Abstract
Background
In Korean women, many of whom have small to moderate-sized breasts, it is difficult to cover a partial breast defect using oncoplastic volume displacement techniques after removal of an adequate volume of tissue during oncologic surgery. In such cases, oncoplastic volume replacement techniques are more useful.
Methods
From January 2007 to December 2011, 104 women underwent a total of 107 breast-conserving surgeries with various kinds of oncoplastic volume replacement techniques. We used latissimus dorsi (LD) myocutaneous flap for cases in which the resection mass was greater than 150 g. In case with a resection mass less than 150 g, we used regional flaps such as a lateral thoracodorsal flap, a thoracoepigastric flap, or perforator flaps such as an intercostal artery perforator (ICAP) flap or a thoracodorsal artery perforator (TDAP) flap.
Results
The mean age was 46.1 years, and the average follow-up interval was 10.3 months. The patients underwent oncoplastic volume replacement techniques with a lateral thoracodorsal flap (n=9), thoracoepigastric flap (n=7), ICAP flap (n=25), TDAP flap (n=12), and LD flap (n=54). There was one case of congestion in an LD flap, and two cases of fat necrosis in an ICAP flap. Most of the patients were satisfied with the cosmetic results.
Conclusions
Oncoplastic volume replacement techniques can be reliable and useful for the correction of breast deformity after breast-conserving surgery, especially in patients with small to moderate-sized breasts.
Keywords: Breast neoplasms, Mammaplasty, Surgical flaps
INTRODUCTION
The increasing incidence of breast cancer has made it one of the most prevalent cancers in Korean women since 2001 [1]. With the recent advancements in diagnostic regimens for breast cancer and the universalized use of regular check-ups, very small, early-stage breast cancers are increasingly being detected. Thus, breast-conserving surgery is frequently performed, and attempts have been made to develop techniques to minimize postoperative breast deformity after partial mastectomy.
The oncoplastic surgery technique, which was first attempted by Audretsch et al. [2], expands on the concept of breast-conserving surgery. In other words, it is a plastic surgical procedure wherein the breast is shaped using the residual breast tissue after an extensive resection of the breast cancer or is reconstructed using the adjacent tissue.
There are two different techniques in oncoplastic surgery depending on the volume of the excised breast tissue. One is the volume displacement procedure, which combines resection with a variety of different breast-reshaping and breast-reduction techniques; the other is the volume replacement procedure in which the volume of excised breast tissue is replaced with autologous tissue [3].
To customize the application of the appropriate oncoplastic procedure to a patient, the breast size and the excised volume should first be considered. In cases where the breast size is relatively large, as in much of the Caucasian population, the amount of residual tissue after the resection of the tumor is sufficient. Therefore, satisfactory cosmetic outcomes can be obtained by glandular reshaping in patients with a relatively small defect size and using reduction mammoplasty when the area of the defect is larger than moderate.
For patients with a relatively small breast size, as in the Asian population, the volume displacement techniques can also be performed after the removal of a small-sized defect. For cases in which the area of the defect is considered larger than moderate, however, satisfactory cosmetic outcomes can only be obtained if the defect area is restored using a volume replacement procedure with adjacent tissue.
Given the above background, we evaluated patients who underwent breast reconstruction using a volume replacement technique after partial mastectomy. In this report, we describe the clinical usefulness and necessity of our surgical procedures.
METHODS
Of the patients who underwent breast-conserving surgery at the Department of Surgery of the author's hospital from January 2007 to December 2011 for breast cancer management, those with small to moderate-sized breasts underwent oncoplastic breast surgery by a single plastic surgeon. If the excised volume was less than 50 g, oncoplastic volume displacement techniques were performed and thus the case was excluded from this series. A total of 104 patients who underwent a total of 107 oncoplastic volume replacement procedures were enrolled in this study. The inclusion criteria were the presence of tumors at all the sites, including under the nipple, and the lack of preoperative anti-cancer chemotherapy. The charts of all the patients who underwent breast reconstruction during the study period were reviewed.
After extensive resection at a distance of approximately 2 cm from the initial tumor margin under general anesthesia, the tumor invasion was evaluated at the resection margin using the frozen section in the operating room. In the patients with a confirmed tumor invasion via a sentinel node biopsy, axillary node dissection was performed in the level I and II nodes. Various volume replacement techniques were performed based on the weight of the excised tumor and its resection margin. We used a latissimus dorsi (LD) myocutaneous flap for cases in which the resection mass was greater than 150 g. For cases in which the resection mass was less than 150 g, we used regional flaps such as a lateral thoracodorsal flap, a thoracoepigastric flap, or perforator flaps such as an intercostal artery perforator (ICAP) flap or a thoracodorsal artery perforator (TDAP) flap.
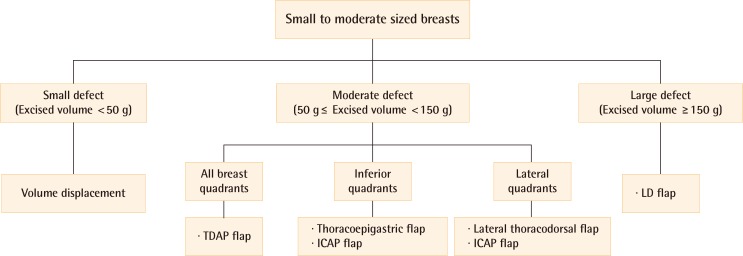
To select the appropriate oncoplastic procedure, the location of the tumor is also considered. When a tumor located in the lateral quadrants (upper outer, lower outer quadrant of the breast), a lateral thoracodorsal flap can be used. A thoracoepigastric flap can be chosen when a tumor is located in the inferior quadrants (lower inner, lower outer quadrant). An ICAP flap can be an alternative choice for lateral and inferior quadrants. A TDAP flap can be also used for lateral and inferior quadrants, even for the upper inner quadrant (Fig. 1).
Fig. 1.
Algorithm of partial breast reconstruction
This figure shows an algorithm of partial breast reconstruction with oncoplastic techniques in small to moderate-sized breasts. ICAP, intercostal artery perforator; TDAP, thoracodorsal artery perforator; LD, latissimus dorsi.
A questionnaire survey was administered six months after surgery to the patients to evaluate their general and aesthetic satisfaction. This standardized questionnaire was adapted from the Michigan Breast Reconstruction Outcomes Survey [4]; it consisted of seven questions (Table 1). Questions 1 to 5 assessed the general satisfaction of the patients with reconstruction, and questions 6 and 7 assessed their aesthetic satisfaction. In question 7, "the implant, transverse rectus abdominis myocutaneous (TRAM), deep inferior epigastric perforator, or latissimus procedure," was changed to "several procedures." Each question was graded on a 5-point Likert scale, and the answers ranged from "very dissatisfied" (1) to "very satisfied" (5). Each answer was then coded in a dichotomous manner, with a "satisfied" score for each question representing answers 4 or 5, and a "dissatisfied" score representing answers 1, 2, or 3. The patients' levels of satisfaction were coded as "generally satisfied" when they had a satisfied score of 5/5 for the questions on their general satisfaction, and as "aesthetically satisfied" when they had a score of 2/2 for the questions on their aesthetic satisfaction.
Table 1.
Modified Michigan Breast Reconstruction Outcomes Survey
From Alderman et al. [4], with permission from American Society of Plastic Surgeons.
Three blinded plastic surgeons performed an aesthetic analysis using photographs of the patients with frontal and bilateral oblique views for each patient. The surgeons reviewed the photographs and scored the results on a 5-point Likert scale that ranged from "poor result" (1) to "excellent result" (5).
RESULTS
A total of 104 patients underwent breast reconstruction using oncoplastic volume replacement techniques after partial mastectomy. In our series, the mean age was 46.1 years (range, 27 to 65 years) and the mean postoperative follow-up period was 10.3 months (range, 4 to 21 months).
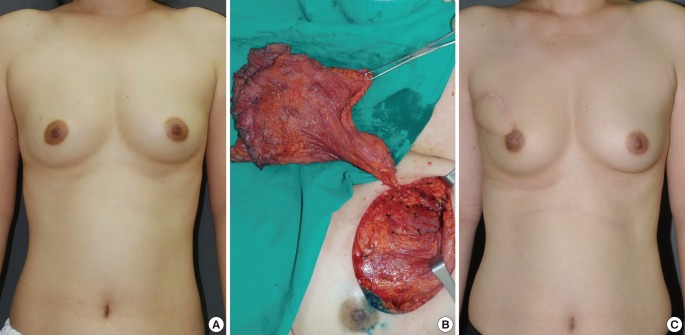
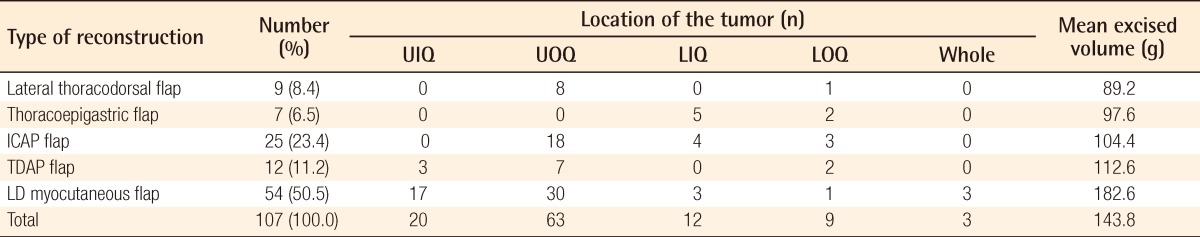
The mean weight of the resected breast tissue was 143.8 g (range, 50 to 390 g). The tumor location in the overall breast area varied (Table 2). Grossly, there was one case of adenocarcinoma, 40 cases of ductal carcinoma in situ, two cases of intraductal papilloma, 63 cases of invasive ductal carcinoma, and one case of invasive mixed ductal and mucinous carcinoma. The oncoplastic procedures were performed using a lateral thoracodorsal flap (Fig. 2) in 9 patients, a thoracoepigastric flap in 7 patients, an ICAP flap in 25 patients (Fig. 3), a TDAP flap in 12 patients (Fig. 4), and an LD myocutaneous flap in 54 patients (Fig. 5). Regarding tumor location, 3 cases of TDAP flap and 17 cases of LD flap were performed for tumors located in the upper inner quadrant of the breast; 8 cases of lateral thoracodorsal flap, 18 cases of ICAP flap, 7 cases of TDAP flap, and 30 cases of LD flap in the upper outer quadrant; 5 cases of thoracoepigastric flap, 4 cases of ICAP flap, and 3 cases of LD flap in the lower inner quadrant; and one case of lateral thoracodorsal flap, 2 cases of thoracoepigastric flap, 3 cases of ICAP flap, 2 cases of TDAP flap, and one case of LD flap in the lower outer quadrant (Table 3).
Table 2.
Characteristics of breast cancers
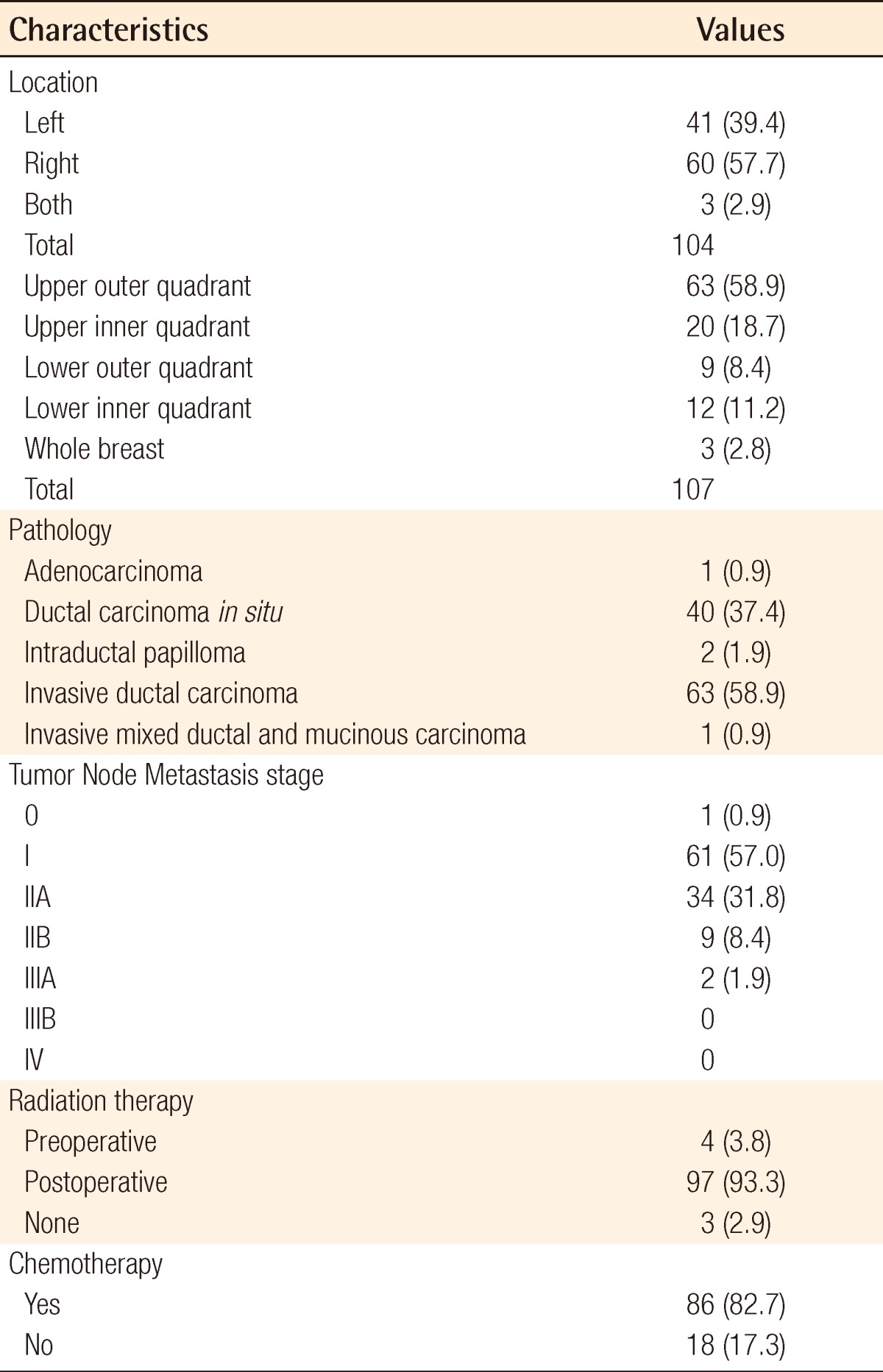
Values are presented as number (%).
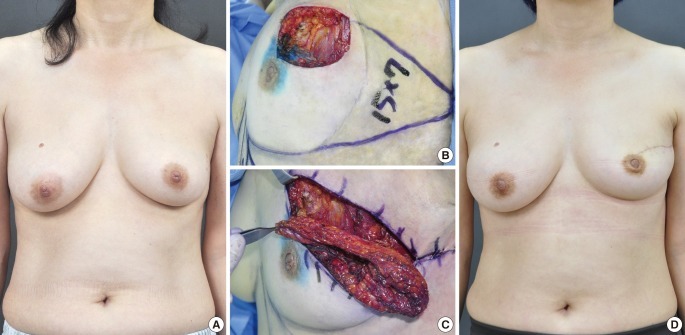
Fig. 2.
Case of a lateral thoracodorsal flap
A 47-year-old woman with invasive ductal carcinoma in the left breast. (A) Preoperative view. (B) Intraoperative view of the designed lateral thoracodorsal flap after partial mastectomy. (C) Intraoperative view of the elevated flap. (D) 3-month postoperative outcome.
Fig. 3.
Case of an intercostal artery perforator flap
A 45-year-old woman with invasive ductal carcinoma in the left breast. (A) Preoperative view. (B) Intraoperative view of the elevated intercostal artery perforator flap. An intercostal artery perforator was found. (C) 6-month postoperative outcome.
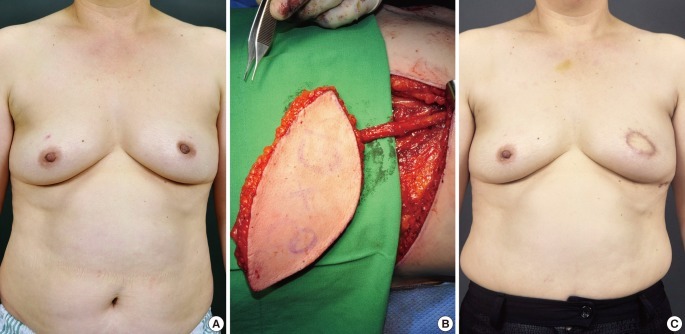
Fig. 4.
Case of a thoracodorsal artery perforator flap
A 55-year-old woman with invasive ductal carcinoma in the left breast. (A) Preoperative view. (B) Intraoperative view of the elevated thoracodorsal artery perforator flap. (C) 4-month postoperative outcome.
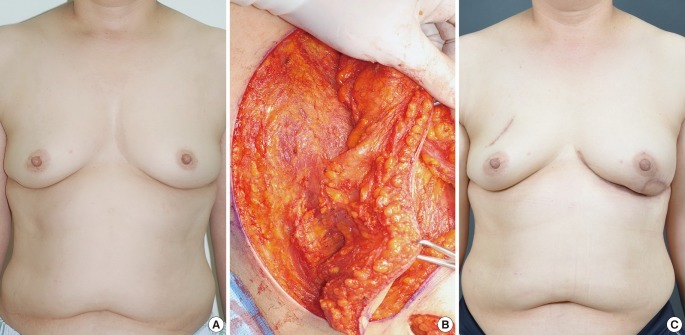
Fig. 5.
Case of a latissimus dorsi myocutaneous flap
A 35-year-old woman with invasive ductal carcinoma in the right breast. (A) Preoperative view. (B) Intraoperative view of the elevated latissimus dorsi myocutaneous flap. (C) 24-month postoperative outcome.
Table 3.
Mean volume of the excised breasts and location of the tumor according to the surgical technique
UIQ, upper inner quadrant; UOQ, upper outer quadrant; LIQ, lower inner quadrant; LOQ, lower outer quadrant; ICAP, intercostal artery perforator; TDAP, thoracodorsal artery perforator; LD, latissimus dorsi.
Postoperatively, there was one case of congestion after the use of the LD flap, and two cases of fat necrosis after the use of the ICAP flap. There were 11 cases (20.4%) of persistent seroma at the donor site of the LD flap. These cases were all resolved, however, using regular needle aspiration. There were no postoperative complications such as flap necrosis or wound infection.
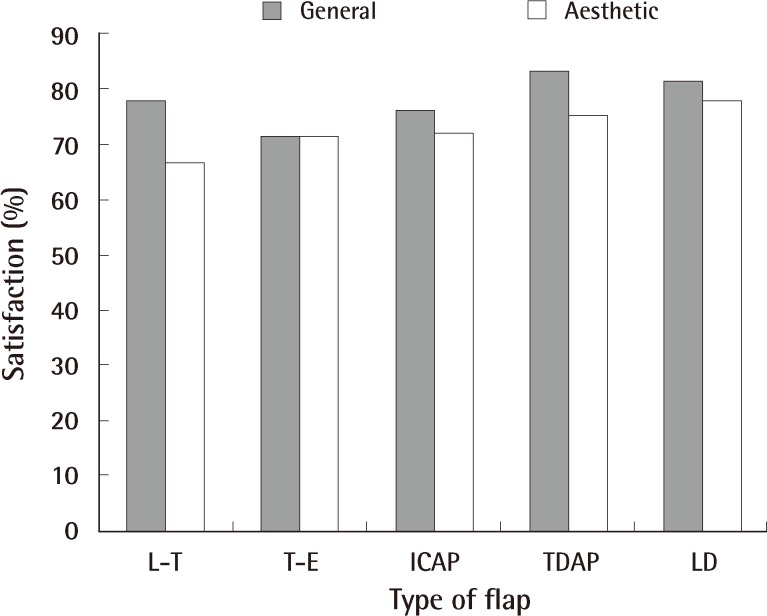
The general satisfaction scores showed that 85 patients (81.7%) were satisfied, and the aesthetic satisfaction scores showed that 80 patients (76.9%) were satisfied. When examining general satisfaction according to type, TDAP flap had the highest scores among the other types of flaps. Aesthetic satisfaction also showed similar results, as LD flap and TDAP flap had higher scores than the others (Fig. 6). The mean aesthetic score of the plastic surgeons was 4.08. These results indicate that the cosmetic outcomes were generally satisfactory.
Fig. 6.
General and aesthetic satisfaction according to the type of flap
L-T, lateral thoracodorsal; T-E, thoracoepigastric; ICAP, intercostal artery perforator; TDAP, thoracodorsal artery perforator; LD, latissimus dorsi.
DISCUSSION
According to a 7-year observation study, the results of which were reported by Veronesi et al. [5] in 1981, the comparison of a group where quadrantectomy was performed concomitantly with radiotherapy with a group that underwent modified radical mastectomy for breast cancer showed no significant difference in their survival rates. According to Fisher et al. [6], in 1985, a comparison of modified radical mastectomy and breast-conserving surgery in cases of negative <4 cm axillary lymph node metastasis showed no significant differences in the local recurrence and survival rates of the two groups. Since then, breast-conserving surgery has been accepted as the primary treatment modality in patients with stages I and II breast cancer, unless there are specific contraindications [7].
In an oncoplastic procedure, an expanded concept of breast-conserving surgery, the breast is shaped using the residual breast tissue following extensive resection of breast cancer, or is reconstructed using the adjacent tissue [8,9]. It can improve the cosmetic outcomes of reconstruction while increasing the distance of the margins from the tumor. The comparison by Kaur et al. [10] of the volumes of the resected tissue and the degrees of infiltration of the surgical margin in the oncoplastic procedure along with standard quadrantectomy in patients with breast cancer showed that the oncoplastic procedure can resect a larger quantity of tissue and thus wider negative surgical margin can be obtained of the surgical margin. Clough et al. [11] reported that the oncoplastic procedure had satisfactory cosmetic and oncologic outcomes in 300 patients. Oncoplastic techniques allow extensive resections for breast-conserving surgery, with favorable cosmesis.
There are two techniques in oncoplastic surgery according to the volume of the excised breast tissue. One includes volume displacement procedures that combine resection with a variety of different breast-reshaping and breast-reduction techniques, and the other is a volume replacement procedure that replaces the volume of excised breast tissue using autologous tissue. To select the appropriate oncoplastic procedure, multiple factors should be considered including the breast size, the excised volume, and the location of the breast tumor. In cases in which the breast size is relatively large, the amount of the residual tissue after the resection of the tumor is sufficient. Therefore, satisfactory cosmetic outcomes can be obtained from the volume displacement procedures such as glandular reshaping and reduction mammoplasty. In cases in which the breast size is relatively small, volume displacement techniques can also be performed after the removal of a small tumor. In cases, however, in which the defect area is larger than moderate, satisfactory cosmetic outcomes can only be obtained after the defect area is restored using volume replacement techniques.
In this study, 5 volume replacement procedures were performed based on the excised volume of the breast and the location of the tumor. We used an LD myocutaneous flap for cases with a resection mass greater than 150 g. For cases with a resection mass less than 150 g, we used regional flaps such as a lateral thoracodorsal flap, a thoracoepigastric flap, or perforator flaps such as an ICAP flap or TDAP flap. If the resection mass was less than 150 g, we selected a lateral thoracodorsal flap or an ICAP flap when a tumor was located in the lateral quadrants. A thoracoepigastric flap or an ICAP flap can be chosen when a tumor is located in the inferior quadrants. A TDAP flap can be used for all breast quadrants.
A lateral thoracodorsal flap is a wedge-shaped transposition flap. Patients with lateral breast cancer and redundant lateral thoracic region might be candidates for this flap. The axis of the flap is in the lateral extension of the inframammary fold. The superior border of the flap begins at the point medial to the anterior axillary fold and extends laterally from there. In elevating the flap, the underlying fascias of the LD and the anterior serratus muscles must be included. This fascia provides viability to the flap because the flap vascular supply is derived from the lateral intercostal perforators and the muscular fascia [12]. These flaps provide excellent skin and tissue matching to the native breast.
A thoracoepigastric flap is designed as a transposition flap. Its upper margin is at the inframammary fold, and its lower margin is drawn with consideration of the pedicle. It is supplied by the musculocutaneous perforating vessels from the superior epigastric artery and vein extending from the underlying rectus abdominis muscle. It can be elevated either above or below the rectus fascia and investing fascia of the external oblique musculature. This flap commonly has a rather broad base and is limited in mobility [13]. It is usually indicated for defects in the lower quadrant of the breast.
An LD flap has excellent blood supply and provides both muscle for filling glandular defects and skin for cutaneous deficiencies. Its disadvantages include a difficult surgical technique as compared to regional flap, long surgical time, and postoperative complications at the donor site such as the restriction of shoulder movement and seroma.
Methods of decreasing donor site morbidity associated with the use of the LD flap have been suggested in several studies. Hamdi et al. [14] recently described the use of a pedicled skin- and fat-only flap over the LD flap, based on the TDAP or ICAP vessels. Therefore, a TDAP flap, which spares the LD muscle, can reduce the occurrence of postoperative complications at the donor sites, and thus shorten the recovery period.
An ICAP flap is designed over the thoracic region at the level of the inframammary fold. The flap width depended on the expected defect and the flap length on the location of the defect. This flap is appropriate for lateral and inferior breast defects. The intercostal vessels form an arcade, which gives numerous perforators. The ICAP flap has a limited range of motion because it can only be used in the adjacent area due to its short perforator. Additionally, it sometimes provides an inadequate perforator, which leads to instability of the flap condition.
A TDAP flap is supplied by perforating vessels from the thoracodorsal artery, which provides the advantage of preserving the LD muscle [15]. When an appropriate perforating vessel is selected, the dissection is continued through the LD muscle up to the thoracodorsal artery and vein. A tunnel is created between the anterior edge of the LD muscle and the defect, through which the flap passes. The flap is then placed on the breast defect. It is often used for defects in the lateral and central, and even medial breast regions.
In cases in which the breast size is relatively large, as in much of the Caucasian population, excellent cosmetic outcomes can be achieved using a volume displacement procedure, one of the oncoplastic procedures. In many cases, however, the breast size is relatively small, as in Korean women, and it is difficult to achieve satisfactory results using a volume displacement procedure. Accordingly, in these cases, the defect areas should be restored using a volume replacement procedure. This may be mandatory to obtain more satisfactory postoperative outcomes from a cosmetic perspective.
Footnotes
This research was supported by Kyungpook National University Research Fund, 2012.
No potential conflict of interest relevant to this article was reported.
References
- 1.Shin HR, Jung KW, Won YJ, et al. 2002 annual report of the Korea Central Cancer Registry: based on registered data from 139 hospitals. Cancer Res Treat. 2004;36:103–114. doi: 10.4143/crt.2004.36.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Audretsch WP, Rezai M, Kolotas C, et al. Tumor-specific immediate reconstruction (TSIR) in breast cancer patients. Perspect Plast Surg. 1998;11:71–100. [Google Scholar]
- 3.Yang JD, Bae SG, Chung HY, et al. The usefulness of oncoplastic volume displacement techniques in the superiorly located breast cancers for Korean patients with small to moderate-sized breasts. Ann Plast Surg. 2011;67:474–480. doi: 10.1097/SAP.0b013e318201fdf4. [DOI] [PubMed] [Google Scholar]
- 4.Alderman AK, Wilkins EG, Lowery JC, et al. Determinants of patient satisfaction in postmastectomy breast reconstruction. Plast Reconstr Surg. 2000;106:769–776. doi: 10.1097/00006534-200009040-00003. [DOI] [PubMed] [Google Scholar]
- 5.Veronesi U, Saccozzi R, Del Vecchio M, et al. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med. 1981;305:6–11. doi: 10.1056/NEJM198107023050102. [DOI] [PubMed] [Google Scholar]
- 6.Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312:665–673. doi: 10.1056/NEJM198503143121101. [DOI] [PubMed] [Google Scholar]
- 7.NIH consensus conference. Treatment of early-stage breast cancer. JAMA. 1991;265:391–395. [PubMed] [Google Scholar]
- 8.Anderson BO, Masetti R, Silverstein MJ. Oncoplastic approaches to partial mastectomy: an overview of volume-displacement techniques. Lancet Oncol. 2005;6:145–157. doi: 10.1016/S1470-2045(05)01765-1. [DOI] [PubMed] [Google Scholar]
- 9.Bae SG, Yang JD, Lee SY, et al. Oncoplastic techniques for treatment of inferiorly located breast cancer. J Korean Soc Plast Reconstr Surg. 2008;35:680–686. [Google Scholar]
- 10.Kaur N, Petit JY, Rietjens M, et al. Comparative study of surgical margins in oncoplastic surgery and quadrantectomy in breast cancer. Ann Surg Oncol. 2005;12:539–545. doi: 10.1245/ASO.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 11.Clough KB, Lewis JS, Couturaud B, et al. Oncoplastic techniques allow extensive resections for breast-conserving therapy of breast carcinomas. Ann Surg. 2003;237:26–34. doi: 10.1097/00000658-200301000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmstrom H, Lossing C. The lateral thoracodorsal flap in breast reconstruction. Plast Reconstr Surg. 1986;77:933–943. doi: 10.1097/00006534-198606000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Huemer GM. Partial mastectomy: breast reconstruction with the pedicled thoracoepigastric flap. In: Fitzal F, Schrenk P, editors. Oncoplastic breast surgery: a guide to clinical practice. Vienna: Springer; 2010. pp. 127–132. [Google Scholar]
- 14.Hamdi M, Salgarello M, Barone-Adesi L, et al. Use of the thoracodorsal artery perforator (TDAP) flap with implant in breast reconstruction. Ann Plast Surg. 2008;61:143–146. doi: 10.1097/SAP.0b013e318158fd7b. [DOI] [PubMed] [Google Scholar]
- 15.Khoobehi K, Allen RJ, Montegut WJ. Thoracodorsal artery perforator flap for reconstruction. South Med J. 1996;89:S110. [Google Scholar]