were compared with patients diagnosed with RA between 1980 and 1994 (n=349). Among patients diagnosed with RA between 1995 and 2007, there were 37 patients with at least one noncardiac vascular disease event (15 with VTE, 11 with cerebrovascular, 6 with peripheral arterial, 1 with VTE and cerebrovascular, 3 with VTE and peripheral arterial, and 1 with cerebrovascular and peripheral arterial) after RA incidence date. Among those patients diagnosed with RA between 1980 and 1994, there were 71 patients with at least one noncardiac vascular disease event (19 VTE, 20 cerebrovascular, 14 peripheral arterial, 6 with VTE and cerebrovascular, 2 with VTE and peripheral arterial, 8 with cerebrovascular and peripheral arterial and 2 with VTE, cerebrovascular and peripheral arterial) that occurred after the RA incidence. Prior to RA incidence, there were a total of 60 noncardiac vascular disease events (17 VTE, 18 cerebrovascular, 19 peripheral arterial, 1 with VTE and cerebrovascular, 3 with VTE and peripheral arterial, and 2 with cerebrovascular and peripheral arterial); patients with each individual noncardiac vascular disease entity were removed from the incidence analysis for that entity.
The incidence of PE was higher in the 1995–2007 time period compared with the 1980–1994 time period (cumulative incidence [±SE] 4.8 ± 1.5 vs 0.6 ± 0.4, respectively; p=0.002). However, the incidence of VTE was not significantly different between the 1995–2007 and 1980–1994 time periods (cumulative incidence [±SE] 6.7 ± 1.7 vs 3.6 ± 1.1, respectively; p=0.12). The incidence of cerebrovascular disease events was similar in the 1995–2007 time period compared to the 1980–1994 time period (cumulative incidence [±SE] 4.1 ± 1.2 vs 3.0 ± 0.9, respectively; p=0.50). The incidence of peripheral arterial events was similar in the 1995–2007 time period compared to the 1980–1994 time period (cumulative incidence [±SE] 3.3 ± 1.1 vs 3.3 ± 1.0, respectively; p=0.85).
Event rates per 1000 person-years for patients with RA diagnosed in 1995–2007 are also of interest. Cerebrovascular events occurred at a rate of 5.0 per 1000 person years (95% CI: 2.6 – 8.5). The rate of VTE was 7.4 per 1000 person-years (95% CI: 4.4 – 11.5). The rate of peripheral arterial events was 3.9 per 1000 person-years (95% CI: 1.9 – 7.1), and the rate of development of first cerebrovascular or peripheral arterial event was 7.6 per 1000 person-years (95% CI: 4.5 – 11.8).
Discussion
Patients with RA diagnosed between 1995 and 2007 appear to have a higher incidence of VTE compared to non-RA subjects from the general population. Over the period 1980–2007, the incidence of VTE among patients with RA has remained stable. Although the technology used to confirm a diagnosis of VTE has evolved and improved over time, the finding that the incidence is different only between patients with RA and non-RA subjects, and did not increase during the study period, suggests that this finding cannot be explained by detection bias alone. While patients with RA may be seen on a more regular basis by a physician due to the chronic nature of their disease, it seems unlikely that VTE would be an incidental finding at a routine office visit that would not have caused the patient to seek medical attention when symptoms began.
The incidence of PE in the general population of Olmsted County appeared to decrease between 1966 and 1990, while the incidence of DVT was unchanged among men and was increased among older women (32). The incidence of all VTE (PE and DVT) occurring between 1991 and 1997 did not changed significantly compared to the period 1981 and 1990 in the Olmsted County population (33).
Reported independent risk factors for VTE include major surgery, hospital or nursing home confinement, trauma, malignancy, chemotherapy, leg paresis due to neurologic disease, previous central venous catheter or pacemaker placement, and superficial vein thromboses (34, 35). One study found that rheumatologic disease approached statistical significance as a risk factor for DVT in outpatients but did not define rheumatologic disease or how it was detected (36).
Our examination of potential time-dependent risk factors for VTE among patients with RA demonstrated that obesity was a time-dependent risk factor for VTE, which is consistent with other studies (37, 38). We did not find an association between congestive heart failure and VTE, which differs from the findings of Heit et al. (35) who found congestive heart failure to be a risk factor for VTE in patients hospitalized for acute medical illness. In the current study, there was an increased risk of VTE within 90 days of hospitalization in both patients with RA and non-RA subjects; adjusting for hospitalization revealed a persistent elevated risk for VTE in patients with RA. Ramagopalan et al. (7) reported an increased relative risk of VTE sustained over both short (≤ 90 days) and long (≥ 91 days) intervals following hospital admission among patients with RA compared to a reference cohort of non-RA patients.
Patients are at higher risk for VTE in the post-operative period in the general population (34). We evaluated this risk in the context of orthopedic surgeries in patients with RA, and found it to be increased as a time-dependent cofactor within 90 days following lower extremity arthroplasty. Matta el al. (4) found the incidence of VTE among hospitalized patients with RA who did not have joint surgery did not change between 1979 and 2005. VTE incidence was higher among hospitalized patients with RA who did not undergo joint surgery compared to both hospitalized RA patients who had joint surgery, and to hospitalized non-RA subjects who did not have joint surgery. Matta et al. (4) used diagnostic codes to identify patients with RA, PE, DVT, and orthopedic procedures. A strength of our study is that we used individual patient record information rather than relying on diagnostic codes, as reliance on database information only for the diagnosis of RA can be misleading (39).
The occurrence of VTE in our patient population was associated with exposure to biologics treatment. In our cohort, more than 95% of the biologic agents used are anti-tumor necrosis factor (TNF) agents. Our findings are similar to Kawakami et al. (5), who reported that DVT occurred more frequently in the group treated with anti-TNF agents compared to those receiving conventional DMARDs; use of TNF blockers was the only statistically significant risk factor for DVT. Petitpain et al. (6) reported the incidence of arterial and VTE events during anti-TNF therapy between 2000 and 2006 in patients with RA, spondyloarthropathies, and inflammatory bowel disease and found that VTE occurred in several patients without other risk factors for VTE. The authors suggest that anti-TNF therapy could favor development of VTE. The reasons for this association are unclear, but use of biologics may be a surrogate for RA and non-RA related factors which will require further exploration.
Antiphospholipid antibodies may contribute to the incidence of VTE among patients with RA. The prevalence of antiphospholipid antibodies among patients with RA ranges from 5% to 75% (40). Anti-TNF agents can induce the development of a variety of autoantibodies, including antiphospholipid antibodies (41). Most of the studies examining VTE in RA, including our study, are retrospective in nature and lack complete data regarding the presence of antiphospholipid antibodies (4–7), hence further research is needed to assess the possible contribution of antiphospholipid antibodies to VTE.
The incidence of cerebrovascular events was not significantly different between patients with RA and non-RA subjects in the current study. Other studies have reported conflicting results in regard to stroke incidence in RA patients (12, 13, 42). Similar to Nadareishvili et al. (11) we found that the presence of coronary heart disease, use of low-dose aspirin, and use of cox-2 inhibitors were risk factors for cerebrovascular events. Our study differs from Solomon et al. (12) who unexpectedly found that patients with RA with a previous history of CVD events did not have an increased risk of subsequent CVD events. Further research is needed to explore these associations.
Strengths of the current study include use of a population-based incident cohort with long-term follow up and record linkage system which permits capture of nearly all of the cases of RA in the community and minimizes referral bias.
Potential limitations of the study include the fact that the population of Olmsted County, Minnesota is predominantly Caucasian, and the results may not be generalized to other patient populations. The use of retrospective methodology limits the analysis to the case record data collected by the managing physician, although most of the factors that we adjusted for have been systematically recorded and utilized in our previous studies (25–27). We were unable to assess the contribution of physical activity level, the effects of biologic and other DMARDs especially with respect to confounding by indication, or NSAID, as these available over the counter and are not accurately recorded in the medical record. The study is also limited by the relative rarity of some individual event types. Hence, trend assessment was only performed for individual noncardiac vascular diseases or combinations of noncardiac vascular diseases with at least 20 events.
The well-recognized increased incidence of CVD in RA has lead to increased efforts at primary prevention as well as ongoing research into areas such as biomarkers that may be related to this increased risk. The results of our study indicate that patients with RA also have an increased risk of VTE compared to the general population. Future directions for research into these questions will include examination of the trends in the identified risk factors for VTE, such as arthroplasty and other types of surgical procedures, cancer and other possible risk factors for VTE. Efforts to identify and treat vascular disease in patients with RA should be extended to awareness and management of the entire vascular system.
Table 2.
Characteristics of 464 patients with incident rheumatoid arthritis (RA) in 1995–2007 and 464 patients without RA in 1995–2007
| Variable | RA | Non-RA | p-value |
|---|---|---|---|
| Age, years, mean ± SD | 55.6 ± 15.5 | 55.5 ± 15.5 | 0.97 |
| Female | 320 (69) | 320 (69) | 1.0 |
| Length of follow-up, years, mean ± SD | 5.9 ± 3.5 | 6.8 ± 3.6 | -- |
| Smoking | 0.19 | ||
| - current | 80 (17) | 69 (15) | |
| - former | 155 (33) | 138 (30) | |
| Alcohol abuse | |||
| At incidence/index date | 37 (8) | 30 (6) | 0.37 |
| Ever during follow-up | 41 (9) | 36 (8) | 0.51 |
| Hypertension | |||
| At incidence/index date | 191 (41) | 174 (38) | 0.25 |
| Ever during follow-up | 281 (67) | 245 (61) | 0.003 |
| Dyslipidemia | |||
| At incidence/index date | 279 (60) | 263 (57) | 0.29 |
| Ever during follow-up | 325 (75) | 317 (72) | 0.32 |
| Obesity (BMI ≥30 kg/m2) | |||
| At incidence/index date | 210 (45) | 192 (41) | 0.23 |
| Ever during follow-up | 238 (54) | 239 (54) | 0.86 |
| Diabetes mellitus | |||
| At incidence/index date | 48 (10) | 43 (9) | 0.58 |
| Ever during follow-up | 77 (20) | 81 (19) | 1.0 |
| Atrial fibrillation | |||
| At incidence/index date | 20 (4) | 22 (5) | 0.75 |
| Ever during follow-up | 47 (15) | 47 (12) | 0.67 |
| Rheumatoid factor positive | 306 (66) | -- | -- |
| Severe extra-articular RA, ever | 40 (11) | -- | -- |
| Total number of hospitalizations | 791 (29) | 640 (20) | <0.001 |
| Ever during follow-up (rate per 100 person-years) |
The values are given as number (%) if not indicated otherwise. Percentages for “ever during follow-up” are estimates of cumulative incidence at 10 years of follow-up with p-values obtained using Gray’s methods (see statistical methods section). P-values for “at incidence/index date” are obtained from chi-square tests.
Abbreviations: RA = rheumatoid arthritis; SD = standard deviation; BMI = body mass index; CHD = coronary heart disease
Table 3.
Cumulative incidence rates of noncardiac vascular events in 464 patients with incident rheumatoid arthritis in 1995–2007 compared to 464 non-RA subjects
| Event | Prior to RA incidence/ non-RA index date, no. (%) |
Number of events after incidence/index in RA / non- RA |
Cumulative incidence (%) at 10 years for RA patients (± SE) |
Cumulative incidence (%) at 10 years for non-RA subjects (± SE) |
p-value |
|---|---|---|---|---|---|
| 1. Hemorrhagic stroke | 0 / 2 | 2 / 1 | 0.8 ± 0.6 | 0.8 ± 0.8 | 0.49 |
| 2. Nonhemorrhagic or nonspecified stroke | 6 / 12 | 9/ 10 | 3.1 ± 1.1 | 4.3 ± 1.5 | 1.0 |
| 3. Transient ischemic attack | 11 / 8 | 3 / 2 | 0.5 ± 0.4 | 0.0 | 0.44 |
| 4. Amaurosis fugax | 1 / 1 | 2 / 0 | 0.7 ± 0.5 | 0.0 | 0.13 |
| Cerebrovascular events (events 1–4 above) | 16 / 17 | 13 / 11 | 4.1 ± 1.2 | 4.3 ± 1.5 | 0.43 |
| 5. Aortic aneurysm | 8 / 4 | 4 / 4 | 1.3 ± 0.7 | 1.8 ± 1.1 | 0.83 |
| 6. Renal artery stenosis | 5 / 1 | 0 / 1 | 0.0 | 0.2 ± 0.2 | 0.34 |
| 7. Peripheral artery disease or atherosclerosis obliterans | 8 / 5 | 5 / 4 | 1.6 ± 0.8 | 1.7 ± 0.9 | 0.60 |
| 8. Arterial thromboembolism | 0 / 0 | 1 / 1 | 0.3 ± 0.3 | 0.3 ± 0.3 | 0.94 |
| Peripheral arterial events (events 5–8 above) | 20 / 10 | 10 / 9 | 3.3 ± 1.1 | 3.8 ± 1.4 | 0.58 |
| Cerebrovascular and peripheral arterial events (events 1–8 above) | 34 / 23 | 19 / 17 | 6.4 ± 1.5 | 7.2 ± 2.0 | 0.39 |
| 9. Deep vein thrombosis | 14 / 10 | 11 / 4 | 3.1 ± 1.0 | 1.7 ± 0.9 | 0.034 |
| 10. Pulmonary embolism | 3 / 5 | 12 / 5 | 4.8 ± 1.5 | 1.6 ± 0.7 | 0.050 |
| Venous thromboembolic events (events 9 and 10 above) | 16 / 12 | 19 / 7 | 6.7 ± 1.7 | 2.8 ± 1.1 | 0.005 |
Acknowledgments
Funding: This work was partially funded by a grant from the National Institutes of Health (R01 AR46849 and HL66216) and made possible by the Rochester Epidemiology Project (R01 AG034676 from the National Institute on Aging). This project was supported by NIH/NCRR CTSA Grant Number UL1 RR024150. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005;52(2):402–411. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 2.Nicola PJ, Maradit-Kremers H, Roger VL, Jacobsen SJ, Crowson CS, Ballman KV, et al. The risk of congestive heart failure in rheumatoid arthritis: a population-based study over 46 years. Arthritis Rheum. 2005;52(2):412–420. doi: 10.1002/art.20855. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez A, Maradit Kremers H, Crowson CS, Nicola PJ, Davis JM, 3rd, Therneau TM, et al. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 2007;56(11):3583–3587. doi: 10.1002/art.22979. [DOI] [PubMed] [Google Scholar]
- 4.Matta F, Singala R, Yaekoub AY, Najjar R, Stein PD. Risk of venous thromboembolism with rheumatoid arthritis. Thromb Haemost. 2009;101(1):134–138. [PubMed] [Google Scholar]
- 5.Kawakami K, Ikari K, Kawamura K, Tsukahara S, Iwamoto T, Yano K, et al. Complications and features after joint surgery in rheumatoid arthritis patients treated with tumour necrosis factor-alpha blockers: perioperative interruption of tumour necrosis factor-alpha blockers decreases complications? Rheumatology (Oxford) 2010;49(2):341–347. doi: 10.1093/rheumatology/kep376. [DOI] [PubMed] [Google Scholar]
- 6.Petitpain N, Gambier N, Wahl D, Chary-Valckenaere I, Loeuille D, Gillet P. Arterial and venous thromboembolic events during anti-TNF therapy: a study of 85 spontaneous reports in the period 2000–2006. Biomed Mater Eng. 2009;19(4–5):355–364. doi: 10.3233/BME-2009-0600. [DOI] [PubMed] [Google Scholar]
- 7.Ramagopalan SV, Wotton CJ, Handel AE, Yeates D, Goldacre MJ. Risk of venous thromboembolism in people admitted to hospital with selected immune-mediated diseases: record-linkage study. BMC Med. 2011;9:1. doi: 10.1186/1741-7015-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casado Naranjo I, Martin Gonzalez R, Sancho Rieger J, Morera Guitart J, Calvo Catala J. Transient ischaemic attacks associated with thrombocytosis in active rheumatoid arthritis. J Neurol Neurosurg Psychiatry. 1988;51(12):1599. doi: 10.1136/jnnp.51.12.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pines A, Kaplinsky N, Olchovsky D, Bregman J, Frankl O. Recurrent transient ischemic attacks associated with thrombocytosis in rheumatoid arthritis. Clin Rheumatol. 1982;1(4):291–293. doi: 10.1007/BF02032089. [DOI] [PubMed] [Google Scholar]
- 10.Liang KP, Liang KV, Matteson EL, McClelland RL, Christianson TJ, Turesson C. Incidence of noncardiac vascular disease in rheumatoid arthritis and relationship to extraarticular disease manifestations. Arthritis Rheum. 2006;54(2):642–648. doi: 10.1002/art.21628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadareishvili Z, Michaud K, Hallenbeck JM, Wolfe F. Cardiovascular, rheumatologic, and pharmacologic predictors of stroke in patients with rheumatoid arthritis: a nested, case-control study. Arthritis Rheum. 2008;59(8):1090–1096. doi: 10.1002/art.23935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solomon DH, Goodson NJ, Katz JN, Weinblatt ME, Avorn J, Setoguchi S, et al. Patterns of cardiovascular risk in rheumatoid arthritis. Ann Rheum Dis. 2006;65(12):1608–1612. doi: 10.1136/ard.2005.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107(9):1303–1307. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 14.Haug ES, Skomsvoll JF, Jacobsen G, Halvorsen TB, Saether OD, Myhre HO. Inflammatory aortic aneurysm is associated with increased incidence of autoimmune disease. J Vasc Surg. 2003;38(3):492–497. doi: 10.1016/s0741-5214(03)00340-9. [DOI] [PubMed] [Google Scholar]
- 15.del Rincon I, Haas RW, Pogosian S, Escalante A. Lower limb arterial incompressibility and obstruction in rheumatoid arthritis. Ann Rheum Dis. 2005;64(3):425–432. doi: 10.1136/ard.2003.018671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Firth J, Helliwell P, Hale C, Hill J, Nelson EA. The predictors of foot ulceration in patients with rheumatoid arthritis: a preliminary investigation. Clin Rheumatol. 2008;27(11):1423–1428. doi: 10.1007/s10067-008-0940-y. [DOI] [PubMed] [Google Scholar]
- 17.Stamatelopoulos KS, Kitas GD, Papamichael CM, Kyrkou K, Zampeli E, Fragiadaki K, et al. Subclinical peripheral arterial disease in rheumatoid arthritis. Atherosclerosis. 2010;212(1):305–309. doi: 10.1016/j.atherosclerosis.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Avina-Zubieta JA, Abrahamowicz M, Choi HK, Rahman MM, Sylvestre MP, Esdaile JM, et al. Risk of cerebrovascular disease associated with the use of glucocorticoids in patients with incident rheumatoid arthritis: a population-based study. Annals of the rheumatic diseases. 2011 doi: 10.1136/ard.2010.140210. [DOI] [PubMed] [Google Scholar]
- 19.Kurland LT, Molgaard CA. The patient record in epidemiology. Sci Am. 1981;245(4):54–63. doi: 10.1038/scientificamerican1081-54. [DOI] [PubMed] [Google Scholar]
- 20.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 21.Doran MF, Pond GR, Crowson CS, O'Fallon WM, Gabriel SE. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis Rheum. 2002;46(3):625–631. doi: 10.1002/art.509. [DOI] [PubMed] [Google Scholar]
- 22.Gabriel SE, Crowson CS, O'Fallon WM. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955–1985. Arthritis Rheum. 1999;42(3):415–420. doi: 10.1002/1529-0131(199904)42:3<415::AID-ANR4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 23.Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising? Results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 25.Kremers HM, Crowson CS, Therneau TM, Roger VL, Gabriel SE. High ten-year risk of cardiovascular disease in newly diagnosed rheumatoid arthritis patients: a population-based cohort study. Arthritis Rheum. 2008;58(8):2268–2274. doi: 10.1002/art.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez A, Maradit Kremers H, Crowson CS, Ballman KV, Roger VL, Jacobsen SJ, et al. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis. 2008;67(1):64–69. doi: 10.1136/ard.2006.059980. [DOI] [PubMed] [Google Scholar]
- 27.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52(3):722–732. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 28.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. Journal of the American College of Cardiology. 1993;22(4 Suppl A):6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 29.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 30.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. The Annals of Statistics. 1988;16(3):1141–1154. [Google Scholar]
- 31.Turesson C, Jacobsson L, Bergstrom U. Extra-articular rheumatoid arthritis: prevalence and mortality. Rheumatology. 1999;38(7):668–674. doi: 10.1093/rheumatology/38.7.668. [DOI] [PubMed] [Google Scholar]
- 32.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158(6):585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 33.Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost. 2005;3(8):1611–1677. doi: 10.1111/j.1538-7836.2005.01415.x. [DOI] [PubMed] [Google Scholar]
- 34.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160(6):809–815. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 35.Heit JA, O'Fallon WM, Petterson TM, Lohse CM, Silverstein MD, Mohr DN, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002;162(11):1245–1248. doi: 10.1001/archinte.162.11.1245. [DOI] [PubMed] [Google Scholar]
- 36.Samama MM. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius study. Arch Intern Med. 2000;160(22):3415–3420. doi: 10.1001/archinte.160.22.3415. [DOI] [PubMed] [Google Scholar]
- 37.Eichinger S, Hron G, Bialonczyk C, Hirschl M, Minar E, Wagner O, et al. Overweight, obesity, and the risk of recurrent venous thromboembolism. Arch Intern Med. 2008;168(15):1678–1683. doi: 10.1001/archinte.168.15.1678. [DOI] [PubMed] [Google Scholar]
- 38.Hunt BJ. The prevention of hospital-acquired venous thromboembolism in the United Kingdom. Br J Haematol. 2009;144(5):642–652. doi: 10.1111/j.1365-2141.2008.07517.x. [DOI] [PubMed] [Google Scholar]
- 39.Gabriel SE. The sensitivity and specificity of computerized databases for the diagnosis of rheumatoid arthritis. Arthritis Rheum. 1994;37(6):821–823. doi: 10.1002/art.1780370607. [DOI] [PubMed] [Google Scholar]
- 40.Olech E, Merrill JT. The prevalence and clinical significance of antiphospholipid antibodies in rheumatoid arthritis. Curr Rheumatol Rep. 2006;8(2):100–108. doi: 10.1007/s11926-006-0049-8. [DOI] [PubMed] [Google Scholar]
- 41.Gladd DA, Olech E. Antiphospholipid antibodies in rheumatoid arthritis: identifying the dominoes. Curr Rheumatol Rep. 2009;11(1):43–51. doi: 10.1007/s11926-009-0007-3. [DOI] [PubMed] [Google Scholar]
- 42.Meune C, Touze E, Trinquart L, Allanore Y. High risk of clinical cardiovascular events in rheumatoid arthritis: Levels of associations of myocardial infarction and stroke through a systematic review and meta-analysis. Archives of cardiovascular diseases. 2010;103(4):253–261. doi: 10.1016/j.acvd.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 43.van der Vliet JA, Boll AP. Abdominal aortic aneurysm. Lancet. 1997;349(9055):863–866. doi: 10.1016/s0140-6736(96)07282-0. [DOI] [PubMed] [Google Scholar]